Abstract
Chemo-immunotherapy has shown great promise as a next-generation treatment strategy for established solid tumors. Cheng et al. first developed the PD-L1- and CD44-responsive multifunctional nanoparticles (MNPs) utilizing a polymer complex of polyethyleneimine and oleic acid (PEI-OA) and loaded with two chemotherapeutic drugs (paclitaxel and chloroquine), an antigen (ovalbumin), an immunopotentiator (CpG), and an immune checkpoint inhibitor (anti-PD-L1 antibody). PEI-OA increases the drug loading capacity and encapsulation efficiency of the nanoplatform. Anti-PD-L1 antibody promotes its cellular uptake, increases the levels of CD4+ and CD8+ T cells and inhibits primary breast cancer at the tumor site. Paclitaxel triggers tumor cells apoptosis directly, and then produces tumor vaccines to promote the dendritic cells (DCs) maturation, finally primes the T cells activation. Ovalbumin as a model antigen and CpG as an immunopotentiator to stimulate the DCs maturation. Moreover, chloroquine turns the “immune-cold” environment and potentiates the anti-tumor effect of both immune checkpoint inhibitor and chemotherapeutics, thus synergically and efficiently improving the breast cancer immunotherapy.
Keywords
Anti-PD-L1 antibody, Autophagy response, Immuno-chemotherapy, Multifunctional nanoparticles
Commentary
Breast cancer is one of the three most common cancers and the most commonly occurring cancer in women, and it presents the leading cause of death in women throughout the world [1]. Anti-cancer therapy for breast tumors is challenging due to the highly metastatic progression of the disease to other organs, such as lung, liver, and bone [2,3]. Chemo-immunotherapy is very promising for its applications in metastatic breast cancer therapy [4].
Among the developed Chemo-immunotherapy strategies, the combination of chemotherapeutics, immune checkpoint therapies, cancer vaccines, and adoptive T-cell transfer may provide an ideal approach to eradicate cancers and prevent tumor recurrence and metastasis [4-6]. The employment of combination therapy is obviously better than individual therapies, such as chances for superior efficacy and dose reduction while maintaining or improving efficacy, decreased toxicity, and delayed or reduced development of drug resistance [4]. Although combination therapy has these advantages, its clinical applications are not satisfying mainly due to the inefficient effector T cells (“immune-cold” tumors) and tumor microenvironment (TME) immunosuppression [6].
Autophagy is a “double-edged sword” in the TME. It can promote or inhibit tumor progression in a context-dependent manner, including variable tumor genetics, the cell and tissue types, and the stages of tumor [7]. For example, autophagy potentiates major histocompatibility complex (MHC) class I (MHC-I) internalization and degradation in DCs and reduces T cells activation [8]. Also, autophagy can improve the MHC-I degradation in tumor cells and then preclude T cell recognition [9]. Remarkably, autophagy inhibitor monotherapy or combination with immunotherapy is able to reshape the TME and reverse its “immune-cold” feature and improve tumor control, thus presenting tremendous promises for cancer therapy [7].
Recently, Cheng et al. [2] first constructed a PD-L1- and CD44-responsive multilayered nanoplatform through a convenient, ultrafast and universal self-assembly route. The developed MNPs were loaded with two chemotherapeutic drugs (paclitaxel (PTX) and Chloroquine (CQ)) in the core, an immunopotentiator (CpG) and an antigen (ovalbumin (OVA)) in the middle, and an immune checkpoint inhibitor (anti-PD-L1 antibody, also known as atezolizumab) at the outer layer (Figure 1A). The constructed MNPs were termed CpG+OVA+PTX+CQ-N/A. The further studies showed that the MNPs were safe with excellent cell permeability and tumor targeting [2].
Then the authors analysed the MNPs-induced 4T1 cell death. The IC50 value was 22.85 μg/mL and 18.12 μg/mL for a solution containing free CpG, OVA, and PTX (CpG+OVA+PTX-S) and CpG/OVA/PTX-loaded MNPs (CpG+OVA+PTX-N). Further loading CQ and atezolizumab CpG+OVA+PTX+CQ-N/A reduced the IC50 value to 5.23 μg/ mL. These results are consistent with the notion that nanotechnology helps with dose reduction while maintaining efficacy, and combination of tumor- sensitive drugs is superior to individual drugs for cancer therapy [4]. Consistently, the cell death and autophagosome induced by CpG+OVA+PTX+CQ-N is higher than CpG+OVA+PTX-N, but less to CpG+OVA+PTX+CQ-N/A, suggesting that CQ promoted cell death by autophagy inhibition, and atezolizumab increased cell death through MNPs uptake [2].
Next, Cheng et al. [2] studied the anti-tumor effect of MNPs by a 4T1 tumor-bearing Balb/c mice model. The results revealed that CpG+OVA+PTX-S did not suppress tumor growth, but CpG+OVA+PTX-N showed a stronger suppressive effect, suggesting that the MNPs targeted the tumor effectively. Moreover, CpG+OVA+PTX+CQ-N could not completely block tumor growth, but the suppression effect was stronger than CpG+OVA+PTX-N, indicating CQ inhibited autophagy and improved the anti-tumor effect. Remarkably, CpG+OVA+CQ+PTX-N/A nearly blocked tumor growth and showed the strongest inhibition of tumor growth, highlighting the therapeutic effect of the anti-PD-L1 antibody. In a mouse lung metastasis model, compared to the control, CpG+OVA+PTX+CQ-N/A partially inhibited lung metastasis and prolonged survival time [2]. Collectively, these results revealed that combination of an autophagy inhibitor with immunoadjuvants and immune-checkpoint blockade (ICB) is a more effective therapy to treat primary breast cancer and inhibit lung metastasis (Figure 1B).
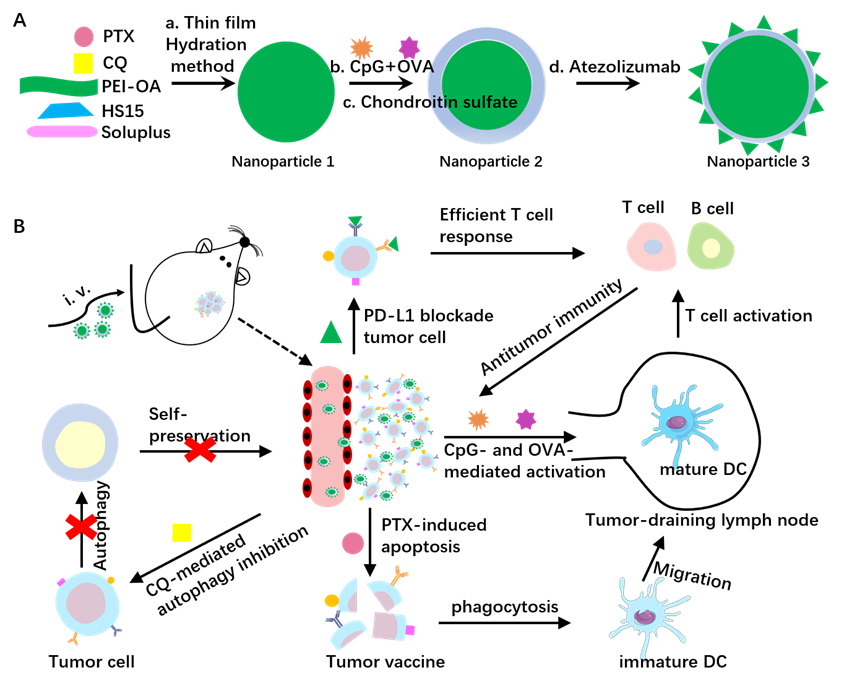
Cheng et al. [2] also addressed the mechanistic basis for the anti-tumor effect of MNPs in vivo. They found that CpG+OVA+PTX+CQ-N/A had higher autophagy flux at the tumor site, higher levels of TNF-α and IFN-γ in serum, and larger number of both CD8+ and CD4+ T cells in tumor and in spleen. These findings suggested that CpG+OVA+PTX+CQ-N/A may exhibit the anti-tumor effect through immunosuppression reversal and triggering a T-cell-mediated anti-tumor immunity by increasing the expression of cytokines and tumor vaccines, promoting DCs maturation, and generating T cells [2].
Although the mechanism by which the MNPs potentiates T cell activation requires further investigation, these results indicate that combination of an autophagy inhibitor with immunoadjuvants and ICB may provide a more effective therapy for patients who are suffering breast cancer. However, there are a few factors to be addressed in order to successfully translate these findings to the clinic. Firstly, considering the promoting or suppression effects of autophagy inhibition on immune cells including DCs, macrophages, and CD4+ and CD8+ T cells [7-9], the net effect on anti-tumor immunity needs further study. Secondly, both CQ and hydroxychloroquine (HCQ) are late-stage inhibitors of autophagy, but HCQ has less toxicity while maintaining efficacy [10]. It is possible that HCQ is a more proper inhibitor for clinical use. Thirdly, maximum benefit from MNPs-mediated treatment may result from proper identification of molecular subclasses and tumor stages of diseases [4], so the dosage and duration of the MNPs-mediated therapy need to be rigorously determined according to the actual situation of the patients. Further investigations to these questions will help to increase the possibility of successful translation of this Chemo-immunotherapy strategy into patients.
Funding
This work was supported by the National Key R&D Program of China (2021YFC 2100100).
References
2. Cheng YB, Wang CX, Wang HH, Zhang ZW, Yang XP, Dong YM, et al. Combination of an autophagy inhibitor with immunoadjuvants and an anti-PD-L1 antibody in multifunctional nanoparticles for enhanced breast cancer immunotherapy. BMC Medicine. 2022 Oct 28;20(1):411.
3. Katsura C, Ogunmwonyi I, Kankam HK, Saha S. Breast cancer: presentation, investigation and management. British Journal of Hospital Medicine (Lond). 2022 Feb 2;83(2):1-7.
4. Fisusi FA, Akala EO. Drug Combinations in Breast Cancer Therapy. Pharmaceutical Nanotechnology. 2019;7(1):3-23.
5. Deng CF, Zhang Q, Jia MD, Zhao J, Sun X, Gong T, et al. Tumors and Their Microenvironment Dual-Targeting Chemotherapy with Local Immune Adjuvant Therapy for Effective Antitumor Immunity against Breast Cancer. Advanced Science. 2019 Jan 30;6(6):1801868.
6. Song C, Phuengkham H, Kim YS, Dinh VV, Lee I, Shin IW, et al. Syringeable immunotherapeutic nanogel reshapes tumor microenvironment and prevents tumor metastasis and recurrence. Nat Commun. 2019 Aug 20;10(1):3745.
7. Xia HJ, Green DR, Zou WP. Autophagy in tumour immunity and therapy. Nat Rev Cancer. 2021 May;21(5):281-297.
8. Yamamoto K, Venida A, Yano JL, Biancur DE, Kakiuchi M, Gupta S, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020 May;581(7806):100-105.
9. Young TM, Reyes C, Pasnikowski E, Castanaro C, Wong C, Decker CE, et al. Autophagy protects tumors from T cell-mediated cytotoxicity via inhibition of TNF?-induced apoptosis. Science Immunology. 2020 Dec 18;5(54): eabb9561.
10. Mudaliar P, Nalawade A, Devarajan S, Aich J. Therapeutic potential of autophagy activators and inhibitors in lung and breast cancer- a review. Molecular Biology Reports. 2022 Nov;49(11):10783-10795.
