Abstract
Malignant peritoneal mesothelioma (MPM) is a rare disease with an unusual natural history. The malignancy progresses within the abdomen and pelvis until gastrointestinal function is lost and a terminal event occurs. Systemic chemotherapy treatments are never curative and usually only have transient benefits. Over the past 2 decades surgical procedures have been developed that allow complete or near complete removal of MPM from the parietal and visceral peritoneal surfaces. The goal of the cytoreductive surgery (CRS) is complete visible removal of MPM. In an attempt to preserve the disease-free status an intraperitoneal chemotherapy lavage of the peritoneal space is performed. The intraoperative chemotherapy is called hyperthermic intraperitoneal chemotherapy (HIPEC). It is performed for 90 minutes with the temperature of the chemotherapy solution at 42-43°C. The CRS plus HIPEC combined treatment is currently a global standard of care with a 50% five-year survival published by multiple peritoneal surface malignancy treatment centers. At the Washington Cancer Institute, additional regional (intraperitoneal) chemotherapy treatments have been added in an attempt to further improve survival. These treatments are early postoperative intraperitoneal chemotherapy (EPIC) with paclitaxel and normothermic intraperitoneal chemotherapy (NIPEC) long-term with pemetrexed and cisplatin. These additional regional chemotherapy strategies added on to CRS and HIPEC caused the five-year survival to be approximately 80%. Although no randomized controlled data is available, propensity matched data are presented. Regional chemotherapy treatments long-term are suggested for further studies with MPM if complete CRS is possible.
Keywords
Hyperthermia, Intraperitoneal Chemotherapy, Perioperative chemotherapy, Intraperitoneal port, Paclitaxel, Pemetrexed, Cisplatin, Cytoreductive surgery
Abbreviations
CC: Completeness of Cytoreduction; CRS: Cytoreductive Surgery; EPIC: Early Postoperative Intraperitoneal Chemotherapy; HIPEC: Hyperthermic Intraperitoneal Chemotherapy; HPLC: High Pressure Liquid Chromatography; HR: Hazard Ratio; MESNA: Sodium Methanethiolate; MPM: Malignant Peritoneal Mesothelioma; NIPEC: Normothermic Intraperitoneal Chemotherapy; PCI: Peritoneal Cancer Index; PSS: Prior Surgical Score
Introduction
In the United States malignant peritoneal mesothelioma (MPM) is a rare disease with approximately 300 new cases each year [1]. The malignancy progresses within the peritoneal space throughout its natural history so that a majority of patients die as a result of massive disease within the abdomen and pelvis. Persistence and then progression of mesothelioma occurs despite extensive surgical procedures to eradicate the disease. A manuscript to describe a new management plan was first published by Loggie et al. who treated 12 patients with cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy (HIPEC). The drug used by Loggie was mitomycin C [2]. Numerous single institution reports support the proposal that a complete or near complete cytoreductive surgery followed by HIPEC improved survival [3-11]. A multi-institutional data registry of 405 patients was performed by Yan and coworkers [12] and a systematic review and meta-analysis by Helm and coworkers [13] further supported that cytoreductive surgery plus HIPEC achieved prolonged survival. This combined treatment was presented as a standard of care in 2016 [14]. Recently, Kusamura et al. published practice guidelines from an extensive literature review. Kusamura et al. concluded that median survival has increased from one year to 7 years when cytoreductive surgery plus HIPEC is used on selected patients treated at experienced centers [15].
To date, no randomized trials regarding an optimal HIPEC and no centralization of these demanding treatments has occurred. Over the last decade additional treatment innovations have occurred. Suggestions for an improved surgical technology for mesenteric peritonectomy and for systematic parietal peritonectomy have been published by Deraco et al. and Baratti et al. [16,17]. However, a major change in management of the regional chemotherapy was proposed by Sugarbaker and Chang in 2017 [18]. This manuscript was based on the pharmacokinetic studies of Pestieau et al. [19]. It showed that long-term combined intraperitoneal and systemic chemotherapy resulted in prolonged survival. As yet, no publications from other institutions to support this treatment plan for MPM similar to that recommended for ovarian cancer have been published [20]. The goal of this manuscript is to present the rationale and data accumulated for HIPEC plus long-term NIPEC. A propensity matched analysis demonstrated an improvement in survival over and above the use of cytoreductive surgery plus HIPEC with prolonged use of NIPEC [21].
Methods
Clinical evaluation of patients preoperatively
All data were prospectively recorded in a standardized database and then collected and statistically analyzed. Prior to commencing this study, permission was obtained from the MedStar Health Research Institute Office of Research Integrity to record and analyze these data. All patients had a diagnosis of epithelial type of MPM. All patients treated with NIPEC paclitaxel signed an informed consent. The first 12 patients receiving NIPEC pemetrexed and intravenous cisplatin signed an informed consent. Subsequent patients were not required to enter an institutional protocol so that the treatments could be administered by the referring medical oncologist. The primary outcome was overall survival which was defined as the time from cytoreductive surgery plus HIPEC and EPIC to death from peritoneal mesothelioma. Postoperative morbidity was the secondary endpoint. Adverse events with NIPEC was also a secondary endpoint.
All patients in the study were assigned a prior surgical score (PSS) as described by Jacquet and Sugarbaker [22]. Patients with a PSS of 0 had biopsy only. PSS of 1 indicated an exploratory laparotomy of a single region. PSS of 2 indicated exploratory laparotomy with resections in 2-5 regions. PSS of 3 indicated an extensive prior cytoreduction with over 5 regions dissected.
Clinical evaluation of patients intraoperatively
The peritoneal cancer index (PCI) was determined prospectively at the time of abdominal exploration [22]. The PCI was an assessment of the distribution and extent of MPM in 13 abdominopelvic regions recorded by the surgeon at the time of abdominal exploration. Patients were grouped by PCI as less than 10, 10 through 30, or greater than 30.
A completeness of cytoreduction (CC) score was determined on all patients [22]. This score was determined by the surgeon at the completion of the cytoreductive surgical procedure. A CC score of 0 indicated no visible evidence of disease. A CC score of 1 indicated tumor nodules less than 2.5 mm in diameter without a confluence of disease at any site. A CC score of 2 indicated tumor nodules between 2.5 mm and 2.5 cm in the absence of a contiguous layer of disease at any anatomic site. A CC score of 3 indicated tumor nodules greater than 2.5 cm in diameter or a confluence of disease layered out at any site within the abdomen or pelvis. Special attention to the CC score on visceral peritoneum (abdominal-pelvic regions 9-12) occurred because of the technical challenge to resect MPM on bowel mesentery.
The lymph node status was determined on all patients. At the time of cytoreductive surgery all enlarged lymph nodes and selected normal sized lymph nodes were resected and submitted for histopathologic examination. At least 4 lymph nodes were resected and submitted for permanent histopathologic examination [23]. If lymph nodes were positive, the patient was excluded from this data analysis.
Clinical evaluation of patients postoperatively
All patients had a standardized morbidity and mortality assessment performed postoperatively [24]. Adverse events were graded 1-5 in a standardized manner.
For grade 1 adverse events, a diagnosis was established that no interventions required for resolution. For grade 2 adverse events, the diagnosis was established and medical treatments were sufficient for resolution. In grade 3 adverse events, the diagnosis was established and an invasive intervention such as a radiologic intervention not requiring general anesthesia was sufficient to resolve the complication. For grade 4 adverse events, the diagnosis was established and an urgent definitive intervention was required, for example, return to the operating room or return to the surgical intensive care unit [25].
Adverse events itemized for the 35 patients who had NIPEC included port site infection, failure to infuse, severe pain upon infusion, generalized abdominal pain, and port rotation [26].
Strategies for cytoreductive surgery in all patients
A cytoreductive surgical procedure was performed on all patients, the goal of which was to remove as much visible disease as was possible. In most patients this was a CC-0 resection. The overall strategy was to clear the abdomen and pelvis using cytoreductive surgery and then maintain that disease-free condition through the use of HIPEC, EPIC and NIPEC. The surgery required a series of five parietal peritonectomy procedures to which visceral resections were added as needed to remove visible evidence of disease [27,28]. Systematic parietal peritonectomy as recommended by Baratti and colleagues was not practiced [17]. MPM layered out on the visceral peritoneal surface of small bowel, colon, or rectum required visceral resections or resection of individual nodules [28]. A single surgeon (PHS) supervised all of the cytoreductions throughout this clinical effort. All peritonectomy procedures and major visceral resections were prospectively recorded. All specimens were submitted to the pathologist to confirm the presence of malignant mesothelioma by histologic study.
Perioperative chemotherapy for patients in the control group was HIPEC plus EPIC
In the control group of patients, the cytoreductive surgery was followed by HIPEC and EPIC. HIPEC required a curled peritoneal dialysis catheter (Covidien, Mansfield, MA) to infuse the chemotherapy solution and four outflow catheters to drain the chemotherapy solution [29]. Heat in the chemotherapy solution was maintained by recirculation of fluid through the heat pump. Two drugs were administered intraperitoneally in 1.5 liters/m2 of 1.5% dextrose peritoneal dialysis solution. The two drugs were doxorubicin at 15 mg/m2 and cisplatin at 50 mg/m2. The chemotherapy solution was administered as rapidly as possible over approximately five minutes with the infused solution maintained between 41.5 and 43.5°C within the whole abdomen by a heat pump (Belmont Surgical Instruments, Billerica, MA). A standardized open abdomen technique with manual distribution of the chemotherapy solution was used [30] (Figure 1). Skin edges were elevated on a fixed retractor that formed a rectangle around the open abdomen (Thompson Surgical Instruments, Lansing, MI). A plastic sheet to cover the open abdomen was secured to the retractor by the skin traction sutures. A cruciate incision in the plastic sheet allowed the surgeon’s double-gloved hand access to all portions of the abdomen to evenly distribute the heat and chemotherapy solution. All HIPEC treatments were for 90 minutes. Following completion of the HIPEC, procedures to repair seromuscular tears, bowel anastomoses, and abdominal closure were performed. At the initiation of the HIPEC, a continuous infusion of ifosfamide at 1300 mg/m2 was started and was continued throughout the 90 minutes of HIPEC treatments. To prevent uroepithelial damage, 256 mg/m2 of sodium methanethiolate (MESNA) was infused intravenously as rapidly as possible 15 minutes prior to the initiation of HIPEC and at 4 hours and 8 hours [31].
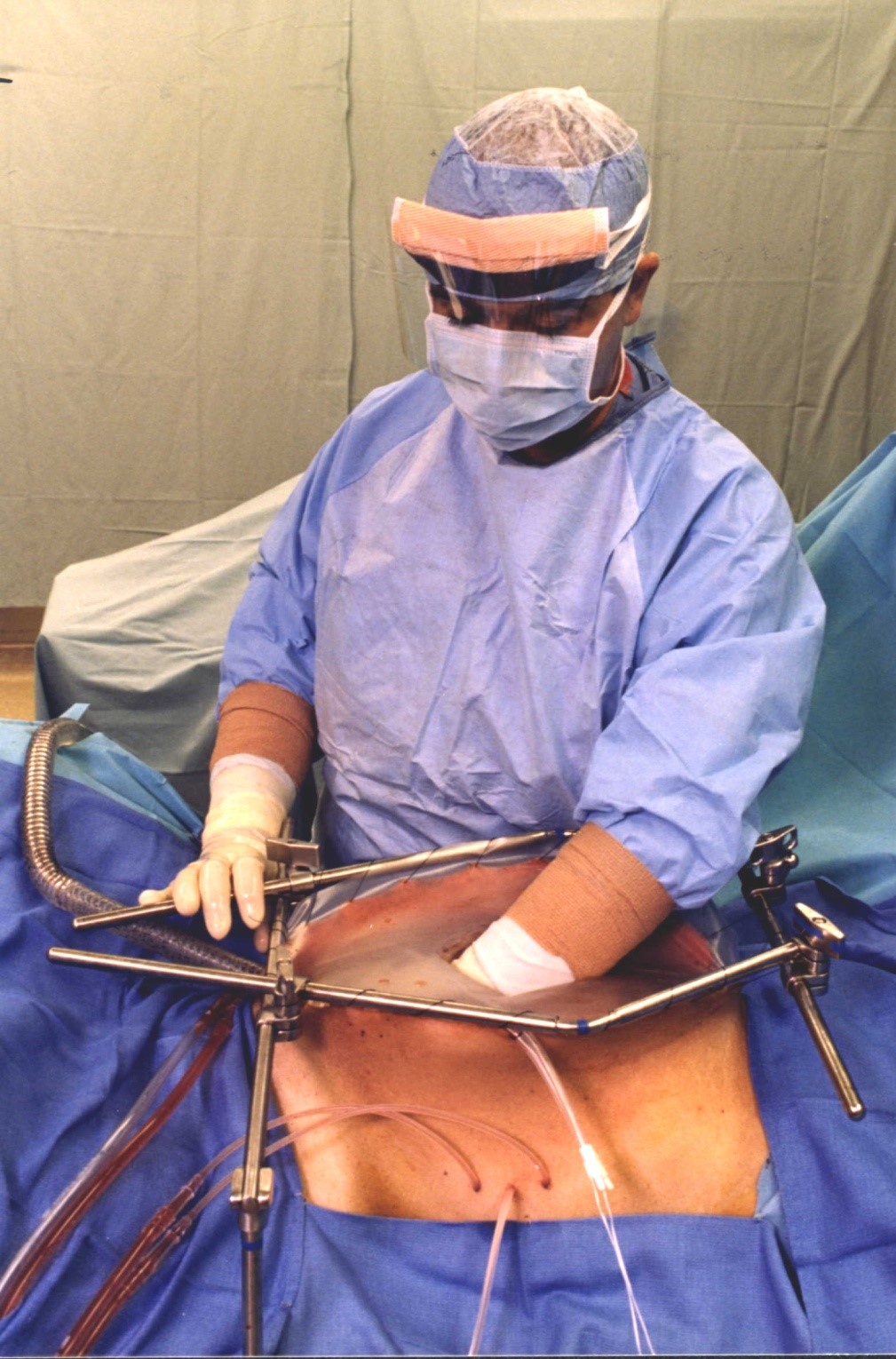
Figure 1: Open technique for hyperthermic intraperitoneal chemotherapy. After placement of tubes, drains and temperature probes, the skin edges are elevated onto the rim of the self-retaining retractor using a running suture. A plastic sheet incorporated into the sutures covers the abdomen to prevents splashing or loss of chemotherapy aerosols into the environment. A slit in the plastic sheet allows the surgeon’s hand access to the abdominal cavity. His continuing activity guarantees that all abdominal surfaces will have access to uniform doses of heat and chemotherapy. A smoke evacuator pulls the air beneath the plastic cover through a charcoal filter to prevent any aerosols from gaining access to the operating room environment.
In order to administer EPIC, the Tenckhoff catheter and closed-suction drains were maintained after the 90 minutes of intraoperative chemotherapy with HIPEC. EPIC administration was initiated on the first postoperative day while the patient was in the surgical intensive care unit. A 1-liter chemotherapy solution containing paclitaxel at 20 mg/m2 was administered intraperitoneally. The carrier solution for the paclitaxel was 6% hetastarch solution (B. Brown, Bethlehem, PA) administered by an infusion pump at 1000 ml/hour [32]. At 23 hours, the drains and Tenckhoff catheter were unclamped and fluid removed by gravity drainage as completely as possible from the peritoneal space. This procedure was repeated for 5 consecutive days for a total dose of 100 mg/m2.
Chemotherapy using NIPEC long-term for patients in the experimental group
The perioperative chemotherapy with HIPEC and EPIC in the experimental group was the same as the control group. Prior to closure of the abdomen, an intraperitoneal port (Smiths Medical ASD Inc., St. Paul, MN) was implanted [33] (Figure 2). At 4-6 weeks postoperatively, the intraperitoneal port was accessed. Five patients received 6 cycles of intraperitoneal paclitaxel given as NIPEC. Paclitaxel dose was 20 mg/m2 five days in a row, one week of every month [34]. These 6 months of additional treatment began 6-8 weeks after cytoreductive surgery thereby adding approximately 8 months to intensive postoperative management. All subsequent patients were treated with intraperitoneal pemetrexed at 500 mg/m2 in 1 liter of 1.5% dextrose peritoneal dialysis solution infused at 1000 ml over 1 hour. After the intraperitoneal pemetrexed has been given, cisplatin at 75 mg/m2 was infused intravenously in 250 ml of normal saline over 120 minutes. These treatments were repeated for a total of 6 cycles with 3 weeks between each treatment adding approximately 6 months of intensive postoperative management [21]. Patients had to receive at least 2 cycles of NIPEC to be included in the experimental group.
Figure 2: Placement of a permanent intraperitoneal port at the completion of cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy (from reference 33 with permission).
Pharmacologic data supporting NIPEC pemetrexed long-term
The pharmacologic rationale for the use of pemetrexed as intraperitoneal chemotherapy for patients with peritoneal metastases was established by Pestieau et al. [19]. The early data was from an animal model. The first step required this group to develop a high-pressure liquid chromatography (HPLC) assay for pemetrexate present within blood, peritoneal fluid and urine. Also, a methodology for extraction of pemetrexed from fresh tissue in order to determine drug concentration within normal and tumor tissue was necessary. Pestieau and coworkers determined the pharmacokinetics of intravenous and intraperitoneal pemetrexed when administered at 10 mg/kg and at 100 mg/kg. After a bolus of intravenous injection of pemetrexed there was a rapid clearance of the drug from the systemic circulation within 60 minutes (Figure 3, top). In sharp contrast, when pemetrexed was administered by the intraperitoneal route there was a delayed clearance of drug from peritoneal fluid (Figure 3, bottom). The mean half-life of intraperitoneal pemetrexed was 127 minutes. The plasma concentration slowly increased to a peak at 60 minutes. The area under the concentration x time curve of intraperitoneal concentration to intravenous concentration was 40.8. After intraperitoneal instillation, 75% of the drug was cleared from the peritoneal space at 6 hours. In summary, a marked increased exposure of peritoneal surfaces to pemetrexate occurred with intraperitoneal administration.
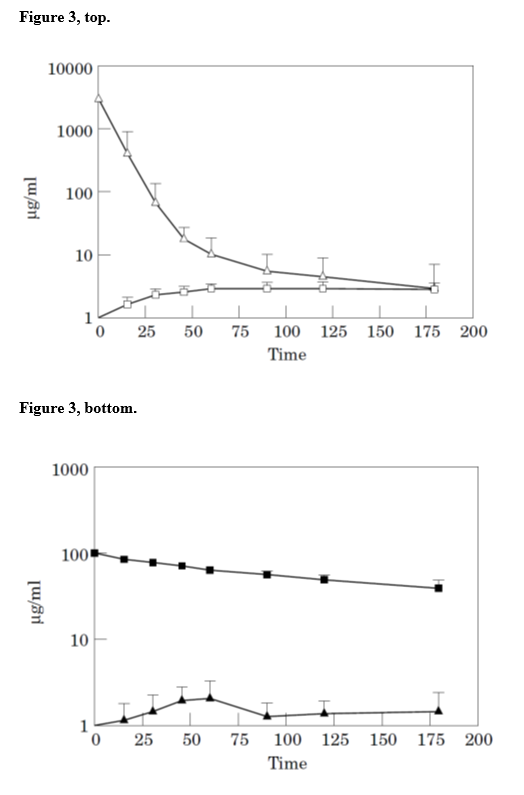
Figure 3: Pharmacokine tic analyses of intravenous (top) and intraperitoneal (bottom) pemetrexed (from reference 19 with permission).
Follow-up
The follow-up was by clinical visits scheduled every 3 months for 3 years. A CT of chest, abdomen, and pelvis was performed every 6 months for 3 years and then yearly for the next 7 years for a total of 10 years of radiologic follow-up. Reoperative surgeries and palliative systemic chemotherapy for recurrence was recorded for these patients but was not part of our analysis.
We elected not to attempt to report disease-free survival. A definitive date for recurrent disease was difficult to establish and was often never confirmed by biopsy.
Data analysis
In an attempt to account for selection bias and potential confounding factors, we performed a propensity matching between control and experimental groups [35]. Major factors that were tested in our univariant analysis were used to match 1:1 as many patients in our two groups as possible. Clinical features utilized included age (± 15 years), gender, PSS 0, 1 or 2 (same), PCI ± 20, and CC score (same). Other important factors including tumor histology and grade, lymph node status and pretreatment chemotherapy were the same in both control and experimental group so did not require matching.
Results
Restriction of clinical features to achieve a uniform cohort of patients
All patients in this data analysis had a histologic diagnosis of epithelial type of MPM. Mesothelioma patients with other histologic types of cystic, papillary, biphasic or sarcomatoid mesothelioma were excluded. Also excluded were patients whose histology showed with poorly differentiated peritoneal mesothelioma with deep invasion (3 patients), patients whose preoperative CT showed small bowel and its mesentery with class 3 changes (4 patients), patients whose CT or surgical exploration showed systemic evidence of disease or direct extension into pleural or pericardial space (4 patients), patients with CC-3 cytoreduction (10 patients), patients receiving neoadjuvant systemic cisplatin and pemetrexed (3 patients) and patients receiving steroids for prolonged periods preoperatively (1 patient). Patients who had positive lymph nodes determined from specimens sent at the time of cytoreductive surgery were excluded. One patient developed an infected intraperitoneal port that required removal. He declined port replacement so could not be included in the experimental group which required at least 2 cycles of NIPEC. These selection factors were designed to accumulate a uniform group of patients to allow the effects of regional chemotherapy to be recognized. After this strict patient selection, there were 39 patients in the control group (CRS plus HIPEC and EPIC) and 35 in the experimental group (CRS plus HIPEC, EPIC and NIPEC). The median follow-up for the 39 patients in the control group was 26.9 years. In the 35 patients in the experimental group it was 20.7 years.
When all 74 selected patients are considered together the impact on survival of the clinical- and treatment-related features is shown in Table 1. Age HR 1.99 (0.929, 4.238 95% CI, p=0.0766), completeness of cytoreduction HR 2.356 (1.113, 4.989 95% CI, p=0.0251) and treatments administered HR 3.497 (1.199, 10.20 95% CI, p=0.0219) were significant determinants of survival.
| Feature | No. (%) | Median Survival (% at 5 years) | Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|
| Gender | 0.4471 | |||
| Male | 37(50%) | 16.2 (85.0%) | 0.748 (0.354, 1.582) | |
| Female | 37(50%) | 15.2 (81.7%) | Reference | |
| Age | 0.0766 | |||
| ≤50 years | 43(58%) | 21.1 (82.3%) | Reference | |
| >50 years | 31(42%) | 9.0 (85.2%) | 1.99 (0.929, 4.238) | |
| Prior surgical score | 0.8860 | |||
| 0, 1 | 51(69%) | 14.7 (84.4%) | Reference | |
| 2, 3 | 23(31%) | 21.1 (81.0%) | 0.943 (0.421, 2.111) | |
| Peritoneal cancer index | 0.4424 | |||
| ≤20 | 36(49%) | --.- (86.6%) | Reference | |
| >20 | 38(51%) | 16.2 (80.6%) | 1.341 (0.634, 2.837) | |
| Completeness of cytoreduction | 0.0251* | |||
| 0, 1 | 48(65%) | --.- (87.2%) | Reference | |
| 2 | 26(35%) | 12.7 (76.9%) | 2.356 (1.113, 4.989) | |
| Treatments administered | 0.0219* | |||
| Control (HIPEC + EPIC) | 39(53%) | 12.7 (76.9%) | 3.497 (1.199, 10.20) | |
| Experimental (HIPEC + EPIC + NIPEC) | 35(47%) | --.- (92.7%) | Reference |
Table 1: Univariant analysis of clinical- and treatment-related features and their impact on overall survival of 74 patients with malignant peritoneal mesothelioma. (From reference 21 with permission).
Comparison of control and experimental groups determined by clinical- and treatment-related variables
A multivariant analysis using the Cox proportional hazards model was applied and the results are shown in Table 2. There were 39 patients in the control group and 35 patients in the experimental group. Model results displayed that gender and age were borderline significant. PCI and CC score were not significant. The treatments administered HIPEC + EPIC vs. HIPEC + EPIC + NIPEC retained significance HR 3.549 (1.157, 10.888 95% CI, p=0.0268).
| Feature | Control (n=39) | Experimental (n=35) | Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|
| Gender | 0.0769 | |||
| Male | 16 | 21 | 0.439 (0.176, 1.093) | |
| Female | 23 | 14 | Reference | |
| Age | 0.0747 | |||
| ≤50 years | 25 | 18 | Reference | |
| >50 years | 14 | 17 | 2.225 (0.923, 5.360) | |
| Prior surgical score | 0.1857 | |||
| 0, 1 | 23 | 28 | Reference | |
| 2, 3 | 16 | 7 | 0.546 (0.223, 1.338) | |
| Peritoneal cancer index | 0.6806 | |||
| ≤20 | 20 | 16 | Reference | |
| >20 | 19 | 19 | 1.221 (0.472, 3.158) | |
| Completeness of cytoreduction | 0.1652 | |||
| 0, 1 | 21 | 27 | Reference | |
| 2 | 18 | 8 | 2.048 (0.744, 5.635) | |
| Treatments administered | 0.0268* | |||
| Control (HIPEC + EPIC) | 39 | 0 | 3.549 (1,157, 10.888) | |
| Experimental (HIPEC + EPIC + NIPEC) | 0 | 35 | Reference |
Table 2: Cox proportional hazards multivariant model with adjustments for all pooled covariates for clinical- and treatment-related features and their impact on overall survival in control and experimental groups. (From reference 21 with permission). Survival of 39 control patients versus 35 experimental patients
Figure 4 shows the product-limit of survival estimates for 39 controls versus 35 experimental patients. The survival benefit of adding NIPEC was significant at p=0.0145.
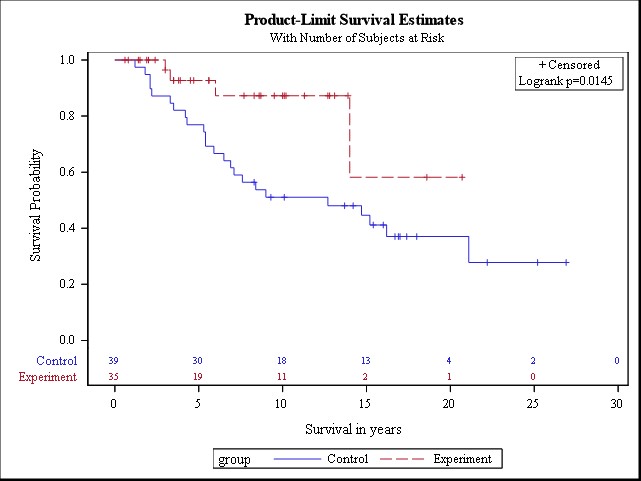
Figure 4: Product-limit of survival estimates of 39 control (CRS + HIPEC + EPIC) versus 35 experimental (CRS + HIPEC + EPIC + NIPEC) patients with epithelial malignant peritoneal mesothelioma (from reference 21 with permission). (CRS: Cytoreductive Surgery; HIPEC: Hyperthermic Intraperitoneal Chemotherapy; EPIC: Early Postoperative Intraperitoneal Chemotherapy; NIPEC: Normothermic Intraperitoneal Chemotherapy)
Propensity matched pairs of controls versus experimental patients
Five factors selected from the univariant analysis of clinical features were used to propensity match as many patients as possible within the two treatment groups. These features were age, gender, PSS, PCI and CC score. Twenty-nine matched pairs were available for an analysis of survival. Therefore, 10 of the control patients and 6 of the experimental patients could not be included in the survival analysis of propensity matched patients. The survival of the experimental group in whom NIPEC was added to HIPEC and EPIC was significant at p=0.0263 (Figure 5).
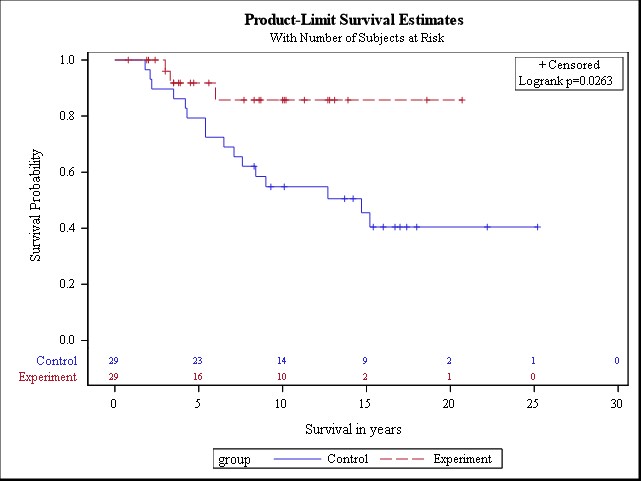
Figure 5: Twenty-nine propensity matched pairs of control (CRS + HIPEC + EPIC) versus experimental (CRS + HIPEC + EPIC + NIPEC) patients with epithelial malignant peritoneal mesothelioma (from reference 21 with permission). (CRS: Cytoreductive Surgery; HIPEC: Hyperthermic Intraperitoneal Chemotherapy; EPIC: Early Postoperative Intraperitoneal Chemotherapy; NIPEC: Normothermic Intraperitoneal Chemotherapy).
Morbidity and mortality
The treatments in the hospital were identical in control and experimental groups except that the experimental group had placement of an intraperitoneal port. A single port became infected and required removal under local anesthesia. The morbidity and mortality of cytoreductive surgery with HIPEC and EPIC has been previously reported and analyzed [25]. There was no mortality in these 74 patients. The grade 3 and 4 adverse events were 20.2 and 9.5%, respectively.
Causes for discontinuation of NIPEC were infected intraperitoneal access after 2 cycles of treatment in one patient, peritoneal inflammation and pain in one patient treated with paclitaxel after 3 cycles of treatment and failure to administer the final cycle of NIPEC at the patient’s request in 6 patients. A single grade 4 adverse event was a negative exploratory laparotomy for abdominal pain to rule out bowel perforation in the patient with a paclitaxel allergic response.
Discussion
Benefits sought by maximizing regional chemotherapy
Our studies have focused on a unique feature of this disease process, the absence of systemic progression throughout the natural history of the disease. Cytoreductive surgery which has as its goal removal of all visible evidence of disease from the abdomen and pelvis is the first step in treatment. Then, HIPEC, a chemotherapy lavage of the abdominal and pelvic space in the operating room is performed. EPIC occurs in the first 5 postoperative days. In the experimental group of patients at the time of the surgical procedure, an access device for the peritoneal space is inserted and prolonged treatments designed to last 6 months are initiated. From a surgical and pharmacologic perspective, this strategy implements an intensive regional treatment on the abdomen and pelvis.
Patient selection to minimize confounding variables
In order to maximize this intense local-regional approach, only the patients considered responsive were selected for inclusion in both the control (CRS + HIPEC + EPIC) and experimental (CRS + HIPEC + EPIC + NIPEC) groups. Patients treated with neoadjuvant systemic chemotherapy using cisplatin and pemetrexed were excluded [36]. Some consider neoadjuvant chemotherapy a means to identify patients with tumor biology most likely to benefit from cytoreductive surgery plus HIPEC. Alternatively, neoadjuvant chemotherapy destroyed responsive cancer cells and preserved resistant cancer cells. The end result would be a reduced or absent response to regional chemotherapy. Patients with residual cancer nodules after cytoreduction greater than 2.5 cm were excluded because of the minimal penetration of intraperitoneal chemotherapy. Patients with positive lymph nodes or an aggressive histology were eliminated thinking they may progress more rapidly than the control possible with our regional chemotherapy. Also, preoperative radiologic studies suggesting disease outside of the abdominal and pelvic space was an exclusion criterion. Seventy-four patients were considered candidates to respond to these regional treatments and underwent statistical analysis of survival. The hypothesis was that a limited benefit of NIPEC should not be expected in all MPM patients, only in those most likely to respond to regional chemotherapy.
In the absence of an RCT, propensity matching was used to show benefit from NIPEC long-term
In a rare disease like MPM, centralization of treatments to a few peritoneal surface malignancy centers would greatly improve outcomes by increasing the volume of patients at dedicated treatment centers and eliminating institutional- and surgeon-related learning curves. This centralization would also facilitate randomized controlled trials to establish optimal treatment strategies. Knowing that centralization is unlikely to occur and that no current plan for a randomized controlled study exists, propensity matching is the statistical tool to evaluate NIPEC [35]. The propensity match seeks to make the patients in the experimental group have all important variables very similar, if not identical, to those in the control group. This is currently the most reliable way to control selection bias between two groups of patients. In this propensity match the most difficult variable to control, the cytoreductive surgical procedure, was uniform in that a single surgeon performed or personally supervised all the cytoreductive surgical procedures. The other 5 clinical and treatment variables were matched. In 29 matches of control and experimental patients, the survival benefit of NIPEC was significant (p=0.0263) (Figure 5).
NIPEC with paclitaxel or pemetrexed/cisplatin
Prolonged intraperitoneal chemotherapy through an intraperitoneal port was begun in MPM patients with clinical and pharmacologic studies with paclitaxel. When pemetrexed/cisplatin was approved for treatment of pleural and peritoneal mesothelioma [37], it was thought necessary to modify the NIPEC component of the protocol for use of intraperitoneal pemetrexed and systemic cisplatin. The data from NIPEC paclitaxel protocol and NIPEC pemetrexed/cisplatin protocol are combined in this analysis. If a randomized controlled trial of NIPEC versus no NIPEC is to be performed, which regional chemotherapy should be tested? A long-term paclitaxel would be recommended. Several other peritoneal surface malignancy groups have shown efficacy of long-term intraperitoneal paclitaxel through an intraperitoneal port [38]. Also, the pharmacology of intraperitoneal paclitaxel recommends this drug over all others for EPIC and NIPEC. Finally, the intraperitoneal paclitaxel is infused in a starch carrier solution (HESPAN or Dextran) which counteracts the progression of peritoneal sclerosis. It may be an intraperitoneal chemotherapy treatment strategy with fewer of the adverse events traditionally associated with drug administration through an intraperitoneal port. Giving intraperitoneal paclitaxel 5 days in a row one week every month is a schedule compatible with travel of a patient for centralized treatment. It may also provide the “peel the onion” concept proposed for intraperitoneal paclitaxel administration [39].
Adverse event-free repeated intraperitoneal access
The improved survival for long-term NIPEC given by an intraperitoneal port to peritoneal metastases patients may not be the best result possible because of port-related interference with drug delivery. Not all patients were able, for logistical reasons, to receive all 6 cycles of NIPEC. To maximize long-term trouble-free intraperitoneal drug delivery, several technical requirements may be suggested. First, insertion of the peritoneal access device at the completion of cytoreductive surgery is recommended. The intraperitoneal catheter should extend from the abdominal wall into the false pelvis amongst small bowel loops. EPIC should be given for the first 5 postoperative days with an aqueous starch carrier solution in an effort to reduce the rapid progression of postoperative adhesions. If chemotherapy administration is interrupted, intraperitoneal catheter placement by the interventional radiologist should be available. Finally, the goal for the number of cycles of NIPEC should be reduced from 6 to 5. The greatest obstacle for implementation of NIPEC long-term for MPM involves the logistical issues that accompany intraperitoneal chemotherapy administration.
Strict patient selection criteria in control and experimental groups
Patient selection allowed the effects of regional chemotherapy to be evident in a comparison of the control and experimental groups. The median survival of 39 control patients treated by cytoreductive surgery, HIPEC and EPIC was 10 years and the 5-year survival was 70% (Figure 4). In the multi-institutional study of Yan and colleagues, the overall median survival in 401 patients was 53 months and the 5-year survival 47% [12]. Selection of patients for a maximal regional chemotherapy response not only improved the survival of control patients treated with HIPEC and EPIC but also allowed the effects of NIPEC to be demonstrated in the experimental group.
More knowledgeable selection of patients for CRS can improve survival
In these control and experimental patients, selection factors that we thought would maximize the responses to regional chemotherapy were used. However, there may be selection factors that will maximize the other component of this combined treatment, that is the cytoreductive surgery. Careful selection of patients by preoperative CT using the concerning CT features will minimize the number of CC-3 cytoreductions [40]. Also, early diagnosis of MPM, a rare disease, requires a high index of suspicion. Persistence in establishing a biopsy-confirmed diagnosis with immunostains calretinin and CK5/6 can eliminate a prolonged delay in definitive diagnosis and treatment. Biopsy-confirmed diagnosis by minimally invasive techniques can prevent a high prior surgical score (PSS) and extensive adhesions that complicate major cytoreductive surgical procedures.
Limitations of the study
The only way to change practice for patients with mesothelioma to a strategy that utilizes long-term intraperitoneal delivery of normothermic chemotherapy is to perform a multi-institutional randomized trial. That being said, there are practical considerations that make our propensity matched data of value in providing a new direction for treatment. Perhaps the major limitation regarding the interpretation of these data revolves around the extended time period over which was required to enter both control patients (18 years) and experimental patients (9 years). Although our continuous efforts were to treat patients in whom the effects of regional chemotherapy could be demonstrated. It is completely possible that confounding and unrecognized variables exist between these two groups. These variables may be responsible for the differences rather than the differences in treatment. These rigid and demanding criteria for including patients in the control and the experimental group cause our control group to be considerably different than that published by other institutions for treatment of peritoneal mesothelioma by cytoreductive surgery plus HIPEC. This severe restriction of eligibility for treatment calls into question the applicability of these data to a substantial proportion of MPM patients. Our subset of MPM patients seemed to profit from NIPEC but the disease as a whole may not be impacted. In this respect, these data on our highly selected patients stand alone and even the control group does not have a comparison at another institution. The lack of disease-free survival which often tends to support data on overall survival is another limitation brought about in large part by the long survival of both the control and experimental group. The data by which to compare disease-free survival in these two groups of patients are not available.
Conclusions
As the unusual natural history of MPM was clarified, the possible use of extensive local/regional treatment options was explored. The surgical treatments utilized were peritonectomy procedures and visceral resections referred to as CRS. Realizing that surgery could not eradicate small volume (microscopic) disease, HIPEC was added to the surgery. At the Washington Cancer Institute, this combination of CRS and HIPEC was further augmented by NIPEC long-term. The treatment strategy required approximately 6 months to complete. However, the results of CRS, HIPEC and NIPEC demonstrate an approximate 80% 5-year survival. Although randomized trials to further document the benefit of CRS, HIPEC and NIPEC are required, the very low incidence of the disease and the lack of an international referral center make progress difficult.
Funding
Administrative and secretarial support was provided by Foundation for Applied Research in Gastrointestinal Oncology (FARGO).
References
2. Loggie BW, Fleming RA, McQuellon RP, Russell GB. Prospective trial for the treatment of malignant peritoneal mesothelioma. The American Surgeon. 2001 Oct 1;67(10):999-1003.
3. Sugarbaker PH, Welch LS, Mohamed F, Glehen O. A review of peritoneal mesothelioma at the Washington Cancer Institute. Surgical Oncology Clinics. 2003 Jul 1;12(3):605-21.
4. Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. Journal of Clinical Oncology. 2003 Dec 15;21(24):4560-7.
5. Deraco M, Nonaka D, Baratti D, Casali P, Rosai J, Younan R, et al. Prognostic analysis of clinicopathologic factors in 49 patients with diffuse malignant peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Annals of Surgical Oncology. 2006 Feb;13(2):229-37.
6. Taub RN, Hesdorffer ME, Keohan ML, Chabot JA, Fountain KS, Talbot S, et al. Combined resection, intraperitoneal chemotherapy, and whole abdominal radiation for malignant peritoneal mesothelioma (MPM). Journal of Clinical Oncology. 2005 Jun 1;23(16_suppl): 664s.
7. Brigand C, Monneuse O, Mohamed F, Sayag-Beaujard AC, Isaac S, Gilly FN, et al. Peritoneal mesothelioma treated by cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: results of a prospective study. Annals of Surgical Oncology. 2006 Mar;13(3):405-12.
8. Elias D, Bedard V, Bouzid T, Duvillard P, Kohneh-Sharhi N, Raynard B, et al. Malignant peritoneal mesothelioma: treatment with maximal cytoreductive surgery plus intraperitoneal chemotherapy. Gastroentérologie Clinique et Biologique. 2007 Oct 1;31(10):784-8.
9. Yano H, Moran BJ, Cecil TD, Murphy EM. Cytoreductive surgery and intraperitoneal chemotherapy for peritoneal mesothelioma. European Journal of Surgical Oncology (EJSO). 2009 Sep 1;35(9):980-5.
10. Blackham AU, Shen P, Stewart JH, Russell GB, Levine EA. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for malignant peritoneal mesothelioma: mitomycin versus cisplatin. Annals of Surgical Oncology. 2010 Oct;17(10):2720-7.
11. Chua TC, Yan TD, Morris DL. Outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal mesothelioma: the Australian experience. Journal of Surgical Oncology. 2009 Feb 1;99(2):109-13.
12. Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, Gilly FN, Levine EA, Shen P, Mohamed F, Moran BJ. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. Journal of Clinical Oncology. 2009 Dec 20;27(36):6237-42.
13. Helm JH, Miura JT, Glenn JA, Marcus RK, Larrieux G, Jayakrishnan TT, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Annals of Surgical Oncology. 2015 May;22(5):1686-93.
14. Sugarbaker PH, Turaga KK, Alexander Jr HR, Deraco M, Hesdorffer M. Management of malignant peritoneal mesothelioma using cytoreductive surgery and perioperative chemotherapy. Journal of Oncology Practice. 2016 Oct;12(10):928-35.
15. Kusamura S, Kepenekian V, Villeneuve L, Lurvink RJ, Govaerts K, De Hingh IH, et al. Peritoneal mesothelioma: PSOGI/EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. European Journal of Surgical Oncology. 2021 Jan 1;47(1):36-59.
16. Deraco M, Baratti D, Kusamura S, Laterza B, Balestra MR. Surgical technique of parietal and visceral peritonectomy for peritoneal surface malignancies. Journal of surgical oncology. 2009 Sep 15;100(4):321-8.
17. Baratti D, Kusamura S, Cabras AD, Deraco M. Cytoreductive surgery with selective versus complete parietal peritonectomy followed by hyperthermic intraperitoneal chemotherapy in patients with diffuse malignant peritoneal mesothelioma: a controlled study. Annals of Surgical Oncology. 2012 May;19(5):1416-24.
18. Sugarbaker PH, Chang D. Long-term regional chemotherapy for patients with epithelial malignant peritoneal mesothelioma results in improved survival. European Journal of Surgical Oncology (EJSO). 2017 Jul 1;43(7):1228-35.
19. Pestieau SR, Stuart OA, Sugarbaker PH. Multi-targeted antifolate (MTA): pharmacokinetics of intraperitoneal administration in a rat model. European Journal of Surgical Oncology (EJSO). 2000 Nov 1;26(7):696-700.
20. Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. New England Journal of Medicine. 2006 Jan 5;354(1):34-43.
21. Sugarbaker PH, Chang D. Cytoreductive Surgery Plus HIPEC With and Without NIPEC for Malignant Peritoneal Mesothelioma: A Propensity-Matched Analysis. Annals of Surgical Oncology. 2021 Nov;28(12):7109-17.
22. Jacquet PP, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. Journal of Experimental & Clinical Cancer Research. 1996 Mar 1;15(1):49-58.
23. Yan TD, Yoo D, Sugarbaker PH. Significance of lymph node metastasis in patients with diffuse malignant peritoneal mesothelioma. European Journal of Surgical Oncology (EJSO). 2006 Nov 1;32(9):948-53.
24. Sugarbaker PH, Alderman R, Edwards G, Marquardt CE, Gushchin V, Esquivel J, et al. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Annals of Surgical Oncology. 2006 May;13(5):635-44.
25. Yan TD, Edwards G, Alderman R, Marquardt CE, Sugarbaker PH. Morbidity and mortality assessment of cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma—a prospective study of 70 consecutive cases. Annals of Surgical Oncology. 2007 Feb;14(2):515-25.
26. Walker JL, Armstrong DK, Huang HQ, Fowler J, Webster K, Burger RA, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group Study. Gynecologic Oncology. 2006 Jan 1;100(1):27-32.
27. Sugarbaker PH. Peritonectomy procedures. Annals of Surgery. 1995 Jan;221(1):29-42.
28. Sugarbaker PH, van der Speeten K. An overview of peritonectomy, visceral resection, and therapeutic laparoscopy for peritoneal surface malignancy. Cytoreductive Surgery & Perioperative Chemotherapy for Peritoneal Surface Malignancy. Textbook and Video Atlas. 2nd edition. Cine-Med Publishing. 2017:17-46.
29. Van der Speeten K, Stuart OA, Sugarbaker PH. Cancer chemotherapy for peritoneal metastases: pharmacology and treatment. Cytoreductive Surgery & Perioperative Chemotherapy for Peritoneal Surface Malignancy. Textbook and Video Atlas. 2nd edition. Cine-Med Publishing. 2017:47-82.
30. Sugarbaker PH, Averbach AM, Jacquet P, Stephens AD, Stuart OA. A simplified approach to hyperthermic intraoperative intraperitoneal chemotherapy (HIIC) using a self retaining retractor. InPeritoneal carcinomatosis: principles of management 1996 (pp. 415-421). Springer, Boston, MA.
31. Van der Speeten K, Stuart OA, Mahteme H, Sugarbaker PH. Pharmacokinetic study of perioperative intravenous Ifosfamide. International Journal of Surgical Oncology. 2011 Sep 21;2011.
32. Mohamed F, Marchettini P, Stuart OA, Sugarbaker PH. Pharmacokinetics and tissue distribution of intraperitoneal paclitaxel with different carrier solutions. Cancer Chemotherapy and Pharmacology. 2003 Nov;52(5):405-10.
33. Sugarbaker PH, Bijelic L. Adjuvant bidirectional chemotherapy using an intraperitoneal port. Gastroenterology Research and Practice. 2012 Jan 1;2012.
34. Sugarbaker PH, Stuart OA. Unusually favorable outcome of 6 consecutive patients with diffuse malignant peritoneal mesothelioma treated with repeated doses of intraperitoneal paclitaxel. A case series. Surgical Oncology. 2020 Jun 1;33:96-9.
35. https://support.sas.com/resources/papers/proceedings17/0689-2017.pdf.
36. Kepenekian V, Elias D, Passot G, Mery E, Goere D, Delroeux D, et al. Diffuse malignant peritoneal mesothelioma: evaluation of systemic chemotherapy with comprehensive treatment through the RENAPE database: multi-institutional retrospective study. European Journal of Cancer. 2016 Sep 1;65:69-79.
37. Jänne PA, Wozniak AJ, Belani CP, Keohan ML, Ross HJ, Polikoff JA, et al. Pemetrexed alone or in combination with cisplatin in previously treated malignant pleural mesothelioma: outcomes from a phase IIIB expanded access program. Journal of Thoracic Oncology. 2006 Jul 1;1(6):506-12.
38. Sugarbaker PH. Intraperitoneal paclitaxel: pharmacology, clinical results and future prospects. Journal of Gastrointestinal Oncology. 2021 Apr;12(Suppl 1):S231.
39. Kuh HJ, Jang SH, Wientjes MG, Weaver JR, Au JL. Determinants of paclitaxel penetration and accumulation in human solid tumor. Journal of Pharmacology and Experimental Therapeutics. 1999 Aug 1;290(2):871-80.
40. Sugarbaker PH, Chang D, Jelinek JS. Concerning CT features predict outcome of treatment in patients with malignant peritoneal mesothelioma. European Journal of Surgical Oncology. 2021 Sep 1;47(9):2212-9.
