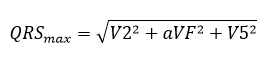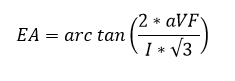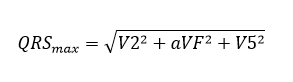Abstract
Background: Cisplatin-based therapy (CBT) represents currently a standard regimen for the testicular cancer treatment leading to longer survival of patients; as a consequence, the late cardiotoxicity can be manifested. In this study we analyzed the effect of CBT on the standard 12-lead ECG parameters in testicular cancer survivors.
Material and Methods: The electrocardiograms (ECG) of 173 patients with the germ cell tumor with a median follow-up duration of 9 years (ranged 5 to 32 years) were retrospectively analyzed. The patients were divided into four groups: Group CT: Orchidectomy plus CBT (n=133); Group AS: Orchidectomy only (n=18); Group RT: Orchidectomy plus adjuvant radiotherapy (n=14); Group CTRT: Orchidectomy plus adjuvant radiotherapy in combination with CBT (n=8). Heart rate (HR), QT and QTc intervals, the maximum spatial QRS vector amplitude, the electrical axis, the QRS complex amplitudes in individual leads and the maximum spatial T vector amplitude were analyzed.
Results: The HR and QT/QTc intervals were within normal limits. The most frequent ECG finding was the left anterior fascicular block, with the highest occurrence in the group CTRT (75%). The amplitudes of aVL, V6 and Tmax differed significantly between the AS group and the CT and RT/CTRT groups. The General Linear Model did not show a significant effect of therapy, but a significant effect of age and the waist circumference, respectively.
Conclusion: Cisplatin-based therapy did not result in pathological ECG changes. The slight ECG changes were associated with age and obesity, but not the therapy.
Keywords
Testicular cancer, Cisplatin-based therapy, Survivors, ECG
Introduction
Introducing cisplatin-based therapy into testicular cancer treatment represents a substantial progress in therapy leading to a longer survival of patients and less adverse effects; currently it represents the standard therapy [1,2]. However, both acute and late adverse effects have been documented, introducing related therapeutic, prognostic and public health problems.
The increased number of testicular cancer survivors living from years to decades after treatment [1] provides opportunity for studying late adverse cardiovascular effects, which are reflected in higher incidence of CV morbidity and mortality. Reported late ECG manifestations of cisplatin-based treatment include severe bradycardia, supraventricular tachycardia, prolonged QT interval, and atrial fibrillation [3], suspected of altered electrical impulse generation and conduction [4]. Survivors of testicular cancer treated cisplatin-based therapy have a higher prevalence of cardiovascular risk factors [5-14] that could further contribute to the increased risk of cardiovascular diseases [5,7,13,15,16].
The acute adverse effects of cisplatin-based therapy include a variety of cardiovascular (CV) events [17-22]. On electrocardiograms (ECG) the transient ECG changes simulating acute myocardial infarction or ischemia [19,23], ST-T changes, atrial fibrillation [24], bradycardia and prolonged QT interval are documented [4]. Animal studies have shown increased serum concentration of cardiac biomarkers of myocardial injury, increased oxidative stress and significant DNA fragmentation, apoptosis after cisplatin [25,26]. These structural and functional changes at the tissue, cellular and subcellular levels can lead to altered electrogenesis. A question arises, to what extent these alterations can be reflected in identifiable ECG changes indicative of late cardiotoxicity.
The aims of this study were: (1) to analyze the effect of cisplatin-based treatment on the ventricular depolarization and repolarization parameters of the standard 12-lead ECG in testicular cancer survivors, (2) and to compare it with ECG findings in testicular cancer survivors with other therapy regimens.
Material and Methods
The data for this study were obtained from the database of the ongoing translational project (Protocol IZLO-1, principal investigator M. M.) focused on the late toxicity of chemotherapy, radiotherapy or their combination in male patients cured for germ cell tumor (the sub-study principal investigator M. Ch.). The study was approved by the Institutional Review Board (IRB) of the National Institute of Oncology, Bratislava, Slovakia. Patients were enrolled between September 2015 and April 2017 and consented according to the IRB-approved protocol.
Study population
The study population was selected from patients diagnosed with the germ cell tumor (GCT) treated in the National Institute of Oncology during the period 1983-2012. All survivors who were at least 5 years after the completion of the last treatment for GCT were included. Patients with an unavailable electrocardiogram or the low technical quality of ECG were excluded.
The total number of 173 patients with the median duration of follow-up 9 years (ranged 5 to 32 years) were divided into four groups:
- Group CT: Orchidectomy plus consequential cisplatin-based treatment, n=133, aging from 25 to 78 years, (average 41.4 years), median follow up 10 years (ranging from 5 to 32 years). There were no significant differences in ECG parameters between cisplatin cumulative dose ≤ 400 mg/m2 and cisplatin > 400 mg/m2 patients (data not presented), therefore all patients treated with cisplatin were pooled into one group.
- Group AS: active surveillance, the control group; Orchidectomy only, without additional chemo- or radiotherapy; n=18, aging from 27 to 57 years, average age 34.7 years, median follow up 5 years (ranging from 5 to 16 years).
- Group RT: Orchidectomy plus consequential adjuvant radiotherapy; n=14, aging 29 to 56 years, average age 43.6 years. median follow up 10 years (ranging from 6 to 16 years).
- Group CTRT: Orchidectomy plus consequential adjuvant radiotherapy in combination with cisplatin-based chemotherapy; n=8, aging 31 to 59 years, average age 46.8 years. median follow up 7 years (ranging from 5 to 15 years).
Electrocardiography
Standard 12-lead electrocardiograms were recorded at the routine annual follow-up visits during the years 2015 and 2016. The time intervals and amplitudes of the QRS and T waves in individual leads were measured manually; the average of three measurements of consecutive QRS complexes was used for analysis. All R wave and S wave measurements were performed to the nearest 0.1 mV.
The following ECG parameters were analyzed:
Heart rate (HR): Bradycardia was defined as HR <60 bpm, tachycardia HR >100 bpm.
Time intervals:
- QRS complex duration (QRSd): the longest QRS duration of all leads;
- QT interval (QT): the longest QT duration of all leads; the corrected QT intervals (QTc) (Bazett correction: QTcB=QT/RR0.5; Fridericia correction: QTcF=QT/RR0.33) [27,28]. The QTcB values were categorized according [29,30] as follows: normal values <430 ms, borderline values 430-450 ms, prolonged values >450 ms.
- The ratio of QT interval and QRS duration: QT/QRSd.
QRS parameters:
- The maximum spatial vector magnitude (QRSmax) calculated as:
where V2 is the maximum QRS deflection in lead V2; aVF is the maximum QRS deflection in lead aVF; V5 is the maximum QRS deflection in lead V5.
The electrical QRS axis (EA) in frontal plane calculated as:
where aVF is the maximum QRS deflection in lead aVF, I is the maximum QRS deflection in lead I.
- Absolute values of the maximum QRS complex deflections in individual leads
T wave parameter:
- The maximum spatial T vector magnitude (Tmax) calculated as:
where TV2 is the T amplitude in lead V2; TaVF is the T amplitude in lead aVF; TV5 is the T amplitude in lead V5.
Clinical and laboratory tests
Clinical and laboratory tests were performed at the time of the follow-up visits, as a part of the routine clinical checkup, data for this study were extracted from the patients’ medical records:
- Body mass index (BMI): calculated as a ratio weight/ height2 (kg/ m2);
- Blood pressure (BP): measured in supine position in a quiet room after a rest period of 10 minute;
- Complete blood count and basic biochemistry, including serum cholesterol, high-density lipoprotein cholesterol (HDL), very-low-density lipoprotein cholesterol (VLD), triglycerides (TAG), fasting glucose and high-sensitivity troponin T levels.
Statistical analysis
The results are presented as mean ± SD for variables normally distributed, and as median and interquartile range for variables that were not normally distributed. The differences between the groups of normally distributed variables were tested using the analysis of variance (ANOVA) with Tukey HSD post hoc test, the non-normally distributed variables were tested using Kruskal-Wallis test. The frequency data of categorical variables were tested using the χ2 test. Univariate regression analysis was performed for selected dependent variables (EA, QRSmax and Tmax) by means of the General Linear Model (GLM), using the therapeutic group, age, BMI and the waist circumference as independent variables. The value p<0.05 was considered statistically significant. Statistical analyses were performed using SPSS-IBM software for windows (version 23, Chicago, Ill, USA).
Results
The basic characteristics of the study population are presented in Table 1. The patients in AS group were significantly younger and had a shorter time of follow-up compared to the CT, RT and CTRT groups. Except of these parameters, there were no statistically significant differences in basic characteristics between groups.
| AS n = 18 |
CT n = 133 |
RT n = 14 |
CTRT n = 8 |
p | |
|---|---|---|---|---|---|
| Age [years] Median (IQR) | 33.0 (29.8-38.3) | 40.0(34.0-48.0)** | 43.0(38.0-50.0)** | 47.0 (41.3-52.8)** | **<0.01 |
| Time since the treatment [years] | 6 (5-7) | 10 (7-14)** | 10 (7-12) *** | 7 (5-9) | **<0.01 ***<0.001 |
| BMI [kg/ m2] | 25.2 (23.6-29.7) | 26.3 (24.9-29.9) | 26.6 (23.8-29.5) | 28.4 (24.7-33.1) | NS |
| Waist circumference [cm] | 95.0 (89.0-105.0) | 99.5 (94.0-105.0) | 98.0 (91.0-110.0) | 100.0 (98.0-113.5) | NS |
| BPs [mmHg] | 136.5 (125.0-145.0) | 136.5 (126.0-149.8) | 150.0 (137.0-154.0) | 125.0 (120.5-151.0) | NS |
| BPd [mmHg] | 82.0 (78.0-92.0) | 87.0 (82.0-97.8) | 92.0 (83.0-100.0) | 90.0 (77.5-100.5) | NS |
| Cholesterol [mmol/l] | 5.2 (4.2-6.0) | 5.3 (4.6-5.9) | 5.2 (4.1-6.5) | 5.2 (4.9-6.2) | NS |
| HDL [mmol/l] | 1.5 (1.3-1.8) | 1.3 (1.1-1.6) | 1.4 (1.2-1.8) | 1.3 (1.0-1.5) | NS |
| LDL [mmol/l] | 2.9 (2.2-3.7) | 2.9 (2.4-3.6) | 3.3 (2.0-3.4) | 3.0 (2.5-3.9) | NS |
| VLDL [mmol/l] | 0.6 (0.4-1.0) | 0.8 (0.5-1.0) | 0.6 (0.5-0.9) | 0.9 (0.8-1.3) | NS |
| TAG [mmol/l] | 1.3 (0.8-2.3) | 1.7 (1.1-2.3) | 1.8 (1.0-5.5) | 2.1 (1.7-2.8) | NS |
| Glucose [mmol/l] | 5.3 (5.1-5.6) | 5.4 (5.0-5.8) | 5.6 (5.3-6.0) | 4.9 (4.8-5.4) | NS |
| hs Troponin T [ng/L] | 1.5 (<3.0-5.5) | 3.4 (<3.0-5.4) | 3.3 (<3.0-4.4) | 4.8 (3.1-6.3) | NS |
|
AS: Active Surveillance, the control group; CT: Orchidectomy plus consequential cisplatin treatment. RT: Orchidectomy plus consequential adjuvant radiotherapy. CTRT: Orchidectomy plus consequential adjuvant radiotherapy in combination with cisplatin. BMI: Body Mass Index; BPs: Systolic Blood Pressure; BPd: Diastolic Blood Pressure; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; VLDL: Very Low Density Lipoprotein; TAG: Triglycerides; hs Troponin T: high-sensitivity troponin T; NS: Not Significant; |
|||||
Heart rate and time intervals
Table 2 presents the heart rate and the time intervals under study. There were no statistically significant differences in the heart rate and the time intervals between the groups. Bradycardia occurred in 28% of CT patients, but the differences between groups were not statistically significant. The occurrence of tachycardia was negligible.
| AS | CT | RT | CTRT | p | |
|---|---|---|---|---|---|
| HR [bpm] | 60.5 (52.7-69.3) | 65.0 (58.0-72.0) | 63.0 (57.8-74.0) | 67 (53.3-91.0) | NS |
| Bradycardia < 60 bpm | 7 (38.9%) | 35 (26.3%) | 5 (35.7%) | 2 (25%) | NS |
| Tachycardia | 0 | 0 | 0 | 0 | NA |
| QTcB [ms] | 383.6 (362.4-413.1) | 401.7 (380.3-424.3) | 395.2 (379.2-431.0) | 405.2(382.1-439.7) | NS |
| QTcF [ms] | 384.0 (371.2-412.1) | 396.5 (378.9-414.1) | 394.5 (374.7-415.7) | 399.7(374.5-414.1) | NS |
| QT/QRSd | 3.7 (3.3-4.1) | 3.8 (3.6-4.1) | 3.7 (3.6-3.9) | 3.5 (3.0-3.8) | NS |
| AS: Active Surveillance, the control group. CT: Orchidectomy plus consequential cisplatin treatment. RT: Orchidectomy plus consequential adjuvant radiotherapy. CTRT: Orchidectomy plus consequential adjuvant radiotherapy in combination with cisplatin. QRSd: QRS complex duration; QTcB: QT interval duration corrected according to Bazett [27]; QTcF: QT interval duration according to Fridericia [28]; NS: Not Significant; NA: Not Applicable due to small numbers. |
|||||
Distinct ECG patterns
The occurrence of distinct ECG patterns is presented in Table 3. All patients were on the sinus rhythm, sporadic premature ventricular contractions were observed in one patient in the AS group. No pathological Q waves were observed in any of the groups.
| AS n = 18 |
CT n = 133 |
RT n = 14 |
CTRT n = 8 |
p | |
|---|---|---|---|---|---|
| PVC | 1 | 0 | 0 | 0 | NA |
| Q wave | 0 | 0 | 0 | 0 | NA |
| LAFB n (%) | 1 (5.6) | 19 (14.3) | 2 (14.3)* | 6 (75)*** | * p<0.05 *** p<0.001# p<0.05 |
| LBBB | 0 | 0 | 1 | 0 | NA |
| RBBB | 0 | 1 | 0 | 0 | NA |
| iRBBB | 0 | 1 | 0 | 0 | NA |
| AS: Active Surveillance, the control group; CT: Orchidectomy plus consequential cisplatin treatment; RT: Orchidectomy plus consequential adjuvant radiotherapy; CTRT: Orchidectomy plus consequential adjuvant radiotherapy in combination with cisplatin; PVC: Premature Ventricular Contractions; LAFB: Left Anterior Fascicular Block; LBBB: Left Bundle Branch Block; RBBB: Right Bundle Branch Block; iRBBB: incomplete Right Bundle Branch Block; ECG-LVH: ECG signs of Left Ventricular Hypertrophy; ECG-RVH: ECG signs of Right Ventricular Hypertrophy. NS: Not Significant; NA: Not Applicable due to small numbers; *: p<0.05; ***: p<0.001, both compared to AS as well as to CT; #: p<0.05, compared to AS. | |||||
The left anterior fascicular block (LAFB) was the most frequent finding, it occurred in 19 (14.3%) cisplatin treated patients. The lower proportion was observed in the AS group, and the highest in the CTRT group. The proportion of patients with LAFB was significantly higher in RT and CTRT group compared to CT and AS groups.
QRSmax
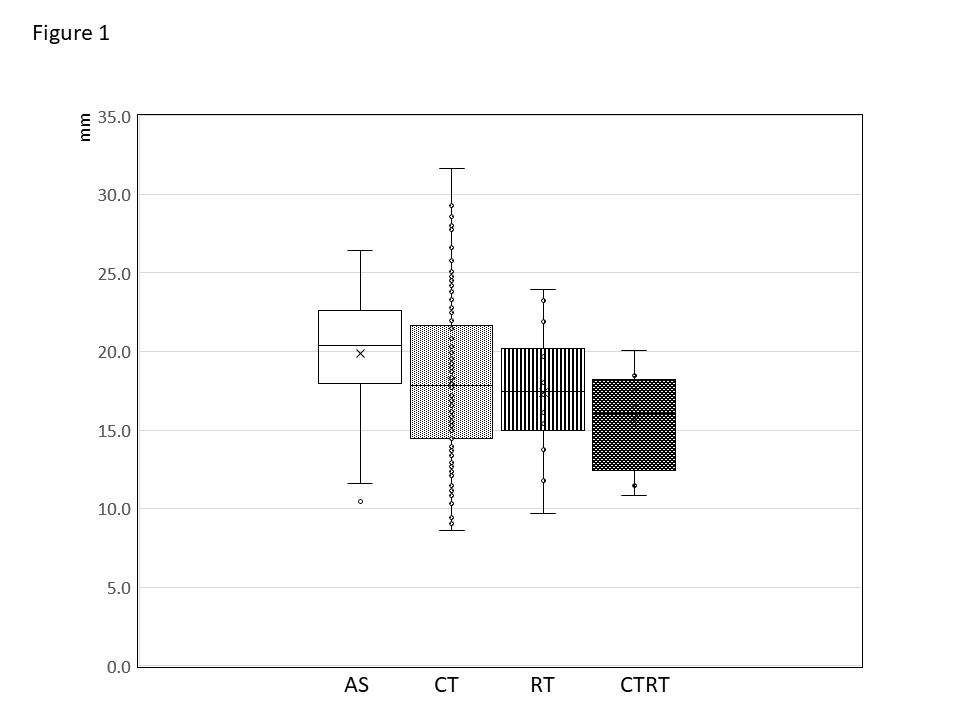
The values of QRSmax were within normal limits. As shown in the Figure 1, the QRSmax values were the highest in the AS group, slightly lower in the CT group, with the lowest values in the CTRT group; however, these differences were not statistically significant. The GLM showed significant effect of age and the waist circumference, but not of the therapeutic group.
Electrical axis of the QRS complex
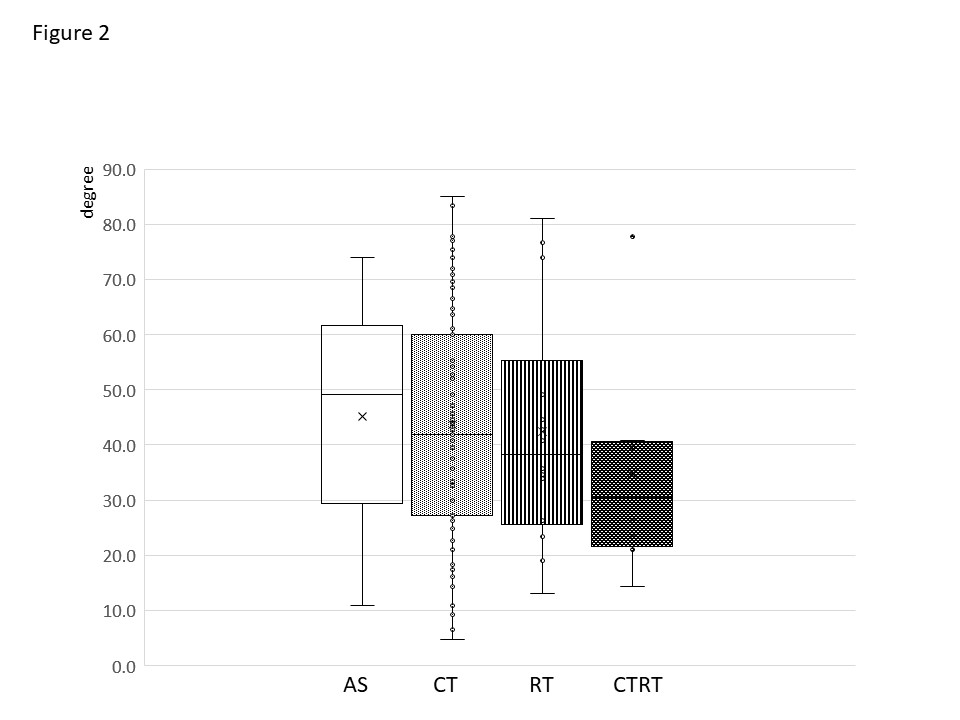
The values of electrical axis in the CT group, as well as in the other groups were within normal limits. The electrical axis values were the highest in the AS group, slightly lower in the CT group, with the lowest values in the CTRT group, however, the differences were not statistically significant (Figure 2). The GLM showed significant effect of age and the waist circumference but not of the therapeutic group.
QRS complex amplitude in the individual leads of the 12-lead ECG
Table 4 presents the values of the maximum QRS complex deflections in the individual leads of the electrocardiogram. The QRS amplitude in leads aVL was significantly higher in CT, RT and CTRT groups compared to the AS group (p=0.007). Significantly lower values in CT, RT and CTRT groups were observed in the lead V6 as compared to AS group (p=0.027). In both aVL and V6, the GLM showed significant effect of the waist circumference, but not of the therapeutic group (Table 5).
| AS | CT | RT | CTRT | p | |
|---|---|---|---|---|---|
| I | 6.0 (4.0-8.0) | 6.0 (4.0-8.0) | 6.0 (3.8-8.5) | 7.5 (5.5-8.8) | NS |
| II | 7.5 (6.0-9.3) | 7.0 (5.0-9.0) | 7.0 (5.5-8.3) | 4.0 (3.0-8.3) | NS |
| III | 4.0 (2.0-5.3) | 5.0 (3.0-7.0) | 3.5 (2.4-6.0) | 5.5 (4.0-8.0) | NS |
| aVR | 7.0 (5.8-8.3) | 7.0 (5.0-8.0) | 6.5 (5.8-8.0) | 4.5 (4-6.5) | NS |
| aVL | 2.3 (1.8-5.0) | 4.0 (3.0-6.0) | 4.0 (3.0-5.8) | 6.5 (4.5-8.8) | 0.007 |
| aVF | 5.0 (3.8-6.3) | 5.0 (3.0-8.0) | 4.5 (3.0-7.5) | 3.5 (3.0-5.8) | NS |
| V1 | 8.0 (7.0-10.0) | 7.0 (5.0-9.5) | 6.0 (4.8-9.0) | 4.5 (3.3-11.5) | NS |
| V2 | 10.0 (7.8-16.0) | 10.0 (7.0-12.0) | 10.0 (4.8-12.3) | 8.0 (3.5-12.5) | NS |
| V3 | 10.5 (7.0-14.0) | 10.0 (7.0-13.0) | 10.0 (9.0-12.5) | 13.0 (8.5-14.0) | NS |
| V4 | 10.5 (8.0-13.0) | 10.0 (8.0-13.5) | 10.5 (9.0-12.0) | 10.5 (7.5-13.5) | NS |
| V5 | 15.0 (10.8-17.3) | 12.0 (9.0-15.0) | 13.5 (9.8-16.0) | 12.5 (9.3-14.5) | NS |
| V6 | 15.0 (12.0-18.0) | 12.0 (9.0-15.0) | 12.5 (10.8-16.8) | 10.0 (7.0-14.3) | 0.027 |
| AS: Active Surveillance, the control group; CT: Orchidectomy plus consequential cisplatin treatment; RT: Orchidectomy plus consequential adjuvant radiotherapy; CTRT: Orchidectomy plus consequential adjuvant radiotherapy in combination with cisplatin. | |||||
| QRSmax | EA | aVL | V6 | Tmax | |
|---|---|---|---|---|---|
| Corr. M. intercept | 0.0001 0.0001 |
0.0001 0.0001 |
0.0001 0.225 |
0.005 0.0001 |
0.0001 0.0001 |
| Th group | 0.163 | 0.772 | 0.106 | 0.187 | 0.116 |
| Age | 0.042 | 0.034 | 0.717 | 0.488 | 0.219 |
| Follow-up | 0.149 | 0.211 | 0.523 | 0.516 | 0.982 |
| Waist | 0.0001 | 0.0001 | 0.0001 | 0.001 | 0.0001 |
The maximum spatial T vector magnitude
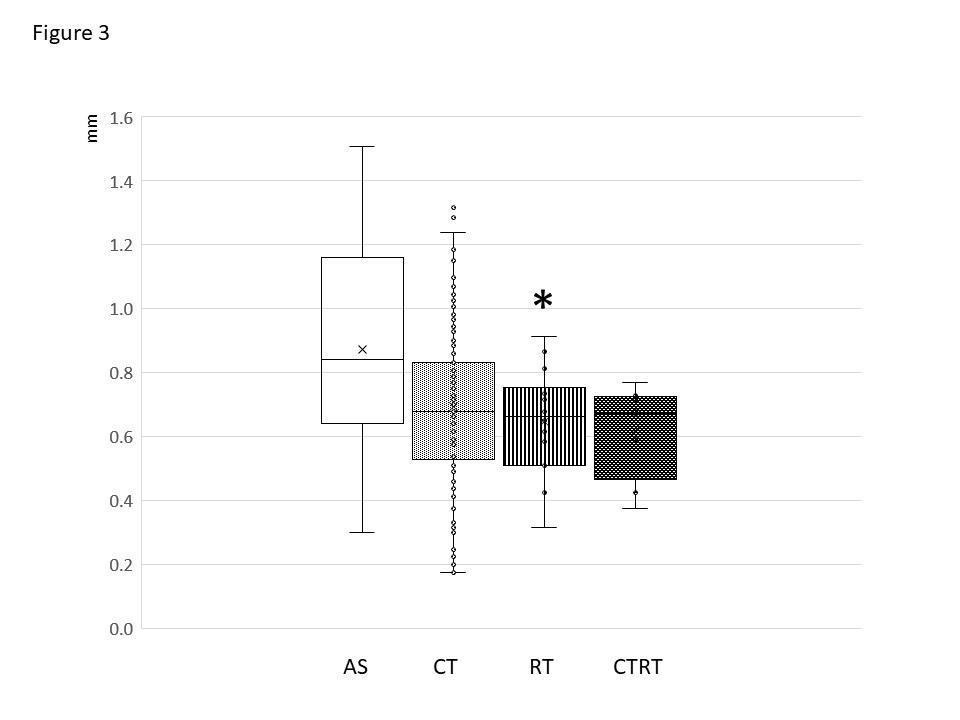
The values of Tmax in the CT group, as well as in the other groups were within normal limits. The Tmax values in CT and CTRT groups were lower compared to the AS group, however, these differences were not statistically significant, the values of Tmax in the RT group were significantly lower compared to the AS group (Figure 3). The GLM showed significant effect of the waist circumference, but not of the therapeutic group (Table 5).
Discussion
In patients with cisplatin-based therapy:
- HR and the time intervals under study were within normal limits and no significant differences between the groups were observed.
- The QRS complex parameters and Tmax values were within normal limits. The GLM did not show a significant effect of therapy, but the significant effect of age and waist circumference;
- The only distinctive pathological ECG finding was LAFB, its significantly highest occurrence was observed in the group CTRT.
The heart rate and the time intervals under study were within normal limits, as well no significant differences were observed between the groups. Changes in HR, both bradycardia and tachycardia, have been reported to be early complications of cisplatin-based therapy: bradycardia can occur as a rare early effect, on the other hand, also tachycardia was reported as an early complication[11,12,31]. In our study however, the occurrence of tachycardia was negligible. Bradycardia occurred in about one third of patients; but there were no differences between groups, therefore we did not assume that bradycardia was related to the treatment. Taking together, no changes in HR were found as a late cisplatin effect in this study.
The QT and QTc values were within normal limits. The QT interval prolongation is used as an indicator of cardiotoxicity [32,33], since it is associated with the risk of ventricular arrhythmias [34]. However, the systematic review showed that the incidence of arrhythmias and sudden cardiac death attributable to QTc prolongation from cancer therapy is extremely rare [33]. Our results are consistent with that statement.
The QT interval is frequently referred to as a parameter of repolarization; however, it contains both depolarization and repolarization. In order to distinguish the primary and secondary repolarization changes we analyzed the QT/QRSd ratio [35]. This ratio was used in experimental and clinical studies for predicting potential risk of drug-induced ventricular arrhythmias [36,37]. In our study, there were no significant differences in QT/QRSd ratio between the groups.
The QRS complex parameters were within normal limits. They did not differ significantly between the groups and the GLM showed significant effect of age and waist, but not of the therapeutic group. QRSmax represent the summary vector of depolarization. The solid angle theorem postulates that the recorded amplitude depends on the extent of the activation front (i.e. dimensions of the right and left ventricles, the sequence of depolarization) and electrical properties of myocardium (i.e. the transmembrane potential, ratio of electrically active and inactive tissue, conduction characteristics). The data on the heart dimensions were not available in this study; however we do not assume considerable changes in the heart dimensions, since papers focused on cardiotoxicity do not report changes in heart dimensions, as oppose to frequently reported alterations in cardiac functions. On the other hand, both cisplatin-based treatment, as well as radiotherapy cause myocardial changes that might alter the electrical properties of myocardium, and consequently might affect the resultant QRS vector [25,26,38-40]. Also obesity and aging were reported to be associated with the decrease in QRS amplitude [41-46]. Our finding is consistent with results documented the leftward shift of the electrical axis in obese subjects [41,47-49], and its occurrence is increased with age [50,51].
The slight differences in QRSmax in the combination with the differences in the electrical axis were reflected in the changes in the individual leads of 12-lead ECG. Although these differences were within normal limits, they could indicate subtle changes in electrical impulse propagation in ventricles. The combination of the decrease in QRS voltage and the electrical axis leftward shift have been observed in conditions with increased cardiovascular risk, such as aging, obesity, diabetes mellitus, metabolic syndrome and obstructive sleep apnea [41-46,52,53]. In this study, the GLM showed that the ECG changes were not associated with therapy, but with the age and the waist circumference. It could be assumed that these ECG findings are associated with the classical cardiovascular risk factors rather than with the cisplatin therapy per se.
In this study we found lower values of T wave amplitude in the CT, RT and CTRT groups as compared to AS group, the difference between AS and RT groups were statistically significant. The decrease in the spatial T vector magnitude was observed also by Wang et al. [54] during the treatment in patients receiving chemotherapy for breast cancer as a possible indicator of electrophysiological abnormalities. However, in this study the GLM did not show a significant effect of the therapeutic group, but of the waist circumference. The low T wave amplitude is a non-specific finding, documented in a variety of cardiac pathologies and associated with CV risk factors. Epidemiological studies have documented that non-specific T wave changes, including the low amplitude T wave, represent a significant independent risk of coronary artery disease and cardiac death [55,56]. Cisplatin has a complex effect on cells through multiple mechanisms [57,58], in combination of age and obesity in patients could affect the repolarization and the subtle Tmax changes might indicate the underlying alterations of electrogenesis [59].
Regarding distinctive QRS patterns, we found minimum pathological ECG findings in this study. We did not find any ECG signs of MI in our study population, such as angina pectoris, myocardial infarction and ECG changes mimicking MI or ischemia documented as early manifestations of cisplatin toxicity [9,60-62].
In the groups CT, RT and CTRT we observed a higher occurrence of patterns of left anterior fascicular block (LAFB), with the highest occurrence in the CTRT group. The ECG pattern of LAFB is typically attributed to the conduction block in the left anterior fascicle. However, this pattern is not unique just for the block in the left anterior fascicle. LAFB is a common nonspecific abnormality that occurs in a variety of left-sided cardiac pathologies, as well as in persons without overt cardiac disease [63]. We have also shown in our simulation studies that LAFB pattern can result also from diffuse and/or regional alteration in the activation propagation in the myocardium of the left ventricle [64,65]. It is not likely that the radiotherapy would affect selectively only the left anterior fasciculus of the left bundle; therefore we assume that this finding might reflect subtle subclinical conduction alteration of myocardium, more pronounced in the CTRT group.
Several factors could contribute to the alteration of the myocardium, and both cisplatin-based treatment and radiotheraphy have been reported to cause structural and functional changes. The effect of cisplatin includes direct damage of myocardium as well as of extracellular matrix, such as oxidative stress and enhanced endoplasmatic reticulum stress, activation of apoptotic processes, nuclear damage and mitochondrial abnormalities, disorganization of cardiomyocytes and widening of intercalated discs, induction of inflammation and fibrosis [25,26,38-40]. Also radiation causes structural and microvascular changes, as was shown in animal as well as in human studies, and late abnormalities can appear months to years later [66-71]. It is also assumed that the combination of chemotherapy and radiotherapy can have a synergetic effect on cardiac function [72].
Additionally, a great proportion on patients in this study were overweight or obese. The structural and functional changes of myocardium in obesity, referred to as “the fatty heart”, include altered glucose and lipid metabolism, the lipid accumulation in myocardium and inflammatory infiltrations [73,74]. And finally, an additional factor is the natural aging. Although none of these conditions were explicitly pronounced in any group of patients, they could have additive effect. A combination of these factors can affect electrogenesis, as well as the ratio of electrically active and inactive myocardial tissue, and consequently the sequence of ventricular activation and the morphology of the QRS complex.
Limitation of the study
There were several limitations of the study. The project was not originally designed for evaluating ECG, therefore not all pre-treatment ECGs were available, and only standard paper ECG recordings were available for the manual measurement. Neither records nor results of the 24-hour Holter monitoring were available in the patients, therefore the occurrence of arrhythmias could not be better evaluated. As well, no clinical events or complaints related to possible arrhythmias, such as syncope or palpitations were found in patients’ medical records.
There were relatively small numbers of patients in the AS , RT and CTRT groups compared to the CT group, since cisplatin is currently the recommended regimen in the treatment of testicular cancer [1,2] Because of the small sample size the results might not achieve statistical significance. In spite of the small numbers it was documented that the subtle ECG changes were pronounced in RT and CTRT groups, suggesting additive effect of radiotherapy, what is consistent with reported subclinical deterioration of cardiac function [22,38,75]. However, because of these limitation these results needs to be taken with caution.
Conclusion
It could be concluded that cisplatin treatment did not show signs of late cardiotoxicity that could be documented by ECG. The ECG findings in patients treated by cisplatin were dominantly within normal limits and the number of pathological ECG findings was negligible. The GLM model did not show a significant effect of cisplatin-based therapy, but the ECG parameters were significantly affected by age and obesity. However, the additive effect of radiotherapy cannot be excluded.
Authors’ Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Andreas Thaler, Lucia Petrikova, Beata Mladosievicova, Daniela Svetlovska, Katarina Kalavska, Zora Krivosikova, Jozef Mardiak, Michal Mego, Michal Chovanec and Ljuba Bacharova. The first draft of the manuscript was written by Ljuba Bacharova and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgment
The study was supported partly by the projects ITMS No. 26240120033, the European Regional Development Fund, and by the projects VEGA 1/0327/19, VEGA 01/0610/18 and APVV-15-0086. We thank Milan Nosko for his valuable input in the statistical analysis.
References
2. Einhorn LH. Treatment of testicular cancer: a new and improved model. J Clin Oncol. 1990;8:1777-81.
3. Kounis NG, Cervellin G, Lippi G. Cisplatin-induced bradycardia: Cardiac toxicity or cardiac hypersensitivity and Kounis syndrome? Int J Cardiol. 2016;202:817-8.
4. Schlumbrecht MP, Hehr K. Cisplatin-induced bradycardia and the importance of the QT interval. J Oncol Pharm Pract. 2015;21:157-60.
5. Meinardi MT, Gietema JA, van der Graaf WT, van Veldhuisen DJ, Runne MA, Sluiter WJ, et al. Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. J Clin Oncol. 2000;18:1725-32.
6. Sagstuen H, Aass N, Fosså SD, Dahl O, Klepp O, Wist EA, et al. Blood pressure and body mass index in long-term survivors of testicular cancer. J Clin Oncol. 2005;23:4980-90.
7. Huddart RA, Norman A, Shahidi M, Horwich A, Coward D, Nicholls J, et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol. 2003;21:1513-23.
8. Nord C, Fosså SD, Egeland T. Excessive annual BMI increase after chemotherapy among young survivors of testicular cancer. Br J Cancer. 2003;88:36-41.
9. Raghavan D, Cox K, Childs A, Grygiel J, Sullivan D. Hypercholesterolemia after chemotherapy for testis cancer. J Clin Oncol. 1992;10:1386-9.
10. Hansen SW, Groth S, Daugaard G, Rossing N, Rørth M. Long-term effects on renal function and blood pressure of treatment with cisplatin, vinblastine, and bleomycin in patients with germ cell cancer. J Clin Oncol. 1988;6:1728-31.
11. Bokemeyer C, Berger CC, Kuczyk MA, Schmoll HJ. Evaluation of long-term toxicity after chemotherapy for testicular cancer. J Clin Oncol. 1996;14:2923-32.
12. Ellis PA, Fitzharris BM, George PM, Robinson BA, Atkinson CH, Colls BM. Fasting plasma lipid measurements following cisplatin chemotherapy in patients with germ cell tumors. J Clin Oncol. 1992;10:1609-14.
13. Fosså SD, Aass N, Harvei S, Tretli S. Increased mortality rates in young and middle-aged patients with malignant germ cell tumours. Br J Cancer. 2004;90:607-12.
14. Nuver J, Smit AJ, Sleijfer DT, van Gessel AI, van Roon AM, van der Meer J, et al. Microalbuminuria, decreased fibrinolysis, and inflammation as early signs of atherosclerosis in long-term survivors of disseminated testicular cancer. Eur J Cancer. 2004;40:701-6.
15. van den Belt-Dusebout AW, de Wit R, Gietema JA, Horenblas S, Louwman MW, Ribot JG, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007;25:4370-8.
16. Lauritsen J, Hansen MK, Bandak M, Kreiberg MB, Skøtt JW, Wagner T, et al. Cardiovascular risk factors and disease after male germ cell cancer. J Clin Oncol. 2020;38:584-92.
17. Gerl A. Vascular toxicity associated with chemotherapy for testicular cancer. Anticancer Drugs. 1994;5:607-14.
18. Stefenelli T, Kuzmits R, Ulrich W, Glogar D. Acute vascular toxicity after combination chemotherapy with cisplatin, vinblastine, and bleomycin for testicular cancer. Eur Heart J. 1988;9:552-56.
19. Jakubowski AA, Kemeny N. Hypotension as a manifestation of cardiotoxicity in three patients receiving cisplatin and 5-fluorouracil. Cancer. 1988;62:266-69.
20. van Schinkel LD, Willemse PM, van der Meer RW, Burggraaf J, van Elderen SG, Smit JW, et al. Chemotherapy for testicular cancer induces acute alterations in diastolic heart function. Br J Cancer. 2013;109:891-6.
21. Doll DC, List AF, Greco FA, Hainsworth JD, Hande KR, Johnson DH. Acute vascular ischemic events after cisplatin-based combination chemotherapy for germ-cell tumors of the testis. Ann Intern Med. 1986;105:48-51.
22. Ozkan TA, Aydin U, Ay D, Cebeci IO. Cisplatin and bleomycin-induced acute peripheral-vascular stenosis in patient with testicular cancer. Urol Ann. 2016;8:483-85.
23. Lee KH, Lee JS, Kim SH. Electrocardiographic changes simulating acute myocardial infarction or ischemia associated with combination chemotherapy with etoposide, cisplatin, and 5-fluorouracil. Korean J Intern Med. 1990;5:112-7.
24. Eskilsson J, Albertsson M, Mercke C. Adverse cardiac effects during induction chemotherapy treatment with cis-platin and 5-fluorouracil. Radiother Oncol. 1988;13:41-46.
25. El-Hawwary AA, Omar NM. The influence of ginger administration on cisplatin-induced cardiotoxicity in rat: Light and electron microscopic study. Acta Histochem. 2019 Jul;121:553-62.
26. Chowdhury S, Sinha K, Banerjee S, Sil PC. Taurine protects cisplatin induced cardiotoxicity by modulating inflammatory and endoplasmic reticulum stress responses. Biofactors. 2016 Nov 12;42:647-64.
27. Bazett HC. An analysis of time relations of electrocardiograms. Heart 1920;7:353-67. Cited from: Salvi V, Karnad DR, Panicker K, Kothari S: Update on the evaluation of a new drug for effects on cardiac repolarization in humans: issues in early drug development. British J Pharmacol 2010; 159: 34-48.
28. Fridericia HJ. Der Systolendaeur in Elektrokardiogram bei normalen Menchen und bei Herzkranken. Acta Med Scand 1920; 53: 469–86. Cited from: Russel T, Stein DS, Kazierad DJ: Design, conduct and analysis of thorough QT studies. In: Bonate PL, Howard DR (eds.): Pharmacokinetics in drug development: Advances and applications, Volume 3, Springer, New York 2011: 211-41.
29. Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e241-50.
30. Baumert M, Porta A, Vos MA, Malik M, Couderc JP, Laguna P, et al. QT interval variability in body surface ECG: measurement, physiological basis, and clinical value: position statement and consensus guidance endorsed by the European Heart Rhythm Association jointly with the ESC Working Group on Cardiac Cellular Electrophysiology. Europace. 2016;18:925-44.
31. Kucharz J, Michalowska-Kaczmarczyk A, Zygulska AL, Wojtak J, Pawlik W, Herman RM, et al. Bradycardia as a rare symptom of cisplatin cardiotoxicity: A case report. Oncol Lett. 2016;11:2297-9.
32. Chandrasekhar S, Fradley MG. QT interval prolongation associated with cytotoxic and targeted cancer therapeutics. Curr Treat Options Oncol. 2019;20:55.
33. Porta-Sánchez A, Gilbert C, Spears D, Amir E, Chan J, Nanthakumar K, et al. Incidence, diagnosis, and management of QT prolongation induced by cancer therapies: A systematic review. J Am Heart Assoc. 2017;6. pii: e007724.
34. Cubeddu LX. QT prolongation and fatal arrhythmias: a review of clinical implications and effects of drugs. Am J Ther. 2003;10:452-7.
35. Bacharova L, Szathmary V, Mateasik A. Primary and secondary T wave changes in LVH: a model study. J Electrocardiology. J Electrocardiol 2010; 43: 624-33.
36. Lu HR, Yan GX, Gallacher DJ. A new biomarker – index of cardiac electrophysiological balance (iCEB) – plays an important role in drug-induced cardiac arrhythmias: beyond QT prolongation and torsade de pointes (TdPs). J Pharmacol Toxical Methods 2013;68:250-9.
37. Robyns T, Lu HR, Gallacher DJ, Garweg C, Ector J, Willems R, et al. Evaluation of cardio-electrophysiological balance (iCEB) as a new biomarker for the identification of patients at increased arrhythmic risk. Ann Noninvasive Electrocardiol 2016; 21:294-304.
38. Altena R, Hummel YM, Nuver J, Smit AJ, Lefrandt JD, de Boer RA, et al. Longitudinal changes in cardiac function after cisplatin-based chemotherapy for testicular cancer. Annals of Oncology 2011;22:2286-93.
39. Dugbartey GJ, Peppone LJ, de raaf IAM. An integrative view of cisplatin-induced renal and cardiac toxicities: molecular mechanisms, current treatment challenges and potential protective measures. Toxicology 2016; 371:58-66.
40. Sancho-Martínez SM, Prieto-García L, Prieto M, López-Novoa JM, López-Hernández FJ. Subcellular targets of cisplatin cytotoxicity: an integrated view. Pharmacol Ther. 2012;136:35-55.
41. Zack PM, Wiens RD, Kennedy HL Left-axis deviation and adiposity: the United States Health and Nutrition Examination Survey. Am J Cardiol. 1984;53:1129-34.
42. Abergel E, Tase M, Menard J, Chatellier G. Influence of obesity on the diagnostic value of electrocardiographic criteria for detecting left ventricular hypertrophy. Am J Cardiol. 1996;77:739–44.
43. Palhares DMF, Marcolino MS, Santos TMM, da Silva JLP, Gomes PR, Ribeiro LB, et al. Normal limits of the electrocardiogram derived from a large database of Brazilian primary care patients. BMC Cardiovasc Disord. 2017;17:152.
44. Macfarlane PW, van Oosterom A, Pahlm O, Kligfield P, Janse J, Camm J. Comprehensive electrocardiology. London: Springer-Verlag; 2011.
45. Rijnbeek PR, van Herpen G, Bots ML, Man S, Verweij N, Hofman A, et al. Normal values of the electrocardiogram for ages 16–90 years. J Electrocardiol. 2014;47:914–21.
46. Elffers TW, de Mutsert R, Lamb HJ, Maan AC, Macfarlane PW, Willems van Dijk K, et al. Relation of overall and abdominal adiposity with electrocardiogram parameters of subclinical cardiovascular disease in individuals aged 45 to 65 years (from the Netherlands Epidemiology of Obesity Study). Am J Cardiol. 2018;121:570-8.
47. Alpert MA, Terry BE, Cohen MV, Fan TM, Painter JA, Massey CV. The electrocardiogram in morbid obesity. Am J Cardiol. 2000;85:908-10.
48. Eisenstein I, Edelstein J, Sarma R, Sanmarco M, Selvester RH. The electrocardiogram in obesity. J Electrocardiol. 1982;15:115–8.
49. Hassing GJ, van der Wall HEC, van Westen GJP, Kemme MJB, Adiyaman A, Elvan A, et al. Body mass index related electrocardiographic findings in healthy young individuals with a normal body mass index. Neth Heart J. 2019;27:506-512.
50. Vicent L, Martínez-Sellés M. Electrocardiogeriatrics: ECG in advanced age. J Electrocardiol. 2017;50:698-700.
51. Molander U, Dey DK, Sundh V, Steen B. ECG abnormalities in the elderly: prevalence, time and generation trends and association with mortality. Aging Clin Exp Res. 2003;15:488-93.
52. Bacharova L, Krivosikova Z, Wsolova L, Gajdos M. Alterations in the QRS complex in the offspring of patients with metabolic syndrome and diabetes mellitus: early evidence of cardiovascular pathology. J Electrocardiol. 2012;45:244-251.
53. Bacharova L, Triantafyllou E, Vazaios C, Tomeckova I, Paranicova I, Tkacova R. The effect of obstructive sleep apnea on QRS complex morphology. J Electrocardiol. 2015;48:164-70.
54. Wang Y, Yin X, Liang X, Chen Y, Pan S, Chen Z, et al. Three-dimensional vectorcardiographic characteristics of breast cancer patients treated with chemotherapy. J Electrocardiol. 2021;67:23-30.
55. Kannel WB, Anderson K, McGee DL, Degatano LS, Stampfer MJ. Nonspecific electrocardiographic abnormality as a predictor of coronary heart disease: the Framingham Study. Am Heart J. 1987;113:370-6.
56. Greenland P, Xie X, Liu K, Colangelo L, Liao Y, Daviglus ML, et al. Impact of minor electrocardiographic ST-segment and/or T-wave abnormalities on cardiovascular mortality during long-term follow-up. Am J Cardiol. 2003;91:1068-74.
57. Martinho N, Santos TCB, Florindo HF, Silva LC. Cisplatin-Membrane Interactions and Their Influence on Platinum Complexes Activity and Toxicity. Front Physiol. 2019;9:1898.
58. El-Awady el-SE, Moustafa YM, Abo-Elmatty DM, Radwan A. Cisplatin-induced cardiotoxicity: Mechanisms and cardioprotective strategies. Eur J Pharmacol. 2011;650:335-41.
59. Alexandre J, Moslehi JJ, Bersell KR, Funck-Brentano C, Roden DM, Salem JE. Anticancer drug-induced cardiac rhythm disorders: Current knowledge and basic underlying mechanisms. Pharmacol Ther. 2018;189:89-103.
60. Spencker S, Schmittel A, Westermann D, Marek A, Schultheiss HP, Witzenbichler B. Angina pectoris and ST-elevation after chemotherapy with 5-fluorouracil. Internist (Berl). 2007;48:69-72.
61. Finsterer J, Ohnsorge P. Influence of mitochondrion-toxic agents on the cardiovascular system. Regul Toxicol Pharmacol. 2013;67:434-45.
62. Bachmeyer C, Joly H, Jorest R. Early myocardial infarction during chemotherapy for testicular cancer. Tumori. 2000;86:428-30.
63. Surawicz B, Knilans TK: Chou’s Electrocardiography in Clinical Practice. 6th Ed. Philadelphia: Saunders Elsevier; 2008.
64. Bacharova L, Mateasik A, Krause R, Prinzen FW, Auricchio A, Potse M. The effect of reduced intercellular coupling on electrocardiographic signs of left ventricular hypertrophy. J Electrocardiol. 2011;44:571-6.
65. Bacharova L, Szathmary V, Mateasik A. QRS complex and ST segment manifestations of ventricular ischemia: the effect of regional slowing of ventricular activation. J Electrocardiol. 2013;46:497-504.
66. Nielsen KM, Offersen BV, Nielsen HM, Vaage-Nilsen M, Yusuf SW. Short and long term radiation induced cardiovascular disease in patients with cancer. Clin Cardiol. 2017;40:255-61.
67. Lenneman CG, Sawyer DB. Cardio-Oncology: An update on cardiotoxicity of cancer-related treatment. Circ Res. 2016;118:1008-20.
68. Zhu Q, Kirova YM, Cao L, Arsene-Henry A, Chen J. Cardiotoxicity associated with radiotherapy in breast cancer: A question-based review with current literatures. Cancer Treat Rev. 2018;68:9-15.
69. Seemann I, Gabriels K, Visser NL, Hoving S, te Poele JA, Pol JF, et al. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiother Oncol. 2012;103:143-50.
70. DeBo RJ, Lees CJ, Dugan GO, Caudell DL, Michalson KT, Hanbury DB, et al. Late effects of total-body gamma irradiation on cardiac structure and function in male Rhesus Macaques. Radiat Res. 2016;186:55-64.
71. Boerma M, Hauer-Jensen M. Preclinical research into basic mechanisms of radiation-induced heart disease. Cardiol Res Pract. 2010;2011. pii: 858262.
72. Hayashi Y, Isohashi F, Tsujil Y, Fujinaga T, Nagal K, Yoshil S, et al. The heart’s exposure to radiation increases the risk of cardiac toxicity after chemotherapy for superficial esopheal cancer: a retrospective kohort study. BMC Cancer 2019; 19:195.
73. Guzzardi MA, Iozzo P. Fatty heart, cardiac damage, and inflammation. Rev Diabet Stud 2011;8:403-417.
74. Iozzo P. Myocardial, perivascular, and epicardial fat. Diabetes Care 2011;34 Suppl 2:S371-379.
75. Altena R, de Haas EC, Nuver J, Brouwer CA, van den Berg MP, Smit AJ, Postma A, Sleijfer DT, Gietema JA. Evaluation of sub-acute changes in cardiac function after cisplatin-based combination chemotherapy for testicular cancer. Br J Cancer. 2009;100:1861-6.