Abstract
Metabolic alterations induced by unhealthy lifestyles, including obesity and insulin resistance are often associated with increased innate immune response and chronic inflammation. Cholesterol has been identified as a key metabolite driving the activation of the inflammasome and the “epigenetic memory” in long-term living hematopoietic stem cells.
In addition to these mechanisms, the physiological aging of short-living neutrophils is a relevant modifier of their immune competency, as while they egress from medullary niches as “fresh”, fully competent, cells, they turn into “aged”, disarmed cells, when they extravasate into peripheral tissues to fight against pathogens or they reach the spleen for disposal. We recently observed that cardio-metabolic alterations induced by a lipid enriched unhealthy diet critically accelerate this process. Indeed, the chronic feeding with a high fat diet (HFD) results in the increase of aged neutrophils in the circulation and their accumulation in liver. This profile is associated with a deteriorated insulin response and obesity. The HFD primes aged, but not fresh neutrophils, to infiltrate in the liver and promotes inflammation coupled to altered cell immune architecture in visceral adipose tissue. Preventing the aging of neutrophils via selective ablation of CXCR2, reduces the development of obesity and improves the sensitivity to insulin. In humans, plasma levels of CXCL1, one of the cytokines binding CXCR2 and promoting neutrophil aging, are directly associated with abdominal adiposity and fatty liver independently of other risk factors.
Together these findings point to a direct role of aged neutrophils in the development of metabolic disorders.
Keywords
Neutrophils, Immunometabolism, High Fat Diet
Commentary
The intake of high calorie food, which is a risk factor for the development of chronic inflammatory and metabolic conditions and comorbidities, impacts the efficiency of the immune system through different mechanisms that can either constrain or exaggeratedly expands immune cells. Indeed, on one hand when the interferon-gamma signaling of T cells is impaired, such as in obesity [1], the feeding reduces the proresolutive migration of effector lymphocytes in response to infections and in presence of tumours [2], impairs the oral tolerance to antigens by T and B cells infiltrating the intestinal Peyer’s patches) [3] and it increases the risk of mortality in experimental models of pulmonary infection with P. Aeruginosa [4]. On the other, the chronic intake of high calorie foods can also promote the acquisition of a hyper-activated of the cell precursors residing in bone marrow (BM), which impacts myeloid cell outputs [5-7]. At the molecular level, this involves an epigenetic reprogramming which promotes the activation of the inflammasome, a complex of cytosolic multiprotein oligomers, that assembles as a consequence of the recognition of either pathogens or inflammatory stimuli [8]. However, an excessive and continuous state of feeding, over-activates the activity of the inflammasome and this results into cellular hyper-proliferation and supra-physiological production of cytokines/chemokines, including interleukin-1β (Il-1β) that, as a result, propagate the inflammatory response over time [7].
Also, cardio-metabolic risk factors trigger an excessive response of the inflammasome, and among them, cholesterol has been identified as a key inducer of the long-term hyper-activation of hematopoietic stem cells (HSCs) residing in the BM. This occurs by establishing a functional epigenetic response known as "trained immunity" [9-11], which affects the activity of a wide set of transcription factors and protein-DNA epigenetic regulators of HSCs physiology [14]. In addition to cholesterol, hyperglycemia also elicits trained immunity of BM-derived macrophages and their precursors, although this occurs via epigenetic activation of the Runt-related transcription factor 1 (Runx1) [12], a crucial transcription factor that regulates the leukemogenic expansion of HSCs. Thus, it is plausible that a sustained intake of refined sugars, which also characterizes the actual dietary patterns [13], can induce inflammatory expansion of hematopoietic progenitor cells via alternative cellular pathways to those triggered by cholesterol. Anyhow, it is to note that triacylglycerols (TGs), rather than cholesterol, represent the main fatty components of foods and, being much more energy-yielding compared to refined sugars, their excessive consumption prominently contributes to the derailment of metabolic homeostasis and to the onset of cardio-metabolic alterations, such as obesity and insulin resistance [14]. Fatty acids (FAs) are released in tissues after the hydrolysis of TGs present in lipoproteins. As such, there is a delicate balance between the delivery of FAs from TG to peripheral cells and their storage in dedicated tissues (for instance, the adipose tissue). In the case of chronic intake of fatty foods, the body initially compensates by increasing the storing capability in specific tissues until a low-grade inflammatory status is generated, which, in turn, triggers the activation of different immune cell subsets via an immunometabolic regulations [15,16]. A role for FAs in the intracellular metabolism of immune cells has been also largely explored. In fact, at cellular level, FAs represent a normal key substrate for the mitochondria to produce energy for different cellular purposes. However, an excess of FAs could drive the overactivation of mitochondria, which results into an increase of oxidative stress and inflammation within the cell [17,18]. Interestingly, these changes in TGs metabolism could be useful when certain immune cells should exert their cytotoxic activity but could be detrimental if it becomes uncontrolled. Also, an excessive substrate of FAs alters the activity of key cellular pathways/components which are essential for the proliferation of the HSCs.
Indeed, long-term living HSCs and the myeloid cell precursors residing in BM (both the common monocytoid progenitors [CMPs], which give rise to monocytes, and the granulocytic progenitor cells [GMPs] which differentiate to neutrophils) largely use oxidative metabolism in the niches [19], but they undergo an immunometabolic reprogramming when the skewing towards the granulocytic lineage is needed to generate short-living neutrophils. Neutrophils, once released from BM predominantly rely on anaerobic metabolism [17,20], despite the availability of oxygen in the blood. Of note, while epigenetic regulations imprint immune competency of neutrophils [5], these cells are programmed to predominantly utilize their DNA as a microbicidal weapon, and generate Neutrophils Extracellular Traps (NETs), to fight against pathogens when they reach target tissues.
It is conceivable to hypothesize that the impact of chronic consumption of calorie-dense, fatty foods on neutrophils goes beyond the activation of the inflammasome and the induction of trained immunity.
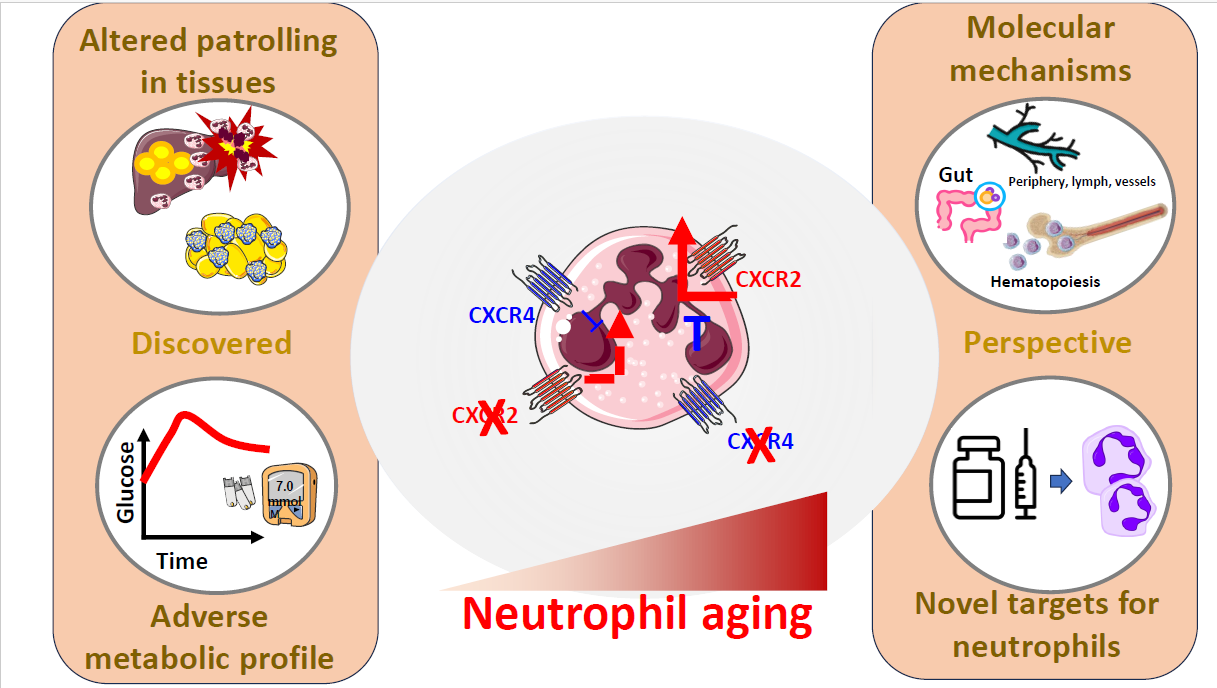
Our knowledge on the biology of neutrophils has been also profoundly expanded over the last decade. The classic vision of neutrophils as protagonists of the acute stages of inflammatory responses against pathogens has now been revisited; and these cells have been implicated in rheumatological, autoimmune diseases, atherosclerosis, diabetes and dysmetabolic comorbidities. Moreover, we now recognize a spectrum of neutrophils entities, presenting with different intracellular architecture and with abilities to aggress from BM niches, to distribute among tissues that might differ according to site-specific pathophysiological demands [21-25]. This phenotypic plasticity is simplified with the term “neutrophil aging”, a process that is coordinated by the finely tuned CXCR4/CXCR2 (C-X-C-motif chemokine receptor 4 and 2) axis. CXCR4, by binding the major ligand Stromal cell-derived factor 1 (SDF-1) strengthen the interaction between integrin α4β1 and its counter receptor VCAM-1 in the stromal vascular cells surrounding the niche [26,27], thus anchoring all the hematopoietic cell lineages, including the GMPs and their neutrophilic developmental subsets in BM [21,22]. By contrast, up to eleven chemokines, being produced by macrophages and epithelial cells in response to an inflammatory stimulus, can bind CXCR2, with half maximal effective concentrations (EC50) ranging by 1 nM (Cxcl3 [28]), 3-7 nM (as the case of Cxcl1, Cxcl2, Cxcl6, Cxcl7, Cxcl8 [28,29]), to 10-11 nM (Cxcl5, which is the weakest ligand [28]). Structural difference for some of these chemokines exist between mice and humans and this could translate in different responses in these two organisms. For instance, the murine form of CXCR2 binds to multiple CXCL8-like CXC chemokines, most notably the macrophage inflammatory protein (MIP)-2, which is the murine orthologue of the human 2 and 3 (GRObeta/gamma) [30-32]. The interaction between these chemokines and CXCR2 activates downstream signals that regulate the chemotaxis, the phagocytotic potential and the release of NETs of neutrophils. In addition, Cxcl1/Cxcl2 also coordinate the mobilization from and the homing back to medullary sites by promoting CXCR4 expression [33] and thus establishing a loop able to control the activity of neutrophils during infections [34], where the balance between “fresh” (i.e. over-expressing CXCR4) and “aged” (CXCR2 bright) becomes critical for the survival of the host. All these processes physiologically occur in neutrophils, following a circadian rhythmicity [35] that is also finely dictated by factors that are affected by the environmental and dietary exposure of the host, such as the case of gut microbiota [36]; however, we showed for the first time, that the derailment of the CXCR4/CXCR2 axis also contributes to the metabolic alterations induced by fats-enriched diet [37], thus extending the relevance of neutrophil aging in the context of chronic diseases.
We indeed characterized the development of obesity and metabolic disorders in mice constitutively expressing fresh neutrophils (neutrophil selective CXCR2 deficiency, MRP8Cre+_CXCR2fl/fl) or aged neutrophils (neutrophil selective CXCR4 deficiency, MRP8Cre+_CXCR4fl/fl) after 20 weeks of feeding with a high fat diet (“HFD”). We found that MRP8Cre+_CXCR2fl/fl mice become less obese as compared to MRP8Cre+_CXCR4fl/fl and WT mice and were more responsive to insulin. This was paralleled by reduced liver steatosis. Of note the contrary was observed in MRP8Cre+_CXCR4fl/fl mice, which presented increased lipid accumulation in the liver (Figure 1).
Interestingly neutrophilia, a common feature of MRP8Cre+_CXCR4fl/fl [34], was partly attenuated upon HFD feeding, a finding coupled to an increased accumulation of neutrophils in the liver of these mice but not in other sites of physiological homing such as the spleen, the lung, or the BM [38].
This might suggest that lipid accumulation in the liver could prime neutrophil hepatic infiltration. To explore whether this could be the case under acute conditions, an oral lipid tolerance test (intragastric olive oil gavage) was performed and MRP8Cre+_CXCR4fl/fl mice, but not the other two experimental models, presented a significant accumulation of neutrophils in liver two hours post gavage compared to fasting. Notably, we cannot exclude the relevance of glucose on the activation of neutrophils. Indeed, the expression of CXCR4 in these cells is regulated by the activity of the glucose-6-phosphate transporter [39], a key enzyme for the pentose phosphate pathway. Its genetic ablation in experimental models results into increased CXCR4 expression in these cells, together with intracellular accumulation of glycogen. This observation recapitulates the findings in glycogen storage disease type 1b [39], where neutropenia could be counteracted by treatment with empagliflozin, a glucose lowering drug that inhibits the sodium-glucose co-transporter-2 [40]. Further data to understand the molecular mechanisms beyond these findings are needed.
The different behaviour of aged and fresh neutrophils under this experimental setting is further reflected in their liver proteome. While that of MRP8Cre+_CXCR4fl/fl points to enrichment of proteins related to oxidative metabolism, NETosis and inflammation, the liver proteome of MRP8Cre+_CXCR2fl/fl mice was characterized by the enrichment of pathways related to physiological patrolling and resolutive inflammation.
The translational relevance of these findings was supported by the association that we found between blood neutrophils count and Cxcl1 plasma levels with markers of fatty liver and visceral adiposity in subjects with metabolic syndrome.
These findings highlight that neutrophil aging is both an active player but also a potential target for either lifestyle interventions or therapeutic tools to limit metabolic diseases and particular liver damage. From a nutritional standpoint, there is evidence that substituting the intake of refined sugars, or high glycaemic index meals, with vegetables, fibre and healthier food patterns, results into reduction of C-Reactive protein (a common marker of low grade-inflammation) in randomized-controlled interventional trials [41], and with reduced risk of developing clonal haematopoiesis of indetermined potential (CHIP) in leukocytes (a surrogate indicator acquired somatic leukemogenic variations in HSCs) in large epidemiological studies [42]. Likewise, several interventional studies support the anti-inflammatory effect of reducing the dietary intake of saturated fats [14]. Furthermore, increasing the intake of omega-3 fatty acids, particularly eicosapentaenoic acid (EPA) reduces the risk of atherosclerotic events in individuals at elevated CVD risk [43] by stabilizing the cell membrane [44] and by potentially reducing the interaction of neutrophils with endothelial cells, their chemotaxis and transmigration potential [45]. Anyhow, identifying which could be the precise nutritional component that, when a meal is ingested, could elicit the activation of neutrophils is complicated, given the hardwired neuro-hormonal circuits that connect the intestinal epithelium, with the enteric nervous system and the brain. The understanding of such aspects could also be of interest in the context of neuroinflammation.
At pharmacological level, CXCR2-directed therapies are already under investigation for the treatment of cardio-metabolic and liver diseases. Specifically, non-competitive allosteric inhibitors of CXCR2, limit systemic inflammation in streptozotocin-treated mice, reverse diabetes in NOD mice [46], improve hepatic insulin resistance, liver damage and inflammation in experimental models of HFD feeding [47] and in models of non-alcoholic fatty liver disease [48]. Despite these promising findings in experimental models, no beneficial effects of CXCR2 inhibition on residual β-cell function were demonstrated in newly diagnosed patients with autoimmune diabetes [49]. As anti-inflammatory therapies focused at restraining the hyperactivation of the innate arm of the immune system, demonstrated anti-atherosclerotic efficacy (e.g., Canakinumab, the antibody against Il-1β [50]) or reached clinical approval or the reduction of cardiovascular risk (as it is the case of Colchicine [51]), identifying strategies to control neutrophil aging without altering their efficacy against infection might help to improve the therapeutic options towards cardioimmunometabolic co-morbidities (Figure 1).
Acknowledgments
The authors are supported by: Telethon Foundation (GGP19146 to GDN); Progetti di Rilevante Interesse Nazionale (PRIN 2017 K55HLC to GDN); Ricerca Finalizzata, Ministry of Health (RF-2019-12370896 to GDN); PNRR Missione 4 (Progetto CN3 - National Center for Gene Therapy and Drugs based on RNA Technology to GDN); PNRR Missione 4 (Progetto MUSA- Multilayered Urban Sustainability Action to GDN); PNRR Missione 6 (PNRR-MAD-2022-12375913 to GDN); Progetti di Rilevante Interesse Nazionale (PRIN-PNRR 2022 P202294PHK to AB).
Author Contribution
Both A.B. and G.D.N drafted and edited the manuscript.
References
2. Collins N, Han SJ, Enamorado M, Link VM, Huang B, Moseman EA, et al. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell. 2019 Aug 22;178(5):1088-101.
3. Nagai M, Noguchi R, Takahashi D, Morikawa T, Koshida K, Komiyama S, Ishihara N, Yamada T, Kawamura YI, Muroi K, Hattori K. Fasting-refeeding impacts immune cell dynamics and mucosal immune responses. Cell. 2019 Aug 22;178(5):1072-87.
4. Janssen H, Kahles F, Liu D, Downey J, Koekkoek LL, Roudko V, et al. Monocytes re-enter the bone marrow during fasting and alter the host response to infection. Immunity. 2023 Apr 11;56(4):783-96.
5. Kalafati L, Kourtzelis I, Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T, et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell. 2020 Oct 29;183(3):771-85.
6. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017 Feb 9;542(7640):177-85.
7. Christ A, Günther P, Lauterbach MA, Duewell P, Biswas D, Pelka K, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell. 2018 Jan 11;172(1):162-75.
8. Baragetti A, Catapano AL, Magni P. Multifactorial activation of NLRP3 inflammasome: relevance for a precision approach to atherosclerotic cardiovascular risk and disease. International Journal of Molecular Sciences. 2020 Jun 23;21(12):4459.
9. Christ A, Lauterbach M, Latz E. Western diet and the immune system: an inflammatory connection. Immunity. 2019 Nov 19;51(5):794-811.
10. Domínguez-Andrés J, Joosten LA, Netea MG. Induction of innate immune memory: the role of cellular metabolism. Current Opinion in Immunology. 2019 Feb 1;56:10-6.
11. Netea MG, Quintin J, Van Der Meer JW. Trained immunity: a memory for innate host defense. Cell Host & Microbe. 2011 May 19;9(5):355-61.
12. Edgar L, Akbar N, Braithwaite AT, Krausgruber T, Gallart-Ayala H, Bailey J, et al. Hyperglycemia induces trained immunity in macrophages and their precursors and promotes atherosclerosis. Circulation. 2021 Sep 21;144(12):961-82.
13. Imamura F, Micha R, Khatibzadeh S, Fahimi S, Shi P, Powles J, et al. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. The Lancet Global Health. 2015 Mar 1;3(3):e132-42.
14. Mattavelli E, Catapano AL, Baragetti A. Molecular immune-inflammatory connections between dietary fats and atherosclerotic cardiovascular disease: which translation into clinics?. Nutrients. 2021 Oct 25;13(11):3768.
15. Palma C, La Rocca C, Gigantino V, Aquino G, Piccaro G, Di Silvestre D, et al. Caloric restriction promotes immunometabolic reprogramming leading to protection from tuberculosis. Cell Metabolism. 2021 Feb 2;33(2):300-18.
16. Mauro C, Smith J, Cucchi D, Coe D, Fu H, Bonacina F, et al. Obesity-induced metabolic stress leads to biased effector memory CD4+ T cell differentiation via PI3K p110δ-Akt-mediated signals. Cell Metabolism. 2017 Mar 7;25(3):593-609.
17. Baragetti A, Bonacina F, Catapano AL, Norata GD. Effect of lipids and lipoproteins on hematopoietic cell metabolism and commitment in atherosclerosis. Immunometabolism. 2021 Mar 3;3(2).
18. Bonacina F, Baragetti A, Catapano AL, Norata GD. The interconnection between immuno-metabolism, diabetes, and CKD. Current Diabetes Reports. 2019 May;19:1-8.
19. Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nature Reviews Immunology. 2020 Jun;20(6):375-88.
20. Kumar S, Dikshit M. Metabolic insight of neutrophils in health and disease. Frontiers in Immunology. 2019 Sep 20;10:2099.
21. Adrover JM, Aroca-Crevillén A, Crainiciuc G, Ostos F, Rojas-Vega Y, Rubio-Ponce A, et al. Programmed ‘disarming’of the neutrophil proteome reduces the magnitude of inflammation. Nature Immunology. 2020 Feb;21(2):135-44.
22. Evrard M, Kwok IW, Chong SZ, Teng KW, Becht E, Chen J, et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity. 2018 Feb 20;48(2):364-79.
23. Casanova-Acebes M, Nicolás-Ávila JA, Li JL, García-Silva S, Balachander A, Rubio-Ponce A, et al. Neutrophils instruct homeostatic and pathological states in naive tissues. Journal of Experimental Medicine. 2018 Nov 5;215(11):2778-95.
24. Grieshaber-Bouyer R, Radtke FA, Cunin P, Stifano G, Levescot A, Vijaykumar B, et al. The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments. Nature Communications. 2021 May 17;12(1):2856.
25. Ballesteros I, Rubio-Ponce A, Genua M, Lusito E, Kwok I, Fernández-Calvo G, et al. Co-option of neutrophil fates by tissue environments. Cell. 2020 Nov 25;183(5):1282-97.
26. Shen W, Bendall LJ, Gottlieb DJ, Bradstock KF. The chemokine receptor CXCR4 enhances integrin-mediated in vitro adhesion and facilitates engraftment of leukemic precursor-B cells in the bone marrow. Experimental Hematology. 2001 Dec 1;29(12):1439-47..
27. Reid JC, Tanasijevic B, Golubeva D, Boyd AL, Porras DP, Collins TJ, et al. CXCL12/CXCR4 signaling enhances human PSC-derived hematopoietic progenitor function and overcomes early in vivo transplantation failure. Stem Cell Reports. 2018 May 8;10(5):1625-41.
28. Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. Journal of Biological Chemistry. 1996; 271, 20545-50.
29. Wuyts A, Proost P, Lenaerts JP, Ben‐Baruch A, Van Damme J, Wang JM. Differential usage of the CXC chemokine receptors 1 and 2 by interleukin‐8, granulocyte chemotactic protein‐2 and epithelial‐cell‐derived neutrophil attractant‐78. European Journal of Biochemistry. 1998 Jul 1;255(1):67-73.
30. Bozic CR, Gerard NP, von Uexkull-Guldenband C, Kolakowski Jr LF, Conklyn MJ, Breslow R, et al. The murine interleukin 8 type B receptor homologue and its ligands. Expression and biological characterization. Journal of Biological chemistry. 1994 Nov 25;269(47):29355-8.
31. Bozic CR, Kolakowski Jr LF, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, et al. Expression and biologic characterization of the murine chemokine KC. Journal of Immunology (Baltimore, Md.: 1950). 1995 Jun 1;154(11):6048-57.
32. Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptor. Journal of Immunology (Baltimore, Md.: 1950). 1995 Aug 15;155(4):2158-64.
33. Adamiak M, Abdel-Latif A, Bujko K, Thapa A, Anusz K, Tracz M, et al. Nlrp3 inflammasome signaling regulates the homing and engraftment of hematopoietic stem cells (HSPCs) by enhancing incorporation of CXCR4 receptor into membrane lipid rafts. Stem Cell Reviews and Reports. 2020 Oct;16:954-67.
34. Adrover JM, Del Fresno C, Crainiciuc G, Cuartero MI, Casanova-Acebes M, Weiss LA, et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity. 2019 Feb 19;50(2):390-402.
35. Aroca-Crevillén A, Adrover JM, Hidalgo A. Circadian features of neutrophil biology. Frontiers in Immunology. 2020 Apr 3;11:576.
36. Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015 Sep 24;525(7570):528-32.
37. Baragetti A, Da Dalt L, Moregola A, Svecla M, Terenghi O, Mattavelli E, et al. Neutrophil aging exacerbates high fat diet induced metabolic alterations. Metabolism. 2023 Jul 1;144:155576.
38. Casanova-Acebes M, Nicolás-Ávila JA, Li JL, García-Silva S, Balachander A, Rubio-Ponce A, et al. Neutrophils instruct homeostatic and pathological states in naive tissues. Journal of Experimental Medicine. 2018 Nov 5;215(11):2778-95.
39. McDermott DH, De Ravin SS, Jun HS, Liu Q, Priel DA, Noel P, et al. Severe congenital neutropenia resulting from G6PC3 deficiency with increased neutrophil CXCR4 expression and myelokathexis. Blood, The Journal of the American Society of Hematology. 2010 Oct 14;116(15):2793-802.
40. Guerra F, Gasperini S, Bonanomi S, Crescitelli V, Pretese R, Da Dalt L, et al. Finding balance between mature and immature neutrophils: The effects of empagliflozin in GSD‐Ib. EJHaem. 2023 May;4(2):551.
41. Navarro SL, Tarkhan A, Shojaie A, Randolph TW, Gu H, Djukovic D, et al. Plasma metabolomics profiles suggest beneficial effects of a low–glycemic load dietary pattern on inflammation and energy metabolism. The American Journal of Clinical Nutrition. 2019 Oct 1;110(4):984-92.
42. Bhattacharya R, Zekavat SM, Uddin MM, Pirruccello J, Niroula A, Gibson C, et al. Association of diet quality with prevalence of clonal hematopoiesis and adverse cardiovascular events. JAMA cardiology. 2021 Sep 1;6(9):1069-77.
43. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. New England Journal of Medicine. 2019 Jan 3;380(1):11-22.
44. Jacobs ML, Faizi HA, Peruzzi JA, Vlahovska PM, Kamat NP. EPA and DHA differentially modulate membrane elasticity in the presence of cholesterol. Biophysical Journal. 2021 Jun 1;120(11):2317-29.
45. Sherratt SC, Dawoud H, Bhatt DL, Malinski T, Mason RP. Omega-3 and omega-6 fatty acids have distinct effects on endothelial fatty acid content and nitric oxide bioavailability. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2021 Oct 1;173:102337.
46. Citro A, Valle A, Cantarelli E, Mercalli A, Pellegrini S, Liberati D, et al. CXCR1/2 inhibition blocks and reverses type 1 diabetes in mice. Diabetes. 2015 Apr 1;64(4):1329-40.
47. Phillips BE, Lantier L, Engman C, Garciafigueroa Y, Singhi A, Trucco M, et al. Improvement in insulin sensitivity and prevention of high fat diet-induced liver pathology using a CXCR2 antagonist. Cardiovascular Diabetology. 2022 Jul 12;21(1):130.
48. Leslie J, Mackey JB, Jamieson T, Ramon-Gil E, Drake TM, Fercoq F, et al. CXCR2 inhibition enables NASH-HCC immunotherapy. Gut. 2022 Oct 1;71(10):2093-106.
49. Piemonti L, Keymeulen B, Gillard P, Linn T, Bosi E, Rose L, et al. Ladarixin, an inhibitor of the interleukin‐8 receptors CXCR1 and CXCR2, in new‐onset type 1 diabetes: A multicentre, randomized, double‐blind, placebo‐controlled trial. Diabetes, Obesity and Metabolism. 2022 Sep;24(9):1840-9.
50. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. New England Journal of Medicine. 2017 Sep 21;377(12):1119-31.
51. U.S. FDA Approves First Anti-Inflammatory Drug for Cardiovascular Disease - Agepha Pharma US. https://us.agephapharma.com/blog/2023/06/20/us-fda-approves-first-anti-inflammatory-drug-for-cardiovascular-disease/.