Abstract
Unesbulin is an orally bioavailable small molecule binding to the colchicine-binding site of tubulin and impeding tubulin polymerization and microtubule formation. It has been investigated as a monotherapy and in combination with other medications for the treatment of cancer, including leiomyosarcoma. This study investigated the effect of food on the pharmacokinetics of unesbulin.
In total, eight leiomyosarcoma (LMS) patients (four males and four females) were enrolled in the food effect study during the phase 1b clinical study (NCT03761095). Patients received dacarbazine 1000 mg/m2 intravenously once every 21 days (Q21D) and unesbulin 300 mg orally twice weekly (BIW) starting the day after dacarbazine infusion in each 21-day cycle. The effect of food was assessed during the first week of a cycle that was most convenient for the patient. During this week, participants received the first unesbulin dose on Day 2 after a 10-hour overnight fast, and the second dose on Day 5 with a low-fat diet following another 10-hour overnight fast. There were no restrictions on food intake for the remainder of the trial. Blood samples were collected up to 72 hours post unesbulin administration, and plasma concentration was measured using a validated HPLC-MS method.
Comparable unesbulin exposures were observed under both fed and fasted conditions with the geometric mean ratios of fed to fasted being 1.18 (90% CI: 0.976, 1.43) for Cmax, 0.934 (0.730, 1.20) for AUC0–48h, and 0.965 (0.711, 1.31) for AUC0–inf. Notably, the inter-subject variability was greatly reduced when unesbulin was administered with food, decreasing from 43.7% to 28.2% for Cmax and from 53.3% to 19.0% for AUC0–48h.
The results indicate that food does not affect the mean oral bioavailability of unesbulin, allowing it to be administered regardless of food intake. However, administering unesbulin with food is encouraged to maintain consistent exposure levels.
Keywords
Unesbulin, Food effect, Oral bioavailability, Pharmacokinetics, PK, Tubulin binding, Anticancer, Leiomyosarcoma, LMS
Introduction
Unesbulin is a small molecule binding to the colchicine-binding site of tubulin. This binding leads to destabilization of tubulin polymerization and microtubule formation, which consequently results in tumor cell arrest in the G2/M phase [1]. The colchicine-binding site of tubulin is different from the site of other approved tubulin-binding agents, such as taxanes, docetaxel, and paclitaxel. Additionally, unesbulin is not a P-glycoprotein substrate, which makes it feasible for oral administration and bioavailable in brain tissues, which was confirmed in the mouse study [1–3]. It is predicted that unesbulin could potentially be effective for drug-resistant cancer patients. Research in xenograft mouse models demonstrates the antitumor effect of unesbulin in combination with dacarbazine for leiomyosarcoma (LMS) and as a monotherapy against diffuse intrinsic pontine glioma (DIPG) [1,4]. The interim analysis from a phase 1b study (NCT03761095) demonstrated a favorable benefit–risk profile with unesbulin 300 mg administered orally twice per week (BIW) in combination with dacarbazine 1000 mg/m2 administered intravenously once every 21 days (Q21D) for the treatment of patients with advanced LMS; this dose regimen was adopted for patients in the expansion cohort of this trial and was recommended for further phase 2 clinical development [5].
Pharmacokinetics (PK) of unesbulin has been investigated in a monotherapy dose-ascending study in adult patients with advanced solid tumors and in the phase 1b study in combination with dacarbazine in adult patients with advanced LMS [6,7]. Following a single oral dose of unesbulin in patients with advanced solid tumors, the median time to peak plasma drug concentration (Tmax) was approximately 4 hours (h), and the terminal half-life (T½) was approximately 10 h [6]. Unesbulin plasma area under the concentration-time curve (AUC) increased dose proportionally (0.65–10.4 mg/kg), whereas Cmax increased dose proportionally up to 2.6 mg/kg and less than dose-proportionally at a dose above 2.6 mg/kg [7]. There was essentially no accumulation or autoinduction following repeated BIW dosing. Unesbulin was demonstrated to be a strong clinical CYP1A2 inhibitor and inducer when coadministered with dacarbazine, a substrate of CYP1A2. However, no impact on unesbulin PK was observed from dacarbazine or repeated unesbulin dosing, although in vitro human liver microsome and hepatocyte studies indicated that unesbulin was a substrate of CYP1A2 [6]. No gender difference in unesbulin was observed following oral administration in a previous clinical study [7].
In all prior clinical studies, patients were instructed to take unesbulin without any restrictions on food intake. It is well recognized that food can significantly impact drugs administered orally, but this effect has not yet been studied and is unknown for unesbulin. This was primarily owing to the recommendation to investigate unesbulin only in cancer patients who could derive clinical benefits. During the phase 1b study, additional patients were enrolled for the expansion cohort post identifying the recommended phase 2 dose to further confirm the clinical benefits. During this part of the study, all patients received consistent unesbulin and dacarbazine doses while dose adjustment was refrained. The opportunity to assess the food effect on unesbulin PK was seized by enrolling patients from the expansion cohort during their ongoing treatment. Previous clinical experience indicated that unesbulin PK was unaffected by repeated dosing or dacarbazine administration; hence, the food effect study was conducted during the first week of the cycle most convenient for the patient. During the food effect study week, unesbulin was dosed under the fasted condition on Day 2 and with a low-fat diet on Day 5. For the remainder of the clinical study, unesbulin was administered without any food restrictions.
Materials and Methods
The food effect study was conducted at two clinical sites, Mayo Clinic (Jacksonville, FL) and Washington University Medical Center in St. Louis School of Medicine (St. Louis, MO), which were among the multiple sites participating in the broader phase 1b study (NCT03761095). The study was conducted in accordance with the International Council for Harmonization (ICH) Guideline on Good Clinical Practice (GCP) and the United States Code of Federal Regulations in conformance with the principles of the Declaration of Helsinki. The study protocol and informed consent form were reviewed and approved by each study site’s institutional review board, and all participants had signed the consent form prior to enrolling in the study. More details of the phase 1b study design, inclusion and exclusion criteria, clinical efficacy and safety, and drug interaction findings have been reported earlier [5].
Food effect on the unesbulin PK was assessed in adult patients (≥18 years) with advanced LMS with Eastern Cooperative Oncology Group (ECOG) performance status 0–1 from the expansion cohort in the phase 1b study. Participants of the food effect study received dacarbazine 1000 mg/m2 intravenously Q21D on Day 1 and unesbulin 300 mg orally BIW starting on Day 2 of every 21-day cycle. During the food effect study cycle, participants received the first dose of unesbulin 300 mg (tablets) with no food on Day 2, after a 10 h overnight fast. On Day 5, participants received the second dose of unesbulin 300 mg (tablets) with a low-fat, low-calorie breakfast after another 10-h overnight fast. The meal was provided and consumed within 30 minutes prior to unesbulin dosing. The contents of the low-fat low-calorie meal was in accordance with the FDA guidance [8]. On Day 2 and Day 5 of unesbulin food effect study, liquid intake was restricted from 1 h prior to unesbulin administration to 1 h postdose; and food intake was restricted for up to 4 h post unesbulin administrations. There was no food/liquid restriction on other unesbulin dosing days during the clinical study.
In total, 12 participants, with a minimum of 6 completers, were planned for the food effect study without formal sample size estimation. This was based on the minimum sample size recommended by FDA guidance for a pilot food effect study [8], and the feasibility of potential available participants in this study.
Safety and clinical efficacy from this whole clinical study have been reported earlier and will not be repeated here [5].
PK assessment
PK sampling: Blood samples for the measurement of unesbulin PK were collected at predose (within 1h prior to dose), 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 36, 48, and 72 h after unesbulin oral administration; plasma was harvested within 30 minutes of blood collection. Unesbulin plasma concentration was measured using a validated LC-MS/MS method. Details of blood sample collection procedures and the LC-MS/MS method were reported in an earlier publication [7]. The bioanalytical method had a lower limit of quantitation of 1.00 ng/mL.
PK analysis and statistical evaluation: The PK of unesbulin was assessed using noncompartmental analysis with Phoenix WinNonlin (version 8.3.5; Certara). PK parameters were derived using the actual dose and time. AUCs were calculated using the linear up logarithm down method. T1/2 was estimated from the terminal phase of the semilogarithmic concentration-time slope with at least three post-Tmax measurable data points. The following criteria were met: the adjusted R2 was greater than or equal to 0.80, the span was greater than or equal to 1.5, and the resulting extrapolated AUC (%) was less than 20%.
The geometric mean ratios (GMRs) for the fed to the fasted condition were calculated for natural logarithm-transformed PK parameters Cmax, AUC0–48h, and AUC0–inf.
Results
Baseline demographics
Despite all the efforts to enroll 12 subjects for the food effect study, only eight subjects (four males and four females) with advanced LMS were enrolled in the study, with a mean age of 58 years (range, 42–67 years). The mean body weight at screening was 82.2 kg (range, 60.3–122 kg) and the mean height was 168.3 cm (range, 142.0–185.4 cm). The mean body mass index at screening was 29.1 kg/m2 (range, 22.8–46.0 kg/m2). In total, six of the participants were White, one was Asian, and one was Black/African American.
Pharmacokinetic analysis
One subject received a reduced unesbulin dose during the fasted treatment period, and data from that period for this subject were excluded from the PK analysis. One subject did not complete PK blood sample collection at the 24 h, 48 h, and 72 h timepoints post-Day 5 unesbulin dosing. However, the last available sample at 8 hours showed a consistent decline in concentration across two consecutive timepoints following the observed peak. Hence, the Tmax and Cmax were derived and included for food effect analysis, whereas AUCs were not calculated. Additionally, unesbulin concentrations at 72 h postdose were missing for five subjects/periods. Consequently, AUC0–48h was used instead of AUC0–72h to assess food effect to maximize the number of subjects included for the analysis. Sufficient total AUC was already captured by 48 h because the ratio of AUC0–48h to AUC0–inf was greater than 80% for all subjects/periods with evaluable AUC0–48h and AUC0–inf. The PK parameters were presented in Table 1.
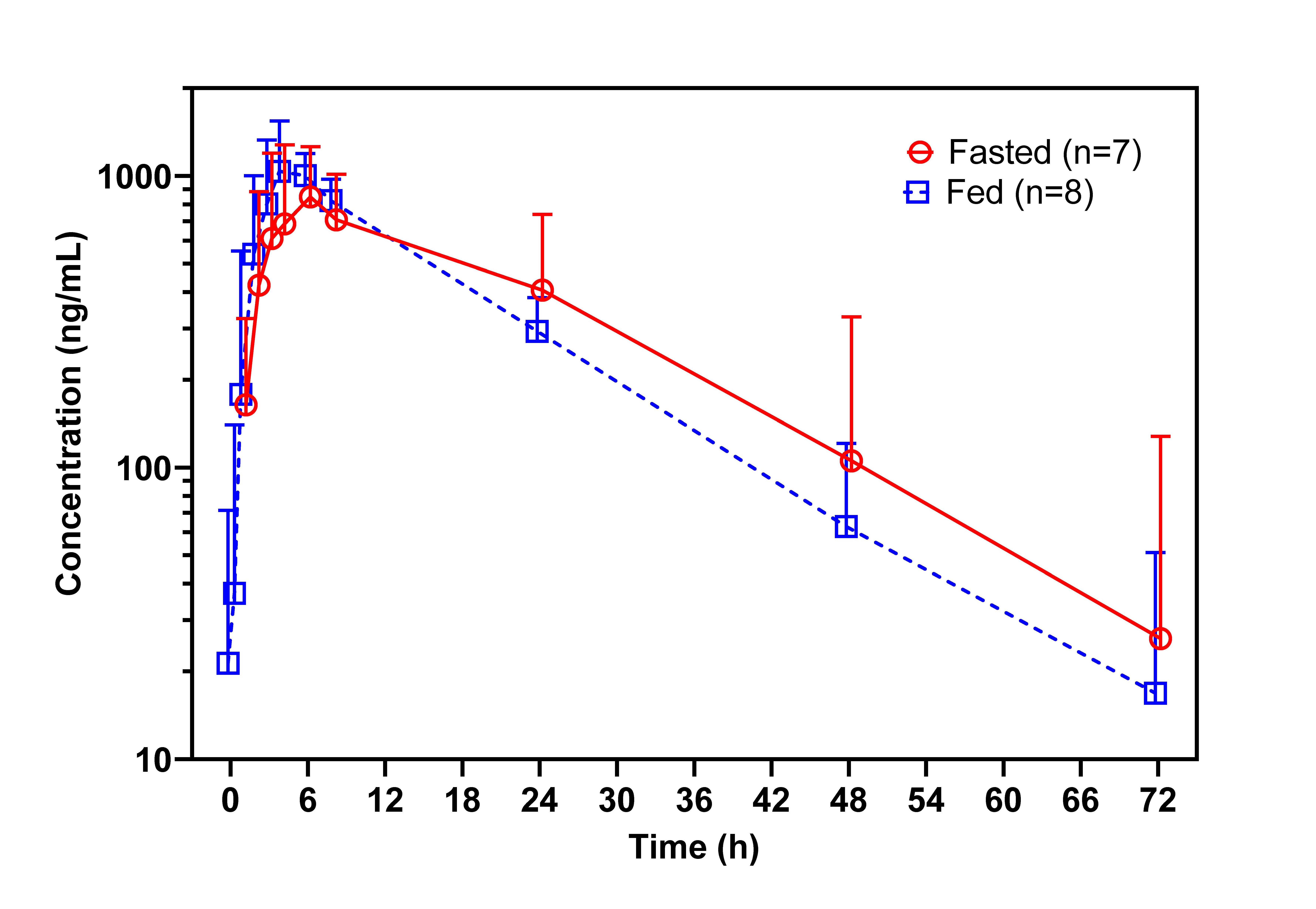
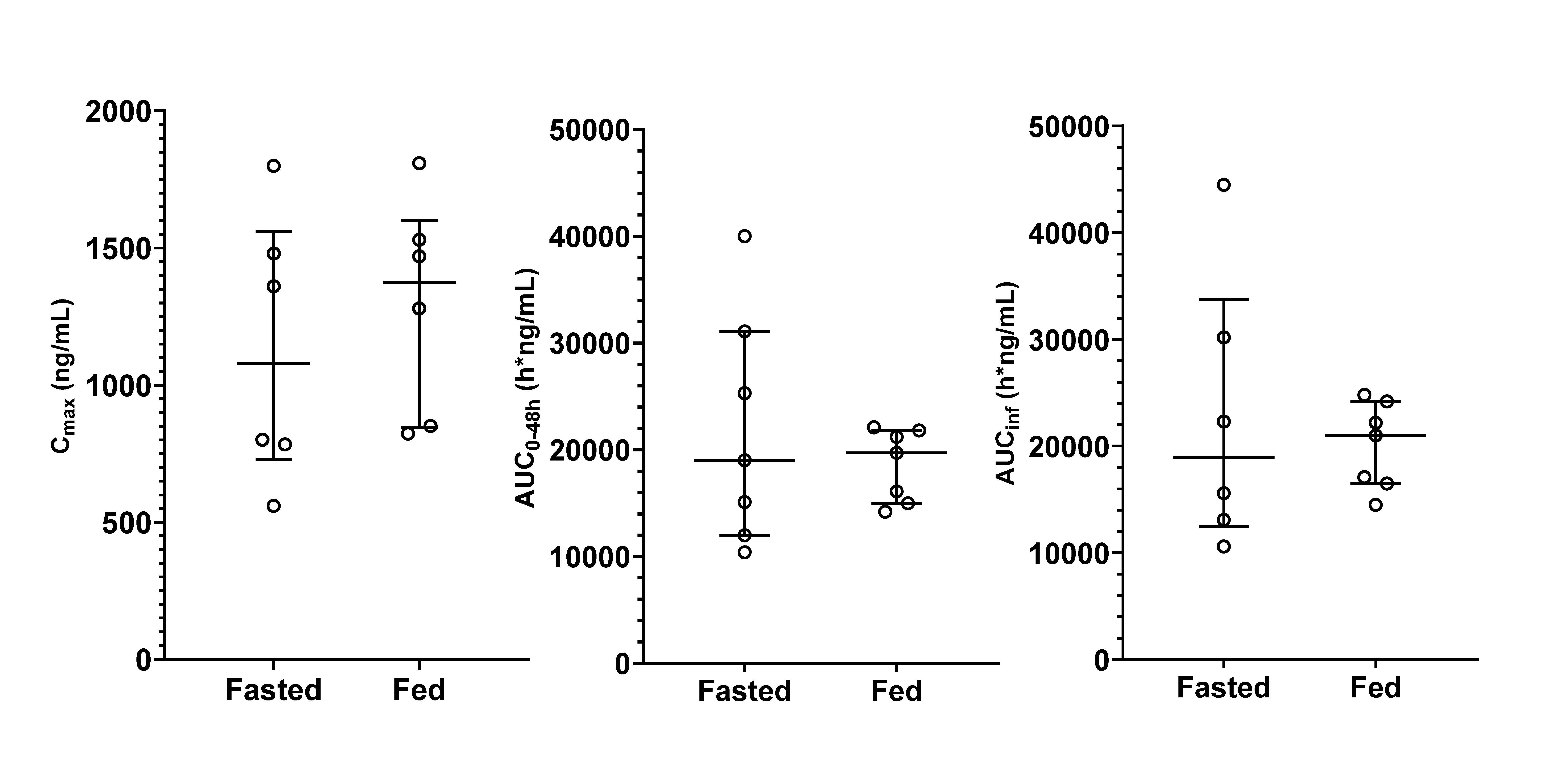
Under both fasted and fed conditions, unesbulin reached its Cmax at the same median time of 4 h following a single oral dose of 300 mg (Figure 1). The Cmax was slightly higher under fed conditions than fasted conditions, with a GMR for natural logarithm-transformed data of 1.18 (90% CI: 0.976, 1.43) (Table 1). The AUC0–48h and AUC0–inf were essentially the same for both food conditions. The GMRs for fed versus fasted conditions for AUC0–48h and AUC0–inf were 0.934 (90% CI: 0.730, 1.20) and 0.965 (90% CI: 0.711, 1.31), respectively. The inter-subject variability was greatly reduced as shown in the inter-subject coefficient of variation (Figure 2). Post Tmax, plasma unesbulin was reduced monophasically. The terminal T1/2 was 12.1 h under fasted conditions and 11.5 h under fed conditions.
|
Parameters |
Fasted (n=7) |
Fed (n=8) |
GMR (90% CI) |
|
AUC0–48h (h*ng/mL) |
19,600 (53.3%) |
18,300 (19.0%)a |
0.934 (0.730, 1.20) |
|
AUC0–inf (h*ng/mL) |
20,000 (58.4%)b |
19,700 (20.9%)a |
0.965 (0.711, 1.31) |
|
Cmax (ng/mL) |
1,020 (43.7%) |
1,230 (28.2%) |
1.18 (0.976, 1.43) |
|
Tmax (h) |
4.00 (2.73, 5.88) |
4.02 (3.00, 6.05) |
|
|
T1/2 (h) |
12.1 (26.0%) |
11.5 (28.0%)a |
|
|
All PK parameters were reported as geometric mean (geometric coefficient of variation, %); except for Tmax, which was reported as median (minimum, maximum). AUC0–48h: Area Under the Concentration-Time Curve from Time Zero to 48 hours; AUC0–inf: Area Under the Concentration-Time Curve from Time Zero to Infinity; CI: Confidence Interval; Cmax: Maximum Observed Concentration; GMR: Geometric Mean Ratio of Natural Logarithm Transformed PK Parameters; LMS: Leiomyosarcoma; an=7; bn=6. |
|||
Figure 1. Geometric mean (+90% confidence interval) unesbulin plasma concentration-time profiles following oral administration of unesbulin 300 mg under fasted (n=6, 7, 7, 7, 7, 7, 7, 7, 7, and 5 at 0.5, 1, 2, 3, 4, 6 , 8, 24, 48, and 72h postdose) and fed (low-fat, low-calorie diet; n=7, 8, 8, 8, 8, 8, 8, 7, 7, and 5 at 0.5, 1, 2, 3, 4, 6 , 8, 24, 48, and 72h postdose) conditions in adult patients with advanced LMS (data were slightly nudged along the x-axis to avoid overlapping).
Figure 2. Distribution of unesbulin PK parameters under fasted and fed (low-fat, low-calorie diet) conditions (the horizontal lines represent the median and the inter-quartile range).
Discussion
Unesbulin has been studied in patients with advanced solid tumors, ovarian cancers, and LMS. During these clinical trials, unesbulin was administered orally BIW without restrictions on food intake. Clinical trials to investigate its clinical pharmacological properties such as the food effect and metabolism in healthy human subjects were restricted. Consequently, patients in the expansion cohort of this phase 1b study were recruited for the food effect study after the phase 2 dose was identified. The absence of food effect criteria was based on the 90% confidence interval of the ratios of log-transformed PK parameters within the bioequivalence limits of 0.80–1.25, as recommended by the FDA guidance for assessing the effects of food [8]. A post hoc analysis of the data indicated that the intra-subject coefficient of variation was 26.2% for AUC0–inf. For a fixed sample size 8, the general bioequivalence limits of 0.80–1.25 should be broadened to 0.578–1.73 to achieve the equivalent 80% power of analysis with 95% confidence in PK parameter assessment [9]. The 90% CI of GMRs for unesbulin Cmax, AUC0–48h, and AUC0–inf under fed-to-fasted dose administration were all within these broadened equivalent limits based on the sample size and intra-subject variability.
Food effects are typically studied following a single dose or at steady-state if the T1/2 is long and with a high-fat high-calorie diet for the worst-case scenario. However, diarrhea and vomiting were among the most common treatment-emergent adverse events during unesbulin treatment [5,7]. Thus, a low-fat, low-calorie diet was selected for this study to reduce the frequency of gastrointestinal discomfort and potential interruption of PK assessments.
Unesbulin was administered BIW orally. Previous clinical studies indicated no unesbulin accumulation following this dosing schedule upto dose 10 mg/kg or 400 mg, allowing all doses to be treated as at the steady state and PK samples could be collected from any cycle during unesbulin treatment. For this food effect study, PK samples were collected from one of the Cycles, ranging from 1 to 7. This hypothesis was confirmed by examining the residual unesbulin concentration prior to unesbulin dosing (predose) relative to Cmax. Except for two cases, one under fasted and one under fed conditions, the predose concentration was less than 5% of Cmax. A post hoc analysis conducted after excluding the two subjects/periods showed the GMRs for Cmax, AUC0–48h, and AUC0–inf were 1.23 (1.00, 1.51), 0.984 (0.696, 1.39), and 1.01 (0.686, 1.49), respectively, comparable to results including the data and all were within the broadened limits of 0.578–1.73.
The absolute bioavailability of unesbulin was studied in nonclinical species, showing high bioavailability in rats (79%) and monkeys (66%) (data on file), suggesting that unesbulin is likely highly bioavailable in humans as well. It was unsurprising that the food effect was minimal for a small molecule with expected absolute bioavailability above 60%. Furthermore, as unesbulin is more soluble in lipids, administration with food did improve consistency and reduced the inter-subject variability as demonstrated by the clinical observation. The inter-subject variability for Cmax, AUC0–48h, and AUC0–inf was reduced from 43.7% to 28.2%, 53.3% to 19.0%, and 58.4% to 20.9%, respectively. This larger inter-subject variability in PK parameters under the fasted condition was reflected in the larger inter-subject variability in the concentrations, as illustrated by the much greater error bars for the fasted condition in Figure 1.
Unesbulin Cmax from the participants in this food effect study was slightly lower, at 1,020 ng/mL and 1,230 ng/mL, under fasted and fed conditions than the Cmax of 1,580 ng/mL in other LMS patients in this study, whereas the Tmax, AUCs, and T1/2 were consistent among all participants [5].
Conclusions
Comparable unesbulin mean exposures were observed under either fasted or fed conditions, whereas the inter-subject variability was greatly reduced when unesbulin was administered with food. Based on the results, it was recommended that unesbulin could be administered with or without food clinically. However, patients were encouraged to take unesbulin with food for more consistent drug exposure.
Conflict of Interest
L. Gao, K. Murase, D. Kaushik, N. Reddy, D. D'Silva, and R. Kong are employed by PTC Therapeutics, Inc. and may own stocks in PTC. B.A. Van Tine and S. Attia have received research funding from PTC Therapeutics.
Funding Statement
The study was funded by PTC Therapeutics, Inc.
Acknowledgments
The authors would like to thank all participants for the study.
Authors Contributions
L. Gao and R. Kong conceptualized and designed the study. B.A. Van Tine and S. Attia monitored the study. D. Kaushik developed the bioanalytical method. D. Kaushik and N. Reddy supervised the sample analysis. L. Gao and K. Murase conducted the data analysis. L. Gao drafted the manuscript. All authors reviewed and approved the manuscript.
References
2. Gao L, Weetall M, O’Keefe K, Goodwin E, Kong R. Unesbulin–a Novel Anti-tubulin Cancer Therapeutic. J Clin Haematol. 2021 Jun 14;2(3):80–5.
3. Eberle-Singh JA, Sagalovskiy I, Maurer HC, Sastra SA, Palermo CF, Decker AR, et al. Effective Delivery of a Microtubule Polymerization Inhibitor Synergizes with Standard Regimens in Models of Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2019 Sep 15;25(18):5548–60.
4. Flamier A, Abdouh M, Hamam R, Barabino A, Patel N, Gao A, et al. Off-target effect of the BMI1 inhibitor PTC596 drives epithelial-mesenchymal transition in glioblastoma multiforme. NPJ Precis Oncol. 2020 Jan 6;4:1.
5. Van Tine BA, Ingham MA, Attia S, Meyer CF, Baird JD, Brooks-Asplund E, et al. Phase Ib Study of Unesbulin (PTC596) Plus Dacarbazine for the Treatment of Locally Recurrent, Unresectable or Metastatic, Relapsed or Refractory Leiomyosarcoma. J Clin Oncol. 2024 Jul 10;42(20):2404–14.
6. Gao L, Kaushik D, Van Tine BA, Ingham MA, Attia S, Meyer CF, et al. Pharmacokinetics of Dacarbazine and Unesbulin and CYP1A2-Mediated Drug Interactions in Patients With Leiomyosarcoma. Clin Transl Sci. 2023 Dec 21;17(2):e13709.
7. Shapiro GI, O'Mara E, Laskin OL, Gao L, Baird JD, Spiegel RJ, et al. Pharmacokinetics and Safety of PTC596, a Novel Tubulin-Binding Agent, in Subjects With Advanced Solid Tumors. Clin Pharmacol Drug Dev. 2021 Aug;10(8):940–9.
8. FDA. Guidance for Industry: Assessing the Effects of Food on Drugs in INDs and NDAs — Clinical Pharmacology Considerations. 2022. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-effects-food-drugs-inds-and-ndas-clinical-pharmacology-considerations.
9. Tothfalusi L, Endrenyi L, Arieta AG. Evaluation of bioequivalence for highly variable drugs with scaled average bioequivalence. Clin Pharmacokinet. 2009;48(11):725–43.