Abstract
Unesbulin (also known as PTC596) is novel tubulin binding drug being developed for the treatment of cancers. The drug binds to the tubulin colchicine binding site to block tumor growth by inhibiting microtubular formation leading to mitotic G2/M phase cell arrest and cell death. Importantly, in contrast to the majority of other tubulin binding compounds, unesbulin is not a substrate for p-glycoprotein transporter and can be administered orally as a tablet. Preclinical studies indicate that the drug has activity both against solid tumor and haemotologic cancers. A recently completed phase 1 study evaluated the safety and pharmacokinetics (PK) of unesbulin in subjects (aged = 18 years [N=31]) with advanced solid tumors. Patients received eight doses per 4-week cycle schedule, and doses were escalated from 0.65, 1.3, 2.6, 5.2, 7.0, to 10.4 mg/kg. Unesbulin was rapidly absorbed and for doses between 0.65 and 7.0 mg/kg reached a maximum plasma concentration 2 to 4 hours after dosing. Area under the plasma concentration–time curve increased proportionally with body weight–adjusted doses and no accumulation occurred after multiple administrations up to 7.0 mg/ kg. For all doses, unesbulin had a terminal half-life ranging from 12 to 20 hours. Overall, unesbulin was well tolerated. The incidences of neutropenia and thrombocytopenia were low; only one patient treated with 10.4 mg/kg experienced dose-limiting toxicity of neutropenia and thrombocytopenia, both of which were reversible. These data support the further development of unesbulin in treating cancers. Currently, several ongoing clinical trials are investigating the drug in other cancers including in women with ovarian cancer, in patients with advanced leiomyosarcoma, and in children with diffuse intrinsic pontine glioma and high-grade glioma.
Background
A hallmark of all cancers is the rapid rates of cell division associated with tumor growth. Rapid cell division is dependent upon the assembly and disassembly of microtubules, which are essential components of the mitotic spindle and are critical for the movement and separation of chromosomes during cell division [1]. Microtubules are dynamic filaments that are composed of αβ-tubulin heterodimers. Microtubules also play key roles in determining cell shape, cell motility, and transportation of organelles within the cell. Blocking normal microtubular function leads to a failure of cells to progress through cell division and ultimately results in cell death.
Drugs known as anti-tubulins block cell division by disrupting mitosis through targeting the assembly and disassembly of microtubules and have been of enormous value in treating diverse forms of cancer [2,3]. Certain compounds, such as taxanes, docetaxel, and paclitaxel, prevent microtubule assembly [2]. Others, such as vinca alkaloids (vinctristine and vinblastine), block tubulin polymerization [2]. Specific sites on tubulin have been identified to which the various ligands bind. The sites bind taxanes, vinca alkaloids, laulimalide/peloruside, pironetin, maytansine, and colchicine. Although, drugs have been approved for treatment of cancer that bind to the taxane, vinca, and maytansine sites, none have yet been approved for treatment of cancer that bind to the colchicine, laulimalide/peloruside, or pironetin sites. It is possible, the drugs that bind to these other sites will have different characteristics with regard to PK, efficacy, and safety than the currently approved drugs.
Although these anti-tubulin drugs have been extensively used over many years to treat cancer in the clinic, antitubulins are clearly imperfect compounds that can limit their use [2]. These drugs tend to have harmful and serious side-effects including neuropathy, myelosuppression, and gastrointestinal distress [1]. The neuropathy following administration of taxanes and vinka alkaloids is often irreversible due to axonal cell death [4]. These clinically relevant limitations highlight the great medical need to develop alternative anti-tubulin drugs.
Many anti-tubulin drugs are substrates for the p-glycoprotein transporter (P-gp) so that they are effluxed from the brain, limiting their use in treating cancer in the central nervous system or organs with high expression of P-gp [5]. Moreover, cancer cells may develop resistance to these chemotherapeutic agents by upregulating P-gp expression further decreasing the intracellular drug concentration and effectiveness [5]. The impact of this on treatment response may be broad as the P-gp drug efflux pump is expressed in a variety of tissues including the intestine, liver, kidney, and the capillary cells that form the blood-brain barrier [5].
The clinical utility of anti-tubulin agents is also often limited by their being administered intravenously. This is necessary, in part, to their high molecular weight, an affinity for the P-gp drug efflux pump in the gut, and vulnerability to first pass hepatic elimination, primarily by cytochrome P450 CYP3A4 [6-8].
Unesbulin: Mechanism of Action
Unesbulin is a novel orally bioavailable small molecule investigational drug being developed for treating solid tumors. Unesbulin blocks cell replication and promotes cell death by binding tubulin and inhibiting microtubular assembly resulting in the arrest of cells in the G2/M phase [9].
The potential utility of unesbulin in treating cancers is supported by the fact that it has demonstrated significant activity in a number of preclinical models of solid tumors and haemotologic cancers. Unesbulin has shown effects in models of pancreatic ductal cancer [10], glioblastoma [11], and diffuse intrinsic pontine glioma (DIPG) [12]. Currently, although progress has been made in treating these cancers, they are still associated with a low 5-year survival rate of <5% [13-15]. Preclinical analysis has also shown that unesbulin is active against in vivo models of multiple myeloma [16] and acute myeloid leukemia (AML) [17-19]. Both cancers are currently difficult to treat, particularly in the relapsed/refractory setting and novel treatments are needed for later stage and high-risk disease.
Unesbulin was initially identified by its ability to lower the activity of the B-cell-specific Moloney murine leukemia virus insertion site 1 (BMI1) protein and to block cancer cell proliferation [9,20,21]. BMI1 is necessary for the selfrenewal of cancer stem cells [22-25]. However, subsequent studies suggest that the effect of unesbulin on BMI1 is likely through the ability of the drug to block the G2/M phase in mitosis; although, other mechanism cannot be ruled out.
Unesbulin binds to the colchicine binding site on tubulin to inhibit tubulin polymerization [26]. The colchicine binding site is located at the interface of the tubulin monomers of the heterodimer and is adjacent to the GTP binding site of α-tubulin and is the most important pocket for potential tubulin polymerization destabilizers [26]. Binding this site blocks polymerization of microtubules by inhibiting the curved-to-straight conformational transition required for microtubule formation [27]. Unesbulin structurally and pharmacologically differs from colchicine and other tubulin binding compounds that bind the colchicine site as it is an amine substituted pyrimidine compound that lacks the trimethoxyphenyl moiety (Figure 1).
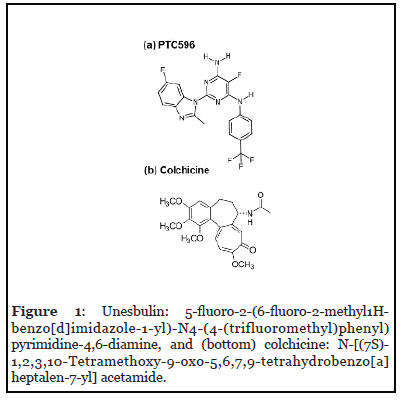
Unesbulin has several advantages over other antitubulin drugs as it (a) can be administered orally as a simple suspension; (b) is not a P-gp substrate; (c) is well tolerated in cancer patients; and (d) has a wellcharacterized pharmacokinetic (PK) profile that allows for the establishment of the PK/pharmacodynamic (PD) relationship.
Unesbulin Pharmacokinetics: Phase 1 First in Human Study
Based on the promising preclinical results indicating unesbulin may have anti-cancer activity, a phase 1 study (PTC-596-ONC-001-AST; Clinical Trial Identifier; NCT02404480) was performed to assess the safety and PK of unesbulin in subjects with advanced solid tumors (Table 1) [28]. Eligible subjects (N=31) were ≥ 18 years of age, diagnosed with metastatic, unresectable solid tumor that had not responded to at least 1 line of standard therapy or in which no standard therapy existed, and who had a life expectancy >3 months. The median age was 62.5 years (range 27 to 81 years) and approximately 60% were female. Most patients (64.5%) had Stage 4 cancer.
| Primary Site of Cancer, n % | Subject Data (N=31) |
|---|---|
| Colorectal | 5 (16.1) |
| Glioblastoma | 1 (3.2) |
| Lung | 3 (9.7) |
| Melanoma | 1 (3.2) |
| Ovary | 1 (3.2) |
| Pancreas | 3 (9.7) |
| Prostate | 1 (3.2) |
| Stomach | 2 (6.5) |
| Uterus | 1 (3.2) |
| Other | 13 (41.9) |
Table 1: Primary Site of Cancer in Study PTC596-ONC-001-AST..
Patients received unesbulin orally bi-weekly during 4-week cycle schedule for a total of 8 doses. The initial dose was based on the human equivalent dose established by pre-clinical studies in rats that showed 40 mg/kg twice weekly was approximately the severely toxic dose in 10% of rats (STD 10). Doses were escalated from 0.65, to 1.3, 2.6, 5.2, 7.0, and 10.4 mg/kg.
Unesbulin was rapidly absorbed, and a maximum plasma concentration (Cmax) was achieved approximately 2 to 4 hours post dosing for doses between 0.6 and 7.0 mg/kg (Figure 2) [28]. Cmax was dose proportional until doses levels were >2.6 mg/kg (Figure 3). Area under the plasma concentration–time curve increased proportionally with body weight–adjusted doses. No accumulation of unesbulin occurred with the bi-weekly dosing regimen even through the highest dose tested of 10.4 mg/kg. Excluding the 10.4 mg/kg dose, the terminal half-life ranged from 12 to 15 hours at all doses; at 10.4 mg/kg the half-life was approximately 20 hours.
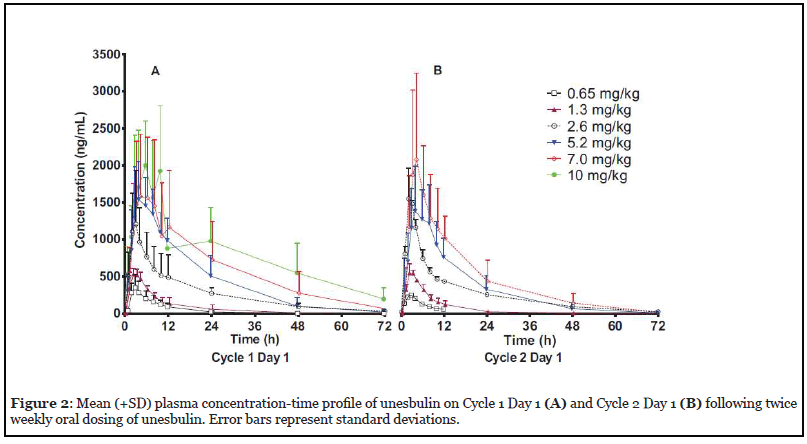
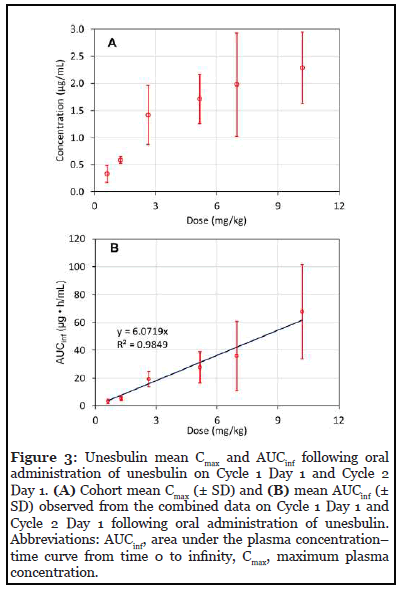
Generally, unesbulin was well tolerated, with the most common drug-related treatment emergent adverse events being mild to moderate gastrointestinal symptoms, such as diarrhea (54.8%), nausea (45.2%), vomiting (35.5%), and fatigue (35.5%) [28]. Only 1 subject, who received the 10.4 mg/kg dose, had dose-limiting toxicity of neutropenia and thrombocytopenia. Importantly both of these adverse events were reversible. The low level of neutropenia and the lack of peripheral neuropathy suggests that the safety profile of unesbulin may differ from that other anti-tubulin drugs.
The study was not powered to evaluate the efficacy of unesbulin in this diverse subject population. No subjects showed tumor response as assessed by Response Evaluation Criteria in Solid Tumors version 1.1. The observation of stable disease as being the best overall response was observed among 7 subjects, with 2 of the 7 subjects receiving the study drug up to 16 weeks. No association was observed between stable disease and tumor type.
Unesbulin is currently being investigated in several ongoing clinic studies. An open label phase 1b doseescalation study (NCT03206645) is evaluating the safety, tolerability, and PK of the drug when combined with and after conventional chemotherapy and maintenance therapy in women with epithelial, fallopian tube, or primary peritoneal cancer that was previously untreated. A phase 1 study (NCT03761095) is investigating the maximum tolerated dose and recommend phase 2 dose of unesbulin in combination with dacarbazine in the treatment of advanced leiomyosarcoma. The study will also evaluate the overall safety profile of the combination of unesbulin with dacarbazine. Another phase 1 study (NCT03605550) is evaluating the safety and efficacy of unesbulin in children with newly diagnosed DIPG and high-grade glioma.
Conclusions
In conclusion, anti-tubulin drugs have been used for years to treat cancer; however, those currently approved have significant limitations that can impact their use in the clinic. Unesbulin differs from these drugs and has compelling pharmacological properties in that it:
• Is not a substrate for P-gp
• Has a predictable PK
• Is well tolerated, with a very low frequency of reversible neutropenia and thrombocytopenia.
• Can be administered orally as a tablet.
These properties may in part depend upon the novel chemical structure and pharmacology of the drug and suggest that unesbulin may have unique characteristics in treating cancers that may result in clinical advantages compared with the currently approved anti-tubulin drugs.
Author Contributions Statement
R.K, L.G., M.W., K.O’K, and E.G. helped draft and review the manuscript.
Disclosures
R.K, L.G., M.W. K.O’K, and E.G. are employed by PTC Therapeutics Inc. and have received salary compensation for time and effort and hold financial interest in the company.
Funding Sources
This work was funded by PTC Therapeutics Inc.
References
2. Denning DP, Hirose T. Anti-tubulins DEPendably induce apoptosis. Nature Cell Biology. 2014 Aug;16(8):741- 3.
3. Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nature Reviews Cancer. 2004 Apr;4(4):253-65.
4. Kerckhove N, Collin A, Condé S, Chaleteix C, Pezet D, Balayssac D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: a comprehensive literature review. Frontiers in Pharmacology. 2017 Feb 24;8:86.
5. Waghray D, Zhang Q. Inhibit or evade multidrug resistance P-glycoprotein in cancer treatment: miniperspective. Journal of Medicinal Chemistry. 2017 Dec 18;61(12):5108-21.
6. van Tellingen O, Kuijpers AV, Beijnen JH, Nooijen WJ, Bult A. Plasma pharmacokinetics, tissue disposition, excretion and metabolism of vinorelbine in mice as determined by high performance liquid chromatography. Investigational New Drugs. 1993 Jun;11(2):141-50.
7. Sparreboom A, Van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, et al. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proceedings of the National Academy of Sciences. 1997 Mar 4;94(5):2031-5.
8. Jibodh RA, Lagas JS, Nuijen B, Beijnen JH, Schellens JH. Taxanes: old drugs, new oral formulations. European Journal of Pharmacology. 2013 Oct 5;717(1-3):40-6.
9. Nagai Y, Mimura N, Rizq O, Isshiki Y, Oshima M, Rizk M, et al. The preclinical activities of PTC596, a novel tubulin binding agent that down-regulates BMI1, alone and in combination with bortezomib in multiple myeloma. American Society of Hematology Washington, DC; 2019.
10. Eberle-Singh JA, Sagalovskiy I, Maurer HC, Sastra SA, Palermo CF, Decker AR, et al. Effective delivery of a microtubule polymerization inhibitor synergizes with standard regimens in models of pancreatic ductal adenocarcinoma. Clinical Cancer Research. 2019 Sep 15;25(18):5548-60.
11. Jin X, Kim LJ, Wu Q, Wallace LC, Prager BC, Sanvoranart T, et al. Targeting glioma stem cells through combined BMI1 and EZH2 inhibition. Nature Medicine. 2017 Nov;23(11):1352-61.
12. Kumar SS, Sengupta S, Zhu X, Mishra DK, Phoenix T, Dyer L, et al. Diffuse intrinsic pontine glioma cells are vulnerable to mitotic abnormalities associated with BMI- 1 modulation. Molecular Cancer Research. 2020 Nov 1;18(11):1711-23.
13. Bengtsson A, Andersson R, Ansari D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Scientific Reports. 2020 Oct 2;10(1):16425.
14. Hoffman LM, Van Zanten SE, Colditz N, Baugh J, Chaney B, Hoffmann M, et al. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the International and European Society for Pediatric Oncology DIPG Registries. Journal of Clinical Oncology. 2018 Jul 1;36(19):1963-72.
15. Poon MT, Sudlow CL, Figueroa JD, Brennan PM. Longer-term (= 2 years) survival in patients with glioblastoma in population-based studies pre-and post- 2005: a systematic review and meta-analysis. Scientific Reports. 2020 Jul 15;10(1):11622.
16. Bolomsky A, Muller J, Stangelberger K, Lejeune M, Duray E, Breid H, et al. The anti-mitotic agents PTC-028 and PTC596 display potent activity in pre-clinical models of multiple myeloma but challenge the role of BMI-1 as an essential tumour gene. British journal of haematology. 2020 Sep;190(6):877-90.
17. Barbosa K, Deshpande A, Chen BR, Ghosh A, Sun Y, Dutta S, et al. Acute myeloid leukemia driven by the CALMAF10 fusion gene is dependent on BMI1. Experimental Hematology. 2019 Jun 1;74:42-51.
18. Nishida Y, Maeda A, Kim MJ, Cao L, Kubota Y, Ishizawa J, et al. The novel BMI-1 inhibitor PTC596 downregulates MCL-1 and induces p53-independent mitochondrial apoptosis in acute myeloid leukemia progenitor cells. Blood Cancer Journal. 2017 Feb;7(2):e527.
19. Seipel K, Kopp B, Bacher U, Pabst T. BMI1-inhibitor PTC596 in combination with MCL1 inhibitor S63845 or MEK inhibitor trametinib in the treatment of acute leukemia. Cancers. 2021 Jan;13(3):581.
20. Maeda A, Nishida Y, Weetall M, Cao L, Branstrom A, Ishizawa J, et al. Targeting of BMI-1 expression by the novel small molecule PTC596 in mantle cell lymphoma. Oncotarget. 2018 Jun 19;9(47):28547.
21. Nishida Y, Maeda A, Kim MJ, Cao L, Kubota Y, Ishizawa J, et al. The novel BMI-1 inhibitor PTC596 downregulates MCL-1 and induces p53-independent mitochondrial apoptosis in acute myeloid leukemia progenitor cells. Blood Cancer Journal. 2017 Feb;7(2):e527.
22. Huber GF, Albinger-Hegyi A, Soltermann A, Roessle M, Graf N, Haerle SK, et al. Expression patterns of BMI-1 and p16 significantly correlate with overall, disease-specific, and recurrence-free survival in oropharyngeal squamous cell carcinoma. Cancer. 2011 Oct 15;117(20):4659-70.
23. Lukacs RU, Memarzadeh S, Wu H, Witte ON. BMI- 1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell. 2010 Dec 3;7(6):682-93.
24. Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011 Oct;478(7368):255-9.
25. Zacharek SJ, Fillmore CM, Lau AN, Gludish DW, Chou A, Ho JW, et al. Lung stem cell self-renewal relies on BMI1-dependent control of expression at imprinted loci. Cell Stem Cell. 2011 Sep 2;9(3):272-81.
26. Lu Y, Chen J, Xiao M, Li W, Miller DD. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharmaceutical Research. 2012 Nov;29(11):2943-71.
27. Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, et al. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004 Mar;428(6979):198-202.
28. Shapiro GI, O’Mara E, Laskin OL, Gao L, Baird JD, Spiegel RJ, et al. Pharmacokinetics and safety of PTC596, a novel tubulin-binding agent, in subjects with advanced solid tumors. Clinical Pharmacology in Drug Development. 2021.
