Short Communication
Breast cancer is the most frequent malignancy in females. Due to its major impact on the population, this disease represents a critical public health problem that requires further research at the molecular level to define its prognosis and specific treatment. Basic research is required to accomplish this task and this involves cell lines as they can be widely used in many aspects of laboratory research and, particularly, as in vitro models in cancer research. MCF-7 is a commonly used breast cancer cell line, that has been promoted for more than 40 years by multiple research groups but its characteristics have never been gathered in a consistent review article. The current paper provides a broad description of the MCF- 7 cell line, including the molecular profile, proliferation, migration, invasion, spheroid formation, its involvement in angiogenesis and lymphangiogenesis, and its interaction with the mesenchymal stem cells [1].
Breast cancer is a commonly diagnosed cancer and a leading cause of cancer-related death in women worldwide [2]. It remains an area of active research both clinically and experimentally. Recent advances in metabolomics show that metabolic profiling can be useful for the identification of biomarkers in breast cancer. Metabolic profiles of human breast cancer show differences among breast cancer subtypes and offer a way to identify and develop strategies for precise prevention and treatment [3-5]. Obesity is a risk factor for breast cancer; its occurrence is positively associated with the risk of breast cancer [6,7]. Obesity is a modern disorder that has resulted, not just from changes in energy balance, but from changes in lifestyle that alter meal times and eating patterns [8,9]. These changes, as environmental factors, disrupt biological rhythms and contribute to metabolic dysfunction [10,11]. Laboratory studies have shown that the feeding timing modifies obesogenic in rodents. For example, mice fed with a high-fat diet (HFD) during the light phase (rest phase for nocturnal animals) gain more weight than mice fed during the dark phase (active phase for nocturnal animals) [12]. Mice fed with an HFD during both light and dark phases exhibit altered daily pattern of energy expenditure and gain body fat [13]. Time-restricted feeding (TRF) is an effective tool in obesity research in rodents. It reinforces the circadian rhythms of energy metabolism by temporal regulation of the feeding/fasting pattern to a fixed time during the dark phase of the day. Available studies have shown that TRF restores the diurnal rhythms of energy metabolism [11] and circadian gene expression [14], improves insulin sensitivity, and reduces body adiposity and inflammation in mice fed with an HFD [13-15].
According to the world health organization (WHO), cancer is an important health problem that claims the level of more than 7 million people worldwide on an annual basis [16,17]. Because of the limitation of surgery and radiotherapy in effecting a cure for cancer, chemotherapy has been increasingly important [16,17]. Therefore, identification of novel potent, selective, and less toxic anticancer agents remains one of the most pressing health problems. In the vast cancer chemotherapeutic space, glycosides have played a very important role as established cancer chemotherapeutic agents, either in their nature, semi-synthetically, or synthetically forms [18-73]. As cited above, among the natural glycosides based antitumor the antibiotic doxorubicin, anthracycline O-glycoside, ranks among the most effective anticancer drug for acute myelocytic leukemia [20-22]. Furthermore, many sugar modified nucleoside analogues are clinically useful chemotherapeutics [18]. For example, capecitabine [29], N-nucleoside and C-nucleoside, are applied in the treatment of metastatic breast cancer and hairy cell leukaemia, respectively. Recently, several S-glycosides, a new non-classical class of nucleosides, have been proved to be potential anticancer agents against many cell Lines [32-37]. Khodair et al. described the synthesis of a series of heterocyclic S-glycosides, thiohydantoins [47-59], rhodanines [60], thioquinazolines [61,62], thiopyridines [63-65], and thiopyrimidine [66] S-glycosides and revealed their potential antitumor activities.
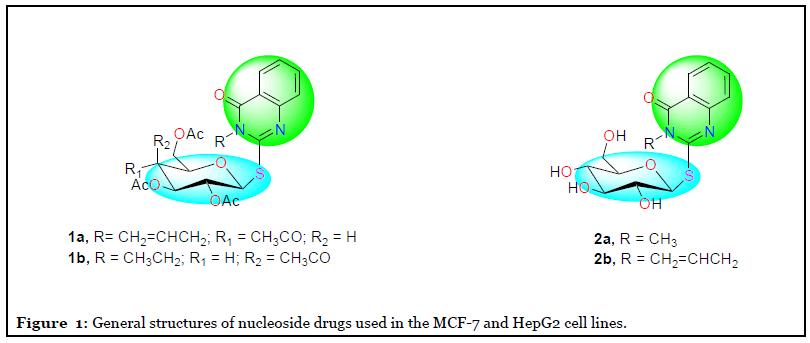
Our research interest focused on the design and synthesis of new small heterocyclic nucleosides targeting cancer especially MCF-7 and HepG2 cell lines. The elaboration of quinazoline derivatives linked with glycopyranose sugars (Figure 1) to form the target nucleosides was our task [61,64]. The in vitro cytotoxic activity against MCF- 7 and HepG2 cell lines showed effective anti-proliferative activity of the analyzed derivatives with lower IC50 values especially 2a with IC50 =2.09 and 2.08 μM against MCF-7 and HepG2, respectively, and their treatments were safe against the normal cell line Gingival mesenchymal stem cells (GMSC). Moreover, RT-PCR reaction investigated the apoptotic pathway for the compound 2a, which activated the P53 genes and its related genes. So, further work is recommended for developing it as a chemotherapeutic drug. We found that anticancer activity of the promising derivatives 1a,b and 2a,b was tested against breast (MCF- 7), liver (HepG2) cell lines by measuring the percentage of cell survival against their serial dilutions (0.01, 0.1, 1, 10, and 100 μM) [61]. Moreover, they were screened against the GMSC as normal cell line to test their safety [66]. We conclude the incorporation of sugar portion to the nucleus, enhanced the cytotoxic activity against the MCF-7 and HepG2 cell lines by having lower IC50 values, as shown in Table 1. Although both compounds 2a and 2b have near IC50 values (2.09 and 2.04 μM, respectively) against HepG2 cells, 2a was considered as the lead compound in our study according to the molecular docking results. It has a higher binding affinity towards the EGFR tyrosine kinase receptor because it forms a larger number of hydrogen bonds with the key amino acid residue Met 769 compared to other derivatives, so it was selected for further testing as the molecular mode of action. An attempt to study the structure-activity relationship using the molecular docking tool for elucidation the binding interactions of the nucleosides which might justify their higher potency [66]. Glycosides of structurally similar heterocyclic systems have been reported before [47-73].
| IC50 (μM) | |||
|---|---|---|---|
| MCF-7 | HepG2 | GMSC | |
| 5-FU | 4.23 | 4.43 | > 50 |
| 1a | 5.93 | 3.79 | > 50 |
| 1b | 2.42 | 1.17 | ND |
| 2a | 2.09 | 2.08 | > 50 |
| 2b | 2.04 | 2.09 | > 50 |
ND: Not Determined
Table 1: Summarized IC50 for the activity of the analyzed compounds against the MCF-7 and HepG2 cell lines.
The nucleoside bases 3-substituted 2-thioxo-2,3-dihydro- 1H-quinazolin-4-ones and 3-substituted 2-thioxo-2,3- dihydro-1H-benzo[g]quinazolin-4-ones can be utilized as starting materials for the synthesis of other carbohydrate derivatives as deoxy, amino and azido nucleosides.
References
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018 Nov;68(6):394-424.
3. Giskeødegård GF, Grinde MT, Sitter B, Axelson DE, Lundgren S, Fjøsne HE, et al. Multivariate modeling and prediction of breast cancer prognostic factors using MR metabolomics. Journal of Proteome Research. 2010 Feb 5;9(2):972-9.
4. Sitter B, Bathen TF, Singstad TE, Fjøsne HE, Lundgren S, Halgunset J, et al. Quantification of metabolites in breast cancer patients with different clinical prognosis using HR MAS MR spectroscopy. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In vivo. 2010 May;23(4):424-31.
5. Tang X, Lin CC, Spasojevic I, Iversen ES, Chi JT, Marks JR. A joint analysis of metabolomics and genetics of breast cancer. Breast Cancer Research. 2014 Aug;16(4):1-5.
6. Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA: A Cancer Journal for Clinicians. 2017 Sep;67(5):378-97.
7. Rohan TE, Heo M, Choi L, Datta M, Freudenheim JL, Kamensky V, et al. Body fat and breast cancer risk in postmenopausal women: a longitudinal study. Journal of Cancer Epidemiology. 2013 Jan 1;2013.
8. Bae SA, Fang MZ, Rustgi V, Zarbl H, Androulakis IP. At the interface of lifestyle, behavior and circadian rhythms: Metabolic implications. Frontiers in Nutrition. 2019;6:132.
9. Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Molecular and Cellular Endocrinology. 2010 Jan 15;314(1):1-16.
10. Branecky KL, Niswender KD, Pendergast JS. Disruption of daily rhythms by high-fat diet is reversible. PlOS One. 2015 Sep 14;10(9):e0137970.
11. Zarrinpar A, Chaix A, Panda S. Daily eating patterns and their impact on health and disease. Trends in Endocrinology & Metabolism. 2016 Feb 1;27(2):69-83.
12. Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity. 2009 Nov;17(11):2100-2.
13. Sundaram S, Yan L. Time-restricted feeding reduces adiposity in mice fed a high-fat diet. Nutrition Research. 2016 Jun 1;36(6):603-11.
14. Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabolism. 2012 Jun 6;15(6):848-60.
15. Chaix A, Lin T, Le HD, Chang MW, Panda S. Timerestricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell metabolism. 2019 Feb 5;29(2):303-19.
16. Gupta SP. Quantitative structure-activity relationship studies on anticancer drugs. Chemical Reviews. 1994 Sep;94(6):1507-51.
17. Keri G, Toth I. In “molecular path mechanisms and new trends in drug research”, London, New York, Taylor and Francis 1st edition. 227 (2003).
18. Kren V, Martínková L. Glycosides in medicine:“The role of glycosidic residue in biological activity”. Current medicinal chemistry. 2001 Sep 1;8(11):1303-28.
19. Buchanan JG, Edgar AR, Hutchison RJ, Stobie A, Wightman RH. A new synthesis of formycin via nitropyrazole derivatives. Journal of the Chemical Society, Chemical Communications. 1980(5):237-8.
20. Monneret C. Recent developments in the field of antitumour anthracyclines. European Journal of Medicinal Chemistry. 2001 Jun 1;36(6):483-93.
21. Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews. 2004 Jun 1;56(2):185-229.
22. Krohn K E. Topics in current chemistry. Anthracycline Chemistry and Biology 282 (2008).
23. Grdadolnik SG, Pristovšek P, Mierke DF. Vancomycin: conformational consequences of the sugar substituent.Journal of Medicinal Chemistry. 1998 Jun 4;41(12):2090-9.
24. Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, et al. Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proceedings of the National Academy of Sciences. 2008 Dec 16;105(50):19579-86.
25. Peterson LB, Blagg BS. Click chemistry to probe Hsp90: synthesis and evaluation of a series of triazolecontaining novobiocin analogues. Bioorganic & Medicinal Chemistry Letters. 2010 Jul 1;20(13):3957-60.
26. Moyer JD, Oliver JT, Handschumacher RE. Salvage of circulating pyrimidine nucleosides in the rat. Cancer Research. 1981 Aug 1;41(8):3010-7.
27. Cadman E, Benz C. Uridine and cytidine metabolism following inhibition of de novo pyrimidine synthesis by pyrazofurin. Biochimica et Biophysica Acta (BBA)-Nucleic Acids and Protein Synthesis. 1980 Oct 17;609(3):372-82.
28. Saran A. Correlation between the conformation of nucleoside antibiotics and their biological activity. International Journal of Quantum Chemistry. 1989 Jan;35(1):193-203.
29. Tiwari KN, Shortnacy-Fowler AT, Parker WB, Waud WR, Secrist III JA. Synthesis and anticancer evaluation of 4′-C-methyl-2′-fluoro arabino nucleosides. Nucleosides, Nucleotides and Nucleic Acids. 2009 Aug 11;28(5-7):657-77.
30. Pomeisl K, Votruba I, Holý A, Pohl R. Syntheses of pyrimidine acyclic nucleoside phosphonates as potent inhibitors of thymidine phosphorylase (PD-ECGF) from SD-lymphoma. Nucleosides, Nucleotides and Nucleic Acids. 2007 Nov 26;26(8-9):1025-8.
31. Elgemeie GH, El-Enany MM, Ismail MM, Ahmed EK. Nucleic Acid Components and Their Analogues: A Novel and Efficient Method for the Synthesis of a New Class of Bipyridyl and Biheterocyclic-nitro Gen Thioglycosides from Pyridine-2 (1 H)-thiones. Nucleosides, Nucleotides and Nucleic Acids. 2002 Nov 1;21(6-7):477-93.
32. Rashad AE, Mahmoud AE, Ali MM. Synthesis and anticancer effects of some novel pyrazolo [3, 4-d] pyrimidine derivatives by generating reactive oxygen species in human breast adenocarcinoma cells. European Journal of Medicinal Chemistry. 2011 Apr 1;46(4):1019- 26.
33. Saad HA, Moustafa AH. Synthesis and anticancer activity of some new S-glycosyl and S-alkyl 1, 2, 4-triazinone derivatives. Molecules. 2011 Jul;16(7):5682-700.
34. Al-Mutairi MS, Al-Abdullah ES, Haiba ME, Khedr MA, Zaghary WA. Synthesis, Molecular Docking and Preliminary in-Vitro Cytotoxic Evaluation of Some Substituted Tetrahydro-naphthalene (2’, 3’, 4’, 6’-Tetra- O-Acetyl-β-D-Gluco/-Galactopyranosyl) Derivatives. Molecules. 2012 Apr;17(4):4717-32.
35. Scala S, Akhmed N, Rao US, Paull K, Lan LB, Dickstein B, et al. P-glycoprotein substrates and antagonists cluster into two distinct groups. Molecular Pharmacology. 1997 Jun 1;51(6):1024-33.
36. Abu-Zaied MA, Nawwar GA, Swellem RH, El-Sayed SH. Synthesis and screening of new 5-substituted-1, 3, 4-oxadiazole-2-thioglycosides as potent anticancer agents. Pharmacol Pharmacy 3, 254 (2012).
37. Ishiwata A, Munemura Y, Ito Y. Synergistic solvent effect in 1, 2-cis-glycoside formation. Tetrahedron. 2008 Jan 1;64(1):92-102.
38. Larsen JS, Zahran MA, Pedersen EB, Nielsen C. Synthesis of triazenopyrazole derivativesas potential inhibitors of HIV-1. Monatshefte für Chemie/Chemical Monthly. 1999 Sep 1;130(9):1167-73.
39. Storer R, Ashton CJ, Baxter AD, Hann MM, Marr CL, Mason AM, et al. The synthesis and antiviral activity of 4-fluoro-1-β-D-ribofuranosyl-1H-pyrazole-3- carboxamide. Nucleosides, Nucleotides & Nucleic Acids. 1999 Feb 1;18(2):203-16.
40. Manfredini S, Baraldi PG, Bazzanini R, Durini E, Vertuani S, Pani A, et al. Pyrazole Related Nucleosides 5.1 Synthesis and Biological Activity of 2′-Deoxy-2′, 3′-dideoxy-and Acyclo-analogues of 4-Iodo-1-β-Dribofuranosyl-3-carboxymethyl Pyrazole (IPCAR). Nucleosides, Nucleotides & Nucleic Acids. 2000 Apr 1;19(4):705-22.
41. Hafez HN, El-Gazzar AR, Nawwar GA. Synthesis, biological and medicinal significance of S-glycosidothieno [2, 3-d]-pyrimidines as new anti-inflammatory and analgesic agents. European Journal of Medicinal Chemistry. 2010 Apr 1;45(4):1485-93.
42. Schmidt RR. New Methods for the Synthesis of Glycosides and Oligosaccharides—Are There Alternatives to the Koenigs-Knorr Method?[New Synthetic Methods (56). Angewandte Chemie International Edition in English. 1986 Mar;25(3):212-35.
43. Elgemeie GH, Zaghary WA, Amin KM, Nasr TM. First synthesis of thiophene thioglycosides. Journal of Carbohydrate Chemistry. 2009 Apr 7;28(3):161-78.
44. Cristescu C, Czobor F. As-Triazine Derivatives with Potential Therapeutic Action. XXVI. 1 Synthesis of 5-Substituted-6-Azauracil Acyclonucleosides. Nucleosides & Nucleotides. 1998 Aug 1;17(8):1319-24.
45. Abu-Zaied MA, El-Telbani EM, Elgemeie GH, Nawwar GA. Synthesis and in vitro anti-tumor activity of new oxadiazole thioglycosides. European Journal of Medicinal Chemistry. 2011 Jan 1;46(1):229-35.
46. Khodair AI, Gesson JP. A new approach for the N-and S-galactosylation of 5-arylidene-2-thioxo-4- thiazolidinones. Carbohydrate Research. 2011 Dec 27;346(18):2831-7.
47. El-Barbary AA, Khodair AI, Pedersen EB, Nielsen C. S-Glucosylated hydantoins as new antiviral agents. Journal of Medicinal Chemistry. 1994 Jan;37(1):73-7.
48. Khodair AI, El-Subbagh HI, El-Emam AA. Synthesis of certain 5-substituted 2-thiohydantoin derivatives as potential cytotoxic and antiviral agents. Bollettino Chimico Farmaceutico. 1997 Sep;136(8):561-567.
49. Al-Obaid AM, El-Subbagh HI, Khodair A, Elmazar MM. 5-substituted-2-thiohydantoin analogs as a novel class of antitumor agents. Anti-Cancer Drugs. 1996 Nov 1;7(8):873-80.
50. Khodair AI. Glycosylation of 2-thiohydantoin derivatives. Synthesis of some novel S-alkylated and S-glucosylated hydantoins. Carbohydrate Research. 2001 Apr 23;331(4):445-53.
51. Khodair AI. Synthesis of 2-thiohydantoins and their S-glucosylated derivatives as potential antiviral and antitumor agents. Nucleosides, Nucleotides and Nucleic Acids. 2001 Sep 30;20(9):1735-50.
52. Khodair AI, Ibrahimb ES. Synthesis of Hydantoin Nucleosides with Naphthylmethylene Substituents in the 5-Position. Nucleosides, Nucleotides & Nucleic Acids. 1996 Nov 1;15(11-12):1927-43.
53. Khodair AI. A Convenient Synthesis of Glycosylated Hydantoins as Potential Antiviral Agents. Phosphorus, Sulfur, and Silicon and the Related Elements. 1997 Mar 1;122(1):9-26.
54. Khodair AI. Synthesis of arylidenehydrazonoand glycopyranosylhydrazino-sulfonylbenzylidene-2, 4-imidazolidinediones as potential antiviral and antitumoral agents. Carbohydrate Research. 1998 Feb;306(4):567-573.
55. Khodair AI, Gesson JP. Sulfur Glycosylation Reactions Involving 3-Allyl-2-thiohydantoin Nucleoside Bases as Potential Antiviral and Antitumor Agents. Phosphorus, Sulfur, and Silicon and the Related Elements. 1998 Nov 1;142(1):167-90.
56. Khodair AI, El-Barbary AA, Abbas YA, Imam DR. Synthesis, reactions and conformational analysis of 5-arylidene-2-thiohydantoins as potential antiviral agents. Phosphorus, Sulfur, and Silicon and the Related Elements. 2001 Mar 1;170(1):261-78.
57. Al-Masoudi IA, Khodair AI, Al-Soud YA, Al-Masoudi NA. Synthesis of N-substituted 1-amino-2, 3-dihydro-1 H-imidazole-2-thione-N-nucleosides and S-glycosylated derivatives. Nucleosides, Nucleotides and Nucleic Acids. 2003 Jun 1;22(3):299-307.
58. Al-Masoudi NA, Al-Soud YA, Khodair AI. Some 2′-Modified 4′-Thionucleosides via Sulfur Participation and Synthesis of Thio-Azt from 4′-Thiofuranoid 1, 2-Glycal. Phosphorus, Sulfur, and Silicon and the Related Elements. 2003 Jun 1;178(6):1199-209.
59. Khodair AI, El Sayed H, Al-Masoudi NA. Thiohydantoin nucleosides. Synthesis approaches. Monatshefte für Chemie/Chemical Monthly. 2004 Sep 1;135(9):1061-79.
60. Khodair AI, Awad MK, Gesson JP, Elshaier YA. New N-ribosides and N-mannosides of rhodanine derivatives with anticancer activity on leukemia cell line: Design, synthesis, DFT and molecular modelling studies. Carbohydrate Research. 2020 Jan 1;487:107894.
61. Khodair AI, Elsafi MA, Al-Issa SA. Simple and Efficient Synthesis of Novel 3-Substituted 2-Thioxo-2, 3-dihydro1H-benzo [g] quinazolin-4-ones and Their Reactions with Alkyl Halides and α-Glycopyranosyl Bromides. Journal of Heterocyclic Chemistry. 2019 Sep;56(9):2358-68.
62. Khodair AI, Alsafi MA, Nafie MS. Synthesis, molecular modeling and anti-cancer evaluation of a series of quinazoline derivatives. Carbohydrate Research. 2019 Dec 1;486:107832.
63. Khodair AI, Al-Masoudi NA, Gesson JP. A new approach to the synthesis of benzothiazole, benzoxazole, and pyridine nucleosides as potential antitumor agents. Nucleosides, Nucleotides and Nucleic Acids. 2003 Nov 1;22(11):2061-76.
64. Khodair AI, Attia AM, Gendy EA, Elshaier YA, El-Magd MA. Discovery of New S-Glycosides and N-Glycosides of Pyridine-biphenyl System with Antiviral Activity and Induction of Apoptosis in MCF 7 Cells. Journal of Heterocyclic Chemistry. 2019 Jun;56(6):1733-47.
65. Attia AM, Khodair AI, Gendy EA, El-Magd MA, Elshaier YA. New 2-oxopyridine/2-thiopyridine derivatives tethered to a benzotriazole with cytotoxicity on MCF7 cell lines and with antiviral activities. Letters in Drug Design & Discovery. 2020 Feb 1;17(2):124-37.
66. Khodair AI, Ibrahim EE, Ashry EE. Glycosylation of 2-thiouracil derivatives. A synthetic approach to 3-glycosyl-2, 4-dioxypyrimidines. Nucleosides & Nucleotides. 1997 Apr 1;16(4):433-44.
67. Khodair AI. A convenient synthesis of 2-Arylidene- 5H-thiazolo [2, 3-b] quinazo-line-3, 5 [2H]-diones and their benzoquinazoline derivatives. Journal of Heterocyclic Chemistry. 2002 Nov;39(6):1153-60.
68. Khodair AI, Pedersen EB, Nielsen C. Synthesis of Uridine with Methylene-2-thiohydantoin as 5-Substituent. Liebigs Annalen der Chemie. 1994 Jun 13;1994(6):619-21.
69. El-Barbary AA, Khodair AI, Pedersen EB, Nielsen C. Synthesis and evaluation of antiviral activity of 2′-deoxyuridines with 5-methylene-2-thiohydantoin substituents in the 5-position. Monatshefte für Chemie/ Chemical Monthly. 1994 May 1;125(5):593-8.
70. El-Barbary AA, Khodair AI, Pedersen EB, Nielsen C. Synthesis of 3′-Amino and 5′-Amino Hydantoin 2′-Deoxynucleosides. Nucleosides, Nucleotides & Nucleic Acids. 1994 Mar 1;13(1-3):707-17.
71. El-Barbary AA, Khodair AI, Pedersen EB. Synthesis and antiviral evaluation of hydantoin analogues of AZT. Archiv Der Pharmazie. 1994;327(10):653-5.
72. El-Barbary AA, Khodair AI, Pedersen EB, Nielsen C. Convergent synthesis of 2′, 3′-dideoxy-3′-mercapto nucleosides—Potential anti-HIV agents. Monatshefte für Chemie/Chemical Monthly. 1994 Aug;125(8):1017-25.
73. Abdel-Bary HM, El-Barbary AA, Khodair AI, Megied AE, Pedersen EB, Nielsen C. Synthesis of hydantoin analogues of 3’-fluoro-3’-deoxythymidine (FLT). Bulletin de la Société Chimique de France. 1995;2(132):149-55.
