Short Communication
According to the report published recently by the World Health Organization, the number of cancer cases in the world will increase to 22 million by 2030. So, the anticancer drug research and development is taking place in the direction where the new entities are developed which are low in toxicity and are with improved activity. Pyridine and their pyridine-biphenyl system derivatives represent a very important class of heterocyclic compounds, which have a diverse therapeutic area. Recently, many active compounds synthesized are very effective; natural products isolated with pyridine moiety have also shown to be potent towards cancer.
In the last few years, many research groups have designed and developed many novel compounds with pyridine as their backbone and checked their anticancer activity. In this short communication, the recent developments made in the direction of design and synthesis of new scaffolds with very potent anticancer activity are briefly described. The effect of various heterocycles attached to the pyridinebiphenyl system and their effect on the anticancer activity are thoroughly studied and recorded in this short communication.
Cancer is a one of the major health problems for human beings with the leading mortality rate [1]. Natural, synthetic, or biological and chemical substances are the cancer-causing agents [2]. Many drugs are used to cure it, but they have their own toxic side effects [3]. Hence, there are lots of research carried out to synthesize new [4-6], effective, and affordable anticancer drugs with more selectivity, minimum dosage, and lesser side effects.
Drug discovery over the years have focussed more on the heterocyclic chemistry due to their huge success rate in forming active pharmaceutical intermediate. Among the heterocyclic compounds, pyridine-biphenyl system is one of the most important heterocyclic compounds which exhibit remarkable pharmacological activities [7-11]. There are many reputations of synthetic compounds and naturally occurring compounds with pyridine-biphenyl system backbone showing a very active anti-cancer activity. Different research groups have done much progress in designing compounds with pyridine-biphenyl system, synthesizing them, and collecting anticancer activity data of those against various human cancer cell lines. An attempt has been made to see how various heterocyclic moiety attached with pyridine-biphenyl system have an effect on the anticancer activity of the various pyridinebiphenyl system compounds synthesized by different groups. A compiled data of all these recent articles helps in providing a direction towards further research.
Breast cancer is considered as the first common leading cause of cancer deaths representing 14.7% of all cancer cases in women recent years [12]. Formation of glycoside bond with heterocyclic compounds is still in the eyes of the majority of chemists and drug design strategies [13]. These compounds containing glycoside bond have been played a vital role as established cancer chemotherapeutic agents [14-27]. For example, N-nucleoside of pyridine-2-one and S-nucleoside of pyridine-2-thione derivatives are applied in the treatment of metastatic breast cancer, hairy cell leukaemia, lung carcinoma cell line, liver carcinoma cell line, and brain carcinoma cell line respectively [25-30]. In this context, dihydropyridine derivatives with glycosides bond were identified as strong P-glycoprotein antagonist with significant cytotoxic activity against human colon carcinoma cells [31] (Figure 1).
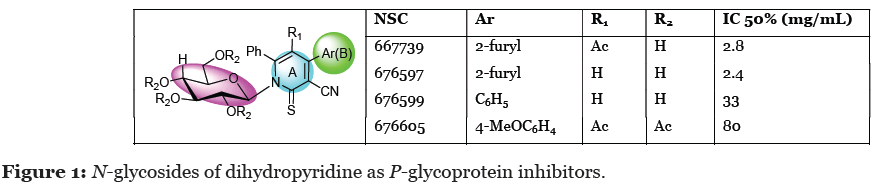
Furthermore, some of glycosides of pyridine-2-thione and pyridine-2-one derivatives illustrated antiviral activities against human immunodeficiency virus 1 (HIV-1) [32-34]. Biphenyl system is an important pharmacophore that are incorporated in different bioactive compounds especially in cancer therapy [35,36]. Several benzotriazole and 1,2,4-triazole derivatives represented an interesting class of heterocyclic compounds [37]. Numerous benzotriazole; including 4-(1H-benzo-1,2,3-triazol-1- ylmethyloxy)-3-methoxybenzaldehyde showed high efficiency against human breast carcinoma cells (MCF7) [38]. Additionally, pyridin-2(1H)-one and pyridine-2(1H)- thione derivatives showed anticancer effect against MCF7, ovarian adenocarcinoma cells (SK-OV-3), and blood cancer cells (CCRF-CEM) [39,40], antiviral activities [41] against human rhinoviruses (HRVs) [42,43] and HIV-1 [32,34,44], anti-inflammatory [45], eukaryotic elongation factor-2 kinase inhibitor [46], antidiabetic [47], potent antiviral [41], and antimicrobial activity [48].
In view of the above observations, our research interest focused on the design and synthesis of small heterocyclic nucleosides targeting cancer especially MCF-7 cell lines, rotavirus WA, HAVHM175, and herpes simplex 1. The elaboration of pyridine-biphenyl system linked with glucopyranose sugars (Figure 1) to form the target nucleosides was our task [49].
The aglycone part is a biphenyl system (ring A and ring B) linked by benzotriazole moiety. The glycone part originated from the two epimerise monosaccharides, glucose, galactose acetylated or deacetylated and examining the effect of N-glycoside or S-glycoside in activity relationship (Figure 2). Their anticancer activities against breast cancer (MC7) including apoptosis studies were evaluated. The cytotoxicity of these compounds against the normal cells and their antiviral activities were also determined. Docking studies and shape similarity studies were also investigated. Glycosides of structurally similar heterocyclic systems have been reported before [50-98].
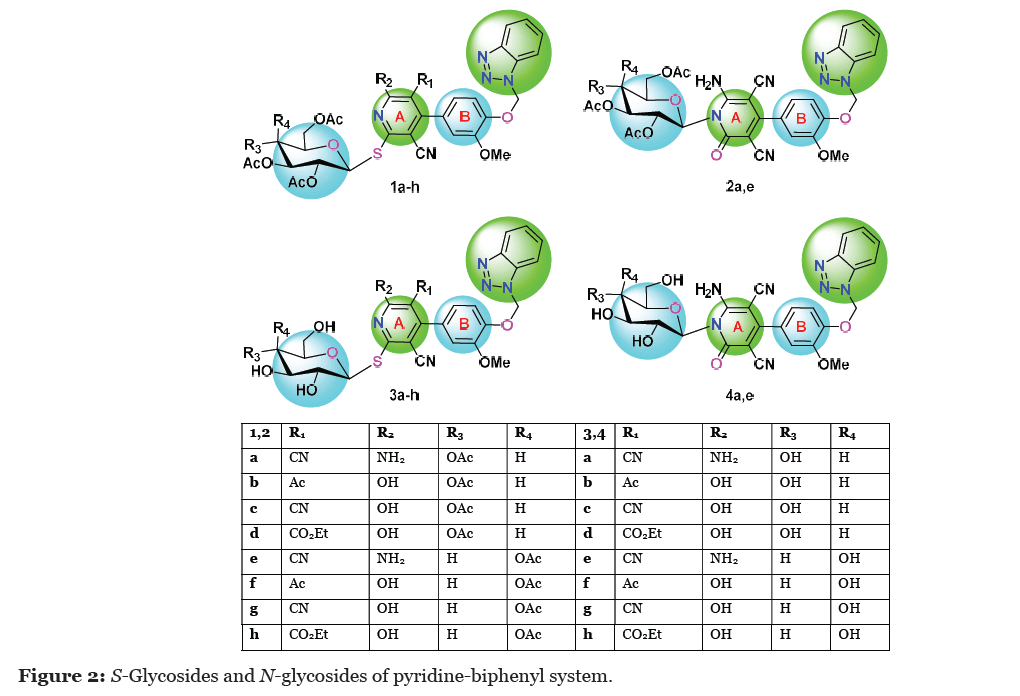
The cytotoxicity of the synthesized compounds was evaluated in vitro against MCF7 cells by MTT assay [99]. The results of novel compounds showed IC50 values compared to the reference cisplatin are shown in Table 1. The results showed compounds 1f, 1g, 3f, 3h moderate activity against MCF7 cells with IC50 = 30.63, 24.39, 27.24, 20.49 respectively. Considering the glycone part, it was observed that compounds contain galactose moiety were more active than others which contain glucose part and this also stratify on their free acetyl of moiety sugar; compound 1f against 1b, compound 1g against to 1c, compound 3f against 3b, and compound 3h against 3d. Regarding aglycone part, compounds contain R1 = CN, R2 = NH2 or OH are least active. We hope that the synthesized compounds serve as lead chemical entities for further modification to render them clinically useful drug agents. N-glycosides are less active in comparison to S-glycosides.
| Compound | IC50 (µM) | Compound | IC50 (µM) | Compound | IC50 (µM) |
|---|---|---|---|---|---|
| Cisplatin | 56.66* | 1g | 32 | 3d | 51 |
| 1a | 88 | 1h | 74 | 3e | 180 |
| 1b | 51 | 2a | 88 | 3f | 44 |
| 1c | 100 | 2e | 60 | 3g | 71 |
| 1d | 59 | 3a | 93 | 3h | 32 |
| 1e | 62 | 3b | 130 | 4a | 170 |
| 1f | 39 | 3c | 100 | 4e | 180 |
Table 1: IC50 values of the cytotoxic effects of compounds 1-4 and standard cisplatin on MCF7 cells as determined by MTT assay.
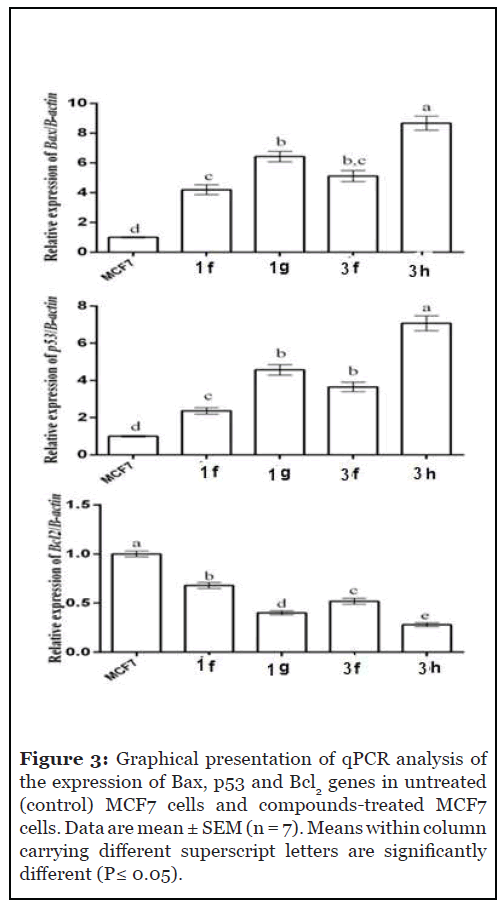
To check whether the cytotoxic effect of compounds 1f, 1g, 3f, and 3h occurred through induction of apoptosis in MCF7 cells, changes in gene expression of apoptotic genes Bax and p53 and the anti-apoptotic gene BCl2 were detected by qPCR before and after addition of these compounds. Addition of these compounds resulted in a significant (P ≤ 0.05) increase in the expression of the Bax and p53 genes in MCF7 cells, with highest expression in 3h followed by 3g, then 3f and finally 1f, as compared to vehicle (DMSO)- treated MCF7 cells (Figure 4). However, BCl2 expression was significantly decreased in MCF7 cells treated by the 4 compounds, with the following order 3h>1g>3f>1f, as compared to vehicle-treated MCF7 cells (Figure 3).
The study used three types of virus namely rotavirus WA, HAVHM175, and herpes simplex 1. The study commenced with examination the nontoxic dose for these compounds against MA104, Hep2, Vero, BGM and FRHK cells as showed in table 2. Most of compounds exhibited nontoxic dose at 100 μg/mL (Table 2).
| Compound | MA104 cells | Hep2 | Vero cells | BGM cell | FRHK4 cells |
|---|---|---|---|---|---|
| 1a | 80 | - | 80 | - | 80 |
| 1b | 90 | 100 | 100 | 90 | - |
| 1c | 70 | 70 | 70 | 70 | - |
| 1d | 70 | 80 | 80 | 70 | - |
| 3a | 100 | 100 | 100 | 100 | - |
| 3b | 100 | 100 | 100 | 100 | - |
| 3c | 90 | 100 | 90 | 90 | - |
| 3d | 70 | 60 | 60 | 70 | - |
Table 2: Non-toxic doses of tested compounds (µg/mL) on MA104, FRHK4, BGM cell, Hep2 and Vero cell line.
The antiviral activities for selected compounds rotavirus WA, HAVHM175, and herpes simplex are illustrated in table 3. Statistical analyses for the percent inhibition of viral titter achieved by each of the title compounds’ non-toxic doses against each of the study viruses were conducted. In our study, only percent inhibition values of viral titres above 50% were considered significant [100]. Compound 3a was more active than other derivatives against Rotavirus WA and Herpes simplex virus type 1 strains by 63.3% and 80% respectively in comparison to acyclovir as standard antiviral drug (IC50% 13.5 mcg/mL). Compounds 1b, 3b and 3c were moderate and similar in activity against Rotavirus WA with range 50%-56%. Compounds 1a, 1b, 3b exhibited good activity against Herpes simplex virus type 1 strain with range 60%-66.5%. In general compounds 3a, 1b, 3b were higher in activity against all three types of virus. While compounds 1c, 3d showed activity less than others are shown in Table 3.
| Compound | Mean % of reduction | ||
|---|---|---|---|
| Rotavirus WA | HAV HM175 | Herpes simplex virus type 1 | |
| 1a | 30 | 30 | 60 |
| 1b | 56.7 | 50 | 66.7 |
| 1c | 0 | 0 | 0 |
| 1d | 10 | 0 | 20 |
| 3a | 63.3 | 50 | 80 |
| 3b | 53.3 | 20 | 60 |
| 3c | 50 | 20 | 56.7 |
| 3d | 0 | 0 | 0 |
| Acyclovir | - | - | 13.5*a) |
a) IC50 % (mcg/mL).
Table 3: Antiviral activity of nontoxic doses of the test compounds against rotavirus Wa, HAV HM175, and herpes simplex virus type 1 strains.
In general, the deacetylated glycosides are more reactive than acetylated analogues. The deacetylated analogues are more reactive while in considering the aglycone part, the compounds contain R1 = nitrile or acetyl functionality and R2 = OH, or NH2 in pyridine part is more reactive.
P53 is an endogenous protein which acts as a tumor suppressor that stop cancer cell from growing and multiplying. The over expression of MDM2 has been observed in a wide range of tumor types. MDM2 interact P53 and so cancer cell blocked the suppression effect of P53 and so avoid apoptosis [101-104]. As our compounds induce apoptosis and increase P53, the goal was directed to examine the docking of these compounds with MDM2 and examine their activity to inhabit P53-MDM2 interactions.
In this regard, a library of target compounds was energy minimized using MMFF94 force field calculations. The catalytic domain of MDM2 (PDB code 5law) [105] was prepared for docking using Open Eye® (Fast Rigid Exhaustive Docking (FRED) Receptor, OMEGA; VIDA. Open Eye Omega application was used to generate different conformations of each ligand. Docking was conducted using FRED, and the data were visualized by the Veda application. This software package generates consensus scoring, a filtering process, to obtain virtual binding affinity; the lower the consensus score, the better the binding affinity of the ligand toward the receptor.
The most active compound 3h binds with the specific receptor of MDM (ID: 5law) with best consensus score 1 and forms hydrogen bonding (HB) through its OH of C-5 of galactose moiety with Tyr 100 AA and through OH of C-4 with both Gly 24 AA and Ala 21 AA. The triazoe ring forms HB with Gly 58 AA, (Figure 4A). The pyridine and the aryl ring from biphenyl system are adopted perpendicularly in the receptor through formation of hydrophobic-hydrophobic interactions. Compound 3f docks with consensus score 5 and overlays completely with 3h but with formation of one HB through its triazole ring with Gly 58 AA (Figure 4B). However, compound 1g showed consensuses score 23 and docked with different pose and mode through formation of HB with Ala 21 AA. Acetylated galactose (1g) forms hydrophobic-hydrophobic interaction with the receptor (Figure 4C). In order to understand the effect of galactose and glucose moiety, the docking pose and mode for compound 1f was compared to 1b, compound 1g was compared to 1c, compound 3f was compared to 3b and compound 3h was compared to 3d. It was clear that the axial position of epimeric hydroxyl in galactose moiety switch the molecule to form HB interactions.
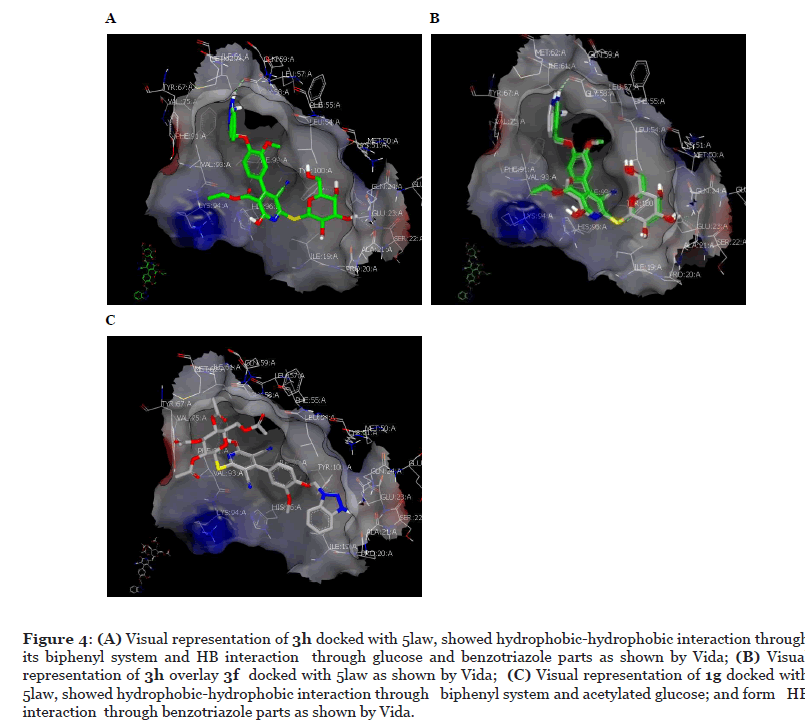
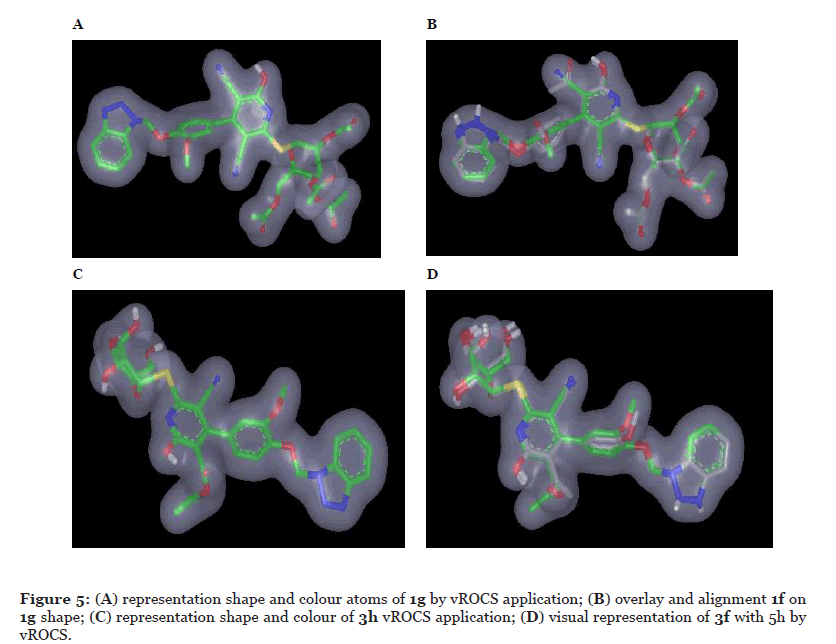
The final compounds behave special manners as the epimeric isomers are not near each other in activity (compound 1g versus 1c, compound 1f versus 1b; and compound 3h versus 1d, compound 3f versus 1b) and also variations of substituent on pyridine ring play an important role in activity. To gain insight about structure activity relationship and understand the compounds activity, ROCS was employed [106-108]. ROCS are a shape-based superposition method and used to perceive similarity between molecules based on their three-dimensional shape. Shape similarity is as a fundamental descriptor in drug design. ROCS alignment requires a) query molecules and those queries here are the most active compounds in both acetylated (1g) and deacetylated (3h) sugar and; b) the database molecules that our final compounds.
The quality of alignment between database and query was calculated using Tanimoto Combo. Tanimoto Combo is the summation of Shape Tanimoto and Colour Tanimoto. Shape Tanimoto represents the shared volume and mismatch volume and has scale from 0 to 1. Colour Tanimoto (also scale from 0 to 1) is reflective of the degree of matching or mismatching of light chemical features in 3diminssions. From ROCs model (shape and colour), quires volume showed many points acceptors, donors and rings. Quality of alignment, using ROCS, between compounds 1g and 3h (queries) Figure 5A, Figure 5C respectively, and database molecules (final compounds) was calculated using Tanimoto Combo (Table 5). Compound 1f overlay complete within the query volume shape Figure 5. Similarly, compound 3f overlay with query 3h, Figure 5D. Based on the ROCs data, Shape Tanimoto data revealed good correlation with biological activities. For examples compounds 4f and 1b exhibited highly Shape Tanimoto score using 1g as query and compounds 3d and 3f using 3h as query.
| Compound | Tanimoto Combo | Shape Tanimoto | Colour Tanimoto |
|---|---|---|---|
| 1a | 1.620 | 0.8750 | 0.7460 |
| 1b | 1.5490 | 0.8700 | 0.6790 |
| 1c | 1.7320 | 0.9090 | 0.8230 |
| 1d | 1.5690 | 0.8580 | 0.711 |
| 1e | 1.981 | 0.997 | 0.9840 |
| 1f | 1.724 | 0.949 | 0.775 |
| 1g | 1.999 | 1 | 0.999 |
| 1h | 0.880 | 0.519 | 0.361 |
| 3a | 1.675 | 0.921 | 0.754 |
| 3b | 1.209 | 0.818 | 0.3910 |
| 3c | 1.563 | 0.896 | 0.667 |
| 3d | 1.769 | 0.979 | 0.7900 |
| 3e | 1.818 | 0.929 | 0.888 |
| 3f | 1.714 | 0.9200 | 0.794 |
| 3g | 1.784 | 0.923 | 0.862 |
| 3h | 2 | 1 | 1 |
Table 5: Tanimoto scales for compounds 1a-h and 3a-h.
Structure activity relationship studies revealed the following features:1) S-glycosides pyridine are more active than N-gycosides probably due to strength and rigidity of N-glycosides; 2) compounds contain deacetylate sugar moiety are more reactive than acetylated derivatives. This hypothesis is clear because the alcoholic hydroxyl groups form HB with the receptor amino acids; 3) the galactosyl compounds are more reactive than glucosyl anaslogs because the epimeric OH forms HB when it located axially; compounds contain glycosides of acetyl galactose is more active than acetylated glucose; 4) the pyridine ring perpendicular to methoxyphenyl ring allowing this biphenyl system to from hydrophobic-hydrophobic interaction.
Conclusion
A biphenyl system from poly functionalized pyridine tethered with benzotriazole moiety was synthesized in very simple method. This system linked to glycoside formation with glucose and galactose epimers. The glycoside side chain was either S-glycosides or N- glycosides. S-Glycosides are more reactive than N-glycosides analogues. In case of antiviral activity, the deacetylated sugars are more active which indicate to importance of lipophilicity. Finally, we recommend further in vivo cancer models for this compound so that it can be developed as chemotherapeutic anti-cancer agent.
References
2. Blackadar CB. Historical review of the causes of cancer. World Journal of Clinical Oncology. 2016 Feb 10;7(1):54.
3. Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of chemotherapyinduced peripheral neuropathy. International Journal of Molecular Sciences. 2019 Jan;20(6):1451.
4. Hitchcock J, White AL, Hondow N, Hughes TA, Dupont H, Biggs S, et al. Metal-shell nanocapsules for the delivery of cancer drugs. Journal of Colloid and Interface Science. 2020 May 1;567: 171-80.
5. Koga Y, Ochiai A. Systematic review of patient-derived xenograft models for preclinical studies of anti-cancer drugs in solid tumors. Cells. 2019 May;8(5):418.
6. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646-74.
7. Rida SM, Ashour FA, El-Hawash SA, ElSemary MM, Badr MH, Shalaby MA. Synthesis of some novel benzoxazole derivatives as anticancer, anti-HIV-1 and antimicrobial agents. European Journal of Medicinal Chemistry. 2005 Sep 1;40(9):949-59.
8. Seth K, Garg SK, Kumar R, Purohit P, Meena VS, G oyal R, et al. 2-(2-Arylphenyl) benzoxazole as a novel antiinflammatory scaffold: synthesis and biological evaluation. ACS Medicinal Chemistry Letters. 2014 May 8;5(5):512-6.
9. Angajala G, Subashini R. Synthesis, molecular modeling, and pharmacological evaluation of new 2-substituted benzoxazole derivatives as potent antiinflammatory agents. Structural Chemistry. 2020 Feb 1;31(1):263-73.
10. Anusha P, Rao JV, Mohan GK. A review on diverse biological activities of benzoxazole molecule. World J Pharm Pharm Sci. 2017 May 18;6(7):1779-94.
11. Singh S, Veeraswamy G, Bhattarai D, Goo JI, Lee K, Choi Y. Recent advances in the development of pharmacologically active compounds that contain a benzoxazole scaffold. Asian Journal of Organic Chemistry. 2015 Dec;4(12):1338-61.
12. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians. 2016 Jan;66(1):7-30.
13. Romero-Ramírez L, García-Álvarez I, Casas J, Barreda- Manso MA, Yanguas-Casás N, Nieto-Sampedro M, et al. New oleyl glycoside as anti-cancer agent that targets on neutral sphingomyelinase. Biochemical Pharmacology. 2015 Sep 15;97(2):158-72.
14. El-Sayed WA, Osman DA, Khalaf HS, Abbas HA, Ali MM. Anticancer Activity of Newly Synthesized Pyrazolyl and Oxadiazolyl Glycosides Based on Thienopyrimidine Nucleus and Their Acyclic Analogs. ACTA POLONIAE PHARMACEUTICA. 2017 Nov 1;74(6):1739-51.
15. Buchanan JG, Edgar AR, Hutchison RJ, Stobie A, Wightman RH. A new synthesis of formycin via nitropyrazole derivatives. Journal of the Chemical Society, Chemical Communications. 1980(5):237-8.
16. Kren V, Martínková L. Glycosides in medicine:“The role of glycosidic residue in biological activity”. Current Medicinal Chemistry. 2001 Sep 1;8(11):1303-28.
17. Elgemeie GH, El-Enany MM, Ismail MM, Ahmed EK. Nucleic acid components and their analogues: A novel and efficient method for the synthesis of a new class of bipyridyl and biheterocyclic-nitro gen thioglycosides from pyridine-2 (1H)-thiones. Nucleosides, Nucleotides and Nucleic Acids. 2002 Nov 1;21(6-7):477-93.
18. Elgemeie GH, Kamal EA. Pyrimidinethione nucleosides and their deaza analogues. Nucleosides, Nucleotides and Nucleic Acids. 2002 Jul 1;21(4-5):287- 325.
19. Hillard EA, de Abreu FC, Ferreira DC, Jaouen G, Goulart MO, Amatore C. Electrochemical parameters and techniques in drug development, with an emphasis on quinones and related compounds. Chemical Communications. 2008(23):2612-28.
20. Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, et al. Digoxin and other cardiac glycosides inhibit HIF1α synthesis and block tumor growth. Proceedings of the National Academy of Sciences. 2008 Dec 16;105(50):19579-86.
21. Tiwari KN, Shortnacy-Fowler AT, Parker WB, Waud WR, Secrist III JA. Synthesis and anticancer evaluation of 4′-C-methyl-2′-fluoro arabino nucleosides. Nucleosides, Nucleotides and Nucleic Acids. 2009 Aug 11;28(5-7):657-77.
22. Peterson LB, Blagg BS. Click chemistry to probe Hsp90: synthesis and evaluation of a series of triazolecontaining novobiocin analogues. Bioorganic & Medicinal Chemistry Letters. 2010 Jul 1;20(13):3957-60.
23. Rashad AE, Mahmoud AE, Ali MM. Synthesis and anticancer effects of some novel pyrazolo [3, 4-d] pyrimidine derivatives by generating reactive oxygen species in human breast adenocarcinoma cells. European Journal of Medicinal Chemistry. 2011 Apr 1;46(4):1019- 26.
24. Saad HA, Moustafa AH. Synthesis and anticancer activity of some new S-glycosyl and S-alkyl 1, 2, 4-triazinone derivatives. Molecules. 2011 Jul;16(7):5682-700.
25. Abu-Zaied MA, Nawwar GA, Swellem RH, El-Sayed SH. Synthesis and screening of new 5-substituted-1,3,4- oxadiazole-2-thioglycosides as potent anticancer agents. Pharmacology & Pharmacy. 2012; 3(2):254-261.
26. Al-Mutairi MS, Al-Abdullah ES, Haiba ME, Khedr MA, Zaghary WA. Synthesis, molecular docking and preliminary in vitro cytotoxic evaluation of some substituted tetrahydronaphthalene(2’,3’,4’,6’- tetra-O-acetyl-β-d-gluco/-galactopyranosyl) derivatives Molecules. 2012 Apr;17(4):4717-32.
27. Siitonen V. Glycosylation and glycodiversification in polyketide antibiotics: Unraveling biosynthetic steps in nogalamycin formation. 2016.
28. Huntley CM, Cotterill AS, Maillard JY, Balzarini J, Simons C. Synthesis and biological evaluation of pyridin- 2-one nucleosides. Nucleosides, Nucleotides and Nucleic Acids. 2001 Mar 31;20(4-7):731-3.
29. Elgemeie GH, Mahdy EM, Elgawish MA, Ahmed MM, Shousha WG, Eldin ME. A new class of antimetabolites: pyridine thioglycosides as potential anticancer agents. Zeitschrift für Naturforschung C. 2010 Oct 1;65(9-10):577- 87.
30. Galal H, Elsayed M, Mona A, Mohammad M, Wafaa G, Mohammad E. New Class of Antimetabolites: Pyridine Thioglycosides as Potential Anticancer Agents. Zeitschrift für Naturforschung C. 2014, 65(9-10): 577-87.
31. Scala S, Akhmed N, Rao US, Paull K, Lan LB, Dickstein B, et al. P-glycoprotein substrates and antagonists cluster into two distinct groups. Molecular Pharmacology. 1997 Jun 1;51(6):1024-33.
32. Li YZ, Li CJ, Pinto AV, Pardee AB. Release of mitochondrial cytochrome C in both apoptosis and necrosis induced by β-lapachone in human carcinoma cells. Molecular Medicine. 1999 Apr 1;5(4):232-9.
33. Cheng Q, Oritani T, Horiguchi T, Yamada T, Mong Y. Synthesis and biological evaluation of novel 9-functional heterocyclic coupled 7-deoxy-9-Dihydropaclitaxel analogue. Bioorganic & medicinal chemistry letters. 2000 Mar 6;10(5):517-21.
34. Hassan GS, Kadry HH, Abou-Seri SM, Ali MM, Mahmoud AE. Synthesis and in vitro cytotoxic activity of novel pyrazolo [3, 4-d] pyrimidines and related pyrazole hydrazones toward breast adenocarcinoma MCF-7 cell line. Bioorganic & Medicinal Chemistry. 2011 Nov 15;19(22):6808-17.
35. Han Z, Pinkner JS, Ford B, Chorell E, Crowley JM, Cusumano CK, et al. Lead optimization studies on FimH antagonists: discovery of potent and orally bioavailable ortho-substituted biphenyl mannosides. Journal of Medicinal Chemistry. 2012 Apr 26;55(8):3945-59.
36. Ghisaidoobe AT, van den Berg RJ, Butt SS, Strijland A, Donker-Koopman WE, et al. Identification and development of biphenyl substituted iminosugars as improved dual glucosylceramide synthase/neutral glucosylceramidase inhibitors. Journal of Medicinal Chemistry. 2014 Nov 13;57(21):9096-104.
37. Wan J, Lv PC, Tian NN, Zhu HL. Facile synthesis of novel benzotriazole derivatives and their antibacterial activities. Journal of Chemical Sciences. 2010 Jul 1;122(4):597-606.
38. Gado, W. S. E. M. Sc. Thesis 2013, Chemistry department, Faculty of Science, Menofia University, Menofia, Egypt. Title: Anticancer activity of newly designed and synthesized 5-arylidene hydantoin, 2-thiohydanton derivatives. http://main.eulc.edu. eg/eulc_v5/Libraries/Thesis/BrowseThesisPages. aspx?fn=PublicDrawThesis&BibID=11761230
39. Hayakawa I, Shioya R, Agatsuma T, Furukawa H, Sugano Y. Thienopyridine and benzofuran derivatives as potent anti-tumor agents possessing different structure– activity relationships. Bioorganic & Medicinal Chemistry Letters. 2004 Jul 5;14(13):3411-4.
40. Chand K. Society and economy in Mughal India a critical analysis of British accounts.
41. Schnute ME, Anderson DJ, Brideau RJ, Ciske FL, Collier SA, Cudahy MM, et al. 2-Aryl-2-hydroxyethylamine substituted 4-oxo-4, 7-dihydrothieno [2, 3-b] pyridines as broad-spectrum inhibitors of human herpesvirus polymerases. Bioorganic & Medicinal Chemistry Letters. 2007 Jun 15;17(12):3349-53.
42. Lv Z, Sheng C, Wang T, Zhang Y, Liu J, Feng J, et al. Design, synthesis, and antihepatitis B virus activities of novel 2-pyridone derivatives. Journal of Medicinal Chemistry. 2010 Jan 28;53(2):660-8.
43. Parreira RL, Abrahão Jr O, Galembeck SE. Conformational preferences of non-nucleoside HIV-1 reverse transcriptase inhibitors. Tetrahedron. 2001 Apr 16;57(16):3243-53.
44. Alaa AM. Novel and versatile methodology for synthesis of cyclic imides and evaluation of their cytotoxic, DNA binding, apoptotic inducing activities and molecular modeling study. European Journal of Medicinal Chemistry. 2007 May 1;42(5):614-26.
45. Tumey LN, Boschelli DH, Lee J, Chaudhary D. 2-Alkenylthieno [2, 3-b] pyridine-5-carbonitriles: Potent and selective inhibitors of PKCθ. Bioorganic & medicinal chemistry letters. 2008 Aug 1;18(15):4420-3.
46. Lockman JW, Reeder MD, Suzuki K, Ostanin K, Hoff R, Bhoite L, et al. Inhibition of eEF2-K by thieno [2, 3-b] pyridine analogues. Bioorganic & Medicinal Chemistry Letters. 2010 Apr 1;20(7):2283-6.
47. Bahekar RH, Jain MR, Jadav PA, Prajapati VM, Patel DN, Gupta AA, et al. Synthesis and antidiabetic activity of 2, 5-disubstituted-3-imidazol-2-yl-pyrrolo [2, 3-b] pyridines and thieno [2, 3-b] pyridines. Bioorganic & Medicinal Chemistry. 2007 Nov 1;15(21):6782-95.
48. Bernardino AM, da Silva Pinheiro LC, Rodrigues CR, Loureiro NI, Castro HC, Lanfredi-Rangel A, et al. Design, synthesis, SAR, and biological evaluation of new 4-(phenylamino) thieno [2, 3-b] pyridine derivatives. Bioorganic & Medicinal Chemistry. 2006 Aug 15;14(16):5765-70.
49. Khodair AI, Attia AM, Gendy EA, Elshaier YA, El- Magd MA. Discovery of new S-glycosides and N-glycosides of pyridine-biphenyl system with antiviral activity and induction of apoptosis in MCF7 cells. Journal of Heterocyclic Chemistry. 2019 Jun;56(6):1733-47.
50. El-Barbary AA, Saffan AA, Sakran MA, Khodair AI. Reactions of some 5-arylidene-3-phenyl-2-thiohydantoins. Delta J. Sci. 1990;14(2):601-22.
51. El-Barbary AA, Saafan AA, Sakran MA, Khodair AI. Mannich reactions on 2-thiohydantoin derivatives, Delta J. Sci., (1990). 14 (2), 623–646.
52. El-Barbary AA, Khodair AI, Pedersen EB. Hydantoin analogs of thymidine. The Journal of Organic Chemistry. 1993 Oct;58(22):5994-9.
53. Pedersen EB, Dueholm KL, El-emam A, Kofoed T, El-Torgoman AA, Motawia MS, et al. Thio nucleoside derivatives as intermediates or target compounds in the attempt of finding new agents against HIV. Phosphorus, Sulfur, and Silicon and the Related Elements. 1993 Jan 1;74(1-4):465-6.
54. El-Barbary AA, Khodair AI, Pedersen EB, Nielsen C. S-Glucosylated hydantoins as new antiviral agents. Journal of Medicinal Chemistry. 1994 Jan;37(1):73-7.
55. El-Barbary AA, Khodair AI, Pedersen EB, Nielsen C. Synthesis of Uridine with Methylene-2-thiohydantoin as 5-Substituent. Liebigs Annalen der Chemie. 1994 Jun 13;1994(6):619-21.
56. El-Barbary AA, Khodair AI, Pedersen EB, Nielsen C. Synthesis and evaluation of antiviral activity of 2′-deoxyuridines with 5-methylene-2-thiohydantoin substituents in the 5-position. Monatshefte für Chemie/ Chemical Monthly. 1994 May 1;125(5):593-8.
57. El-Barbary AA, Khodair AI, Pedersen EB, Nielsen C. Synthesis of 3′-Amino and 5′-Amino Hydantoin 2′-Deoxynucleosides. Nucleosides, Nucleotides & Nucleic Acids. 1994 Mar 1;13(1-3):707-17.
58. El-Barbary AA, Khodair AI, Pedersen EB. Synthesis and antiviral evaluation of hydantoin analogues of AZT. Archiv der Pharmazie. 1994;327(10):653-5.
59. El-Barbary AA, Khodair AI, Pedersen EB, Nielsen C. Convergent synthesis of 2′, 3′-dideoxy-3′-mercapto nucleosides—Potential anti-HIV agents. Monatshefte für Chemie/Chemical Monthly. 1994 Aug;125(8):1017-25.
60. Mahmoud KR, Khodair AI, Shaban SY. Positron annihilation lifetime studies of changes in free volume on some biorelevant nitrogen heterocyclic compounds and their S-glycosylation. Applied Radiation and Isotopes. 2015 Jul 31; 105:303–307.
61. Abdel-Bary HM, El-Barbary AA, Khodair AI, Megied AE, Pedersen EB, Nielsen C. Synthesis of hydantoin analogues of 3’-fluoro-3’-deoxythymidine (FLT). Bulletin de la Société chimique de France. 1995;2(132):149-55.
62. Al-Obaid AM, El-Subbagh HI, Khodair A, Elmazar MM. 5-Substituted-2-thiohydantoin analogs as a novel class of antitumor agents. Anti-Cancer Drugs. 1996 Nov 1;7(8):873-80.
63. Khodair AI, Ibrahimb ES. Synthesis of hydantoin nucleosides with naphthylmethylene substituents in the 5-position. Nucleosides, Nucleotides & Nucleic Acids. 1996 Nov 1;15(11-12):1927-43.
64. Mansour H, Khodair AI, Elsiginy SM, Elghanam AE. Design, synthesis, characterization and biological evaluation of thieno[2,3-b]pyridines-chitosan nanocomposites as drug delivery systems for colon targeting. Carbohydrate Research. 2020 Mar 29; 492:107990.
65. Khodair AI. A convenient synthesis of glycosylated hydantoins as potential antiviral agents.. Phosphorus, Sulfur, and Silicon and the Related Elements. 1997 Mar 1;122(1):9-26.
66. Khodair AI, El-Subbagh HI, El-Emam AA. Synthesis of certain 5-substituted 2-thiohydantoin derivatives as potential cytotoxic and antiviral agents. Bollettino chimico farmaceutico. 1997 Sep;136(8):561-7.
67. Khodair AI, Ibrahim EE, Ashry EE. Glycosylation of 2-thiouracil derivatives. A synthetic approach to 3-glycosyl-2, 4-dioxypyrimidines. Nucleosides & Nucleotides. 1997 Apr 1;16(4):433-44.
68. Khodair AIA, El-Ashry ESH, Al-Masoudi NAL. Thiohydantoin nucleosides. Synthesis approaches, Monatshefte für Chemie/Chemical Monthly. 2004 Jun 24;135:1061-79.
69. Ahmed AI. Synthesis of arylidenehydrazano- and glycopyranosylhydrazino sulfonyl benzylidenediones as potential antiviral and antitumoral agents. Carbohydr Res. 1998;306(4):567-73.
70. Khodair AI, Gesson JP. Sulfur glycosylation reactions involving 3-allyl-2-thiohydantoin nucleoside bases as potential antiviral and antitumor agents. Phosphorus, Sulfur, and Silicon and the Related Elements. 1998 Nov 1;142(1):167-90.
71. Khodair AI, Bertrand P. A new approach to the synthesis of substituted 4-imidazolidinones as potential antiviral and antitumor agents. Tetrahedron. 1998 May 7;54(19):4859-72.
72. Khodair AI, El-Subbagh HI, Al-Obaid AM. Synthesis, conformational analysis and antitumor testing of 5-(Z)- arylidene-4-imidazolidinone derivatives. Phosphorus, Sulfur, and Silicon and the Related Elements. 1998 Sep 1;140(1):159-81.
73. Khodair AI. Glycosylation of 2-thiohydantoin derivatives. Synthesis of some novel S-alkylated and S-glucosylated hydantoins. Carbohydrate research. 2001 Apr 23;331(4):445-53.
74. Khodair AI. Synthesis of 2-thiohydantoins and their S-glucosylated derivatives as potential antiviral and antitumor agents. Nucleosides, Nucleotides and Nucleic Acids. 2001 Sep 30;20(9):1735-50.
75. Khodair AI, El-Barbary AA, Abbas YA, Imam DR. Synthesis, reactions and conformational analysis of 5-arylidene-2-thiohydantoins as potential antiviral agents. Phosphorus, Sulfur, and Silicon and the Related Elements. 2001 Mar 1;170(1):261-78.
76. Khodair AI. A convenient synthesis of 2-arylidene5Hthiazolo [2,3-b]quinazoline-3,5[2H]-diones and their benzoquinazoline derivatives. Journal of Heterocyclic Chemistry. 2002 Nov;39(6):1153-60.
77. Al-Masoudi IA, Khodair AI, Al-Soud YA, Al-Masoudi NA. Synthesis of N-substituted 1-amino-2, 3-dihydro-1 H-imidazole-2-thione-N-nucleosides and S-glycosylated derivatives. Nucleosides, Nucleotides and Nucleic Acids. 2003 Jun 1;22(3):299-307.
78. Al-Masoudi NA, Al-Soud YA, Khodair AI. Some 2′-modified 4′-thionucleosides via sulfur participation and synthesis of thio-azt from 4′-thiofuranoid 1,2-glycal. Phosphorus, Sulfur, and Silicon and the Related Elements. 2003 Jun 1;178(6):1199-209
79. Khodair AI, Al-Masoudi NA, Gesson JP. A new approach to the synthesis of benzothiazole, benzoxazole, and pyridine nucleosides as potential antitumor agents. Nucleosides, Nucleotides and Nucleic Acids. 2003 Nov 1;22(11):2061-76.
80. Khodair AI, Schmidt RR. Synthesis of C-glycosyl compounds of N-acetylneuraminic acid from D-gluconolactone. Carbohydrate research. 2002 Nov 19;337(21-23):1967-78.
81. Khodair AI. A convenient preparation of 2-(2-arylidene)-and 2-(2-polyhydroxyalkylidene) hydrazono-4-imidazolidinones with various heterocyclic side chain substituents at position 5 as potential antiviral and antitumor agents. Phosphorus, Sulfur, and Silicon and the Related Elements. 2002 May 1;177(5):1157-73.
82. Khodari AI, Nielsen J. Synthesis of 3-substituted 5-arylidene-1-methyl-2-thiohydantoins under microwave irradiation. Heterocycles. 2002;57(6):1017-32.
83. Khodair AI, Winterfeld GA, Schmidt RR. Conjugate addition of phenols to 2-nitrogalactal− synthesis of O-(2-acetamido-2-deoxygalactosyl)tyrosine. European Journal of Organic Chemistry. 2003 May 1;2003(10):1847-52.
84. Winterfeld GA, Khodair AI, Schmidt RR. O-Glycosyl amino acids by 2-nitrogalactal concatenation- synthesis of a mucin-type O-glycan. European Journal of Organic Chemistry. 2003 Mar;2003(6):1009-21.
85. Khodair AI, Gesson JP, El-Ashry ES. Synthesis of 15H-isoquino[2′,3′:3,4]imidazo[2,1-b]quinazoline-7, 13,15-triones and 14H-isoquino[2′,3′:3,4]imidazo[2,1-b] benzo[g]quinazoline-8,14,16-trione as new polycyclic fused-ring systems. Phosphorus, Sulfur, and Silicon and the Related Elements. 2004 Dec 1;179(12):2653-65.
86. Khodair AI, El Sayed H, Al-Masoudi NA. Thiohydantoin nucleosides. Synthesis approaches. Monatshefte für Chemie/Chemical Monthly. 2004 Sep 1;135(9):1061-79.
87. Khodair AI, Pachamuthu K, Schmidt RR. A convenient route to O-glycosyl lactates via conjugate addition to 2-nitroglycals: ring closure to novel pyrano [2.3-b][1, 4]-oxazines. Synthesis. 2004 Jan 1(1):53-8.
88. Khodair AI, Gesson JP. A new approach for the N-and S-galactosylation of 5-arylidene-2-thioxo-4- thiazolidinones. Carbohydrate research. 2011 Dec 27;346(18):2831-7.
89. Khodair A, Schmidt R. A convenient synthesis of novel pyrano [2, 3-b][1, 5] oxazepines via ring closure of O-glycosyl amino acids. European Journal of Organic Chemistry. 2011;2011(36):7407-13.
90. Khodair AI, Elbadawi MM, Elsaady MT, Abdellatif KR. Design, synthesis, molecular docking and cytotoxicity evaluation of some novel 5-arylidene-3-(substituted phenyl)-2-(p-tolylamino)-4-imidazolones. Journal of Applied Pharmaceutical Science. 2017 Sep;7(09):058-68.
91. Abdellatif KRA, Elbadawi MM, Elsaady MT, El- Hafeez AAA, Fujimura T, Kawamoto S, Khodair AI. Design, synthesis and cytotoxicity evaluation of new 3,5-disubstituted-2-thioxoimidazolidinones, Anti-Cancer Agents in Medicinal Chemistry.2018 Apr 1;18(4): 573-82.
92. Khodair AI, Elsafi MA, Al-Issa SA. Simple and efficient synthesis of novel 3-substituted 2-thioxo-2,3-dihydro- 1H-benzo[g]quinazolin-4-ones and their reactions with alkyl halides and α-glycopyranosyl bromides. Journal of Heterocyclic Chemistry. 2019 Sep;56(9):2358-68.
93. Khodair AI, Alsafi MA, Nafie MS. Synthesis, molecular modeling and anti-cancer evaluation of a series of quinazoline derivatives. Carbohydrate Research. 2019 Dec 1;486:107832.
94. Bakare SB, Awad MK, Nafie MS, Design, synthesis, DFT, molecular modelling studies and biological evaluation of novel 3-substituted (E)-5-(arylidene)-1-methyl-2- thioxoimidazolidin-4-ones with potent cytotoxic activities against breast MCF-7, liver HepG2, and lung A549. Journal of Molecular Structure. 2021 Apr 5;1229:129805.
95. Khodair AI, Awad MK, Gesson JP, Elshaier YA. New N-ribosides and N-mannosides of rhodanine derivatives with anticancer activity on leukemia cell line: Design, synthesis, DFT and molecular modelling studies. Carbohydrate Research. 2020 Jan 1;487:107894.
96. Khodair AI, Awad MK, Gesson JP, Elshaier YA. New N-ribosides and N-mannosides of rhodamine derivatives for suppressing leukemia cell line growth. J Clin Haematol. 2020;1(1), 7-9 (2020).
97. Khodair AI, Alsafi MA. Anticancer activity of S-glycosylated quinazoline derivatives. J Clin Haematol. 2020;1(3):72-77.
98. Attia AM, Khodair AI, Gendy EA, El-Magd MA, Elshaier YA. New 2-oxopyridine/2-thiopyridine derivatives tethered to a benzotriazole with cytotoxicity on MCF7 cell lines and with antiviral activities. Letters in Drug Design & Discovery. 2020 Feb 1;17(2):124-37.
99. Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Journal of immunological methods. 1986 May 22;89(2):271-7.
100. Mohamed MS, Zohny YM, El-Senousy WM, Abou AM. Synthesis and biological screening of novel pyrazoles and their precursors as potential antiviral agents. Pharmacophore. 2018 Jan 1;9(1):126-39.
101. Sun Y, Sun YI, Wenger L, Rutter JL, Brinckerhoff CE, Cheung HS. p53 down-regulates human matrix metalloproteinase-1 (collagenase-1) gene expression. Journal of Biological Chemistry. 1999 Apr 23;274(17):11535- 40.
102. Cummins JM, Rago C, Kohli M, Kinzler KW, Lengauer C, Vogelstein B. Disruption of HAUSP gene stabilizes p53. Nature. 2004 Apr;428(6982):1-2.
103. Everett RD, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. The EMBO journal. 1997 Feb 1;16(3):566-77.
104. Liao G, Yang D, Ma L, Li W, Hu L, Zeng L, et al. The development of piperidinones as potent MDM2-P53 protein-protein interaction inhibitors for cancer therapy. European Journal of Medicinal Chemistry. 2018 Nov 5;159:1-9.
105. Gollner A, Rudolph D, Arnhof H, Bauer M, Blake SM, Boehmelt G, et al. Discovery of novel spiro[3H-indole- 3,2′-pyrrolidin]-2(1H)-one compounds as chemically stable and orally active inhibitors of the MDM2–p53 interaction. Journal of Medicinal Chemistry. 2016 Nov 23;59(22):10147-62.
106. Hawkins PC, Skillman AG, Nicholls A. Comparison of shape-matching and docking as virtual screening tools. Journal of Medicinal Chemistry. 2007 Jan 11;50(1):74-82.
107. Sutherland JJ, Nandigam RK, Erickson JA, Vieth M. Lessons in molecular recognition. 2. Assessing and improving cross-docking accuracy. Journal of Chemical Information and Modelling. 2007 Nov 26;47(6):2293-302.
108. Huang H, Zhang G, Zhou Y, Lin C, Chen S, Lin Y, et al. Reverse screening methods to search for the protein targets of chemopreventive compounds. Frontiers in Chemistry. 2018 May 9;6:138.
