Commentary
Various studies have reported a strong association between different autoimmune diseases or infection-mediated disorders and an increased risk for venous thromboembolism (VTE). Particularly, patients with rheumatoid arthritis (RA) [1,2], ankylosing spondylitis (AS) [3] and psoriatic arthritis (PsA) [4] have been found to be associated with VTE. The aforementioned studies posed an intriguing question concerning the putative role of a shared genetic background as regards with the co-occurrence of VTE with RA, AS or PsA. In this context, we managed to shed light in this issue recently by conducting extended literature searches [5-7]. VTE, the next to coronary heart disease (CHD) and stroke, represents the third most common cardiovascular disorder, is a multifactorial vascular disease, representing a worldwide health problem affecting people of all ages, sexes and races, exhibiting 2 major subtypes, deep vein thrombosis (DVT) and pulmonary embolism (PE) [8]. Particularly, VTE pathogenesis includes clots that have either formed in the veins of the legs and arms, known as DVT, or embolized and travelled to the lungs, known as pulmonary embolism PE. Data based on histological and electron microscopic investigations have shown that venous thrombi have more fibrin and erythrocytes while arterial thrombi have more platelets [9]. VTE is a disorder with significant genetic predisposition, with its heritability being approximately 50% in studies of families, twins, siblings, and half-siblings [10], while the risk of a sibling developing VTE if another sibling has VTE is approximately 2.5-fold [11], considering that genetic risk factors play the most important etiopathogenic role in venous thromboembolism (VTE) in people younger than 50 years old [12]. Approximately 10% of patients suffering from DVT develop PE later in their life, and 10% of these patients die [8]. The clinical outcome from VTE disease represents the major source of morbidity and mortality. VTE is the third leading cause of cardiovascular death in the United States [13]. Noteworthy, about 900,000 people have VTE each year in the United States, and between 60,000 and 100,000 die from this disorder [13]. Lifetime risk of VTE is rather high and has been estimated to be 5–10%. VTE recurrence risk is also high [8] and the risk of recurrent VTE is around 25% in 5 years [10].
Notably, an interesting issue emerged recently focusing on the frequency, incidence rates, risk factors and outcomes of a first venous thromboembolic event (VTE) between patients with systemic lupus erythematosus (SLE) and controls [14]. Nossent et al. [14] conducted a study to compare the incidence, odds and consequences of multiple VTE types in an observational long-term study comprising Australian patients with SLE and controls [14]. The aforementioned study revealed that VTE affected 12.8% of patients with SLE at six times the VTE rate in controls with antiphospholipid antibodies (aPL) but VTE did not associate with an increased risk of arterial events. The increased frequency of VTE between SLE patients and controls is an issue, considering that similar results were collected from previous population-based or observational studies conducted worldwide (i.e. in Australia, USA, Canada, France, the Netherlands) (for references see [14]). However, the existing information refers to epidemiological or clinical aspects of this association. SLE is the prototypic multisystemic, multifactorial autoimmune disease, characterized by a loss of self-tolerance leading to the presence of autoantibodies directed towards ubiquitous nuclear antigens, immune complex deposition resulting in organ damage, as well as chronic inflammation at classic target organs such as skin, joints, and kidneys. Most patients, constitutional, mucocutaneous, and musculoskeletal symptoms appear, including include fatigue, lupus-specific rash, mouth ulcers, alopecia, joint pain, and myalgia [15]. This disease affects more than 3.4 million people worldwide, predominantly affecting women, with a ratio of about 9 women to 1 man [16]. Considering the long-term interest of our research group in the investigation of the genetic components that are involved in the co-occurrence of VTE with various autoimmune diseases, we attempted to delineate the genetic basis of the co-occurrence of SLE and VTE by searching in the literature for known genes involved in the development of both conditions, aiming to reveal a partially shared genetic background.
To our knowledge, it is the first attempt in the literature focusing on this scientific issue thus far. The method used for this article included case-control, genome wide association studies (GWAS), systematic reviews and cross-sectional studies. The papers included were written in English language and published from 2006 to 2024. The database used were PubMed, PubMed Central (PMC), Google Scholar, Web of Science and Base, while the keywords that were included in the search study were venous thromboembolism, systemic lupus erythematosus, genetics, gene polymorphisms, association studies, GWAS. Both authors have worked individually to extract the data following the specified criteria and no disagreements arose during the process.
Thus, we found certain genes that represent potential risk factors for developing both diseases, including the interleukin-4 (IL-4) –589 C/T (rs2243250) and the insertion/deletion (ID) of the angiotensin-converting enzyme (ACE) polymorphisms [17-20], the endothelial nitric oxide synthase (eNOS) intron 4 VNTR [21,22] as well as the methylenetetrahydrofolate reductase (MTHFR) rs1801133 [23,24], tumor necrosis factor-α (TNF-α) rs1800629 [24,25], vitamin D receptor (VDR) rs7975232 [24,26], interleukin-1 beta (IL-1B) rs16944 [17,27], SH2B adapter protein 3 (SH2B3) rs3184504 [28,29], signal transducer and activator of transcription-4 (STAT4) rs7574865 [17,30] and rs7582694 [17,30] single nucleotide polymorphisms (SNPs). In Figure 1, a Venn diagram shows the number of the shared gene polymorphisms between VTE and SLE, based on the aforementioned articles as well as others selected upon an extensive, careful literature search [10,31-41]. Moreover, Table 1 demonstrates the shared gene polymorphisms in detail.
|
dbSNP ID |
SLE- and VTE -associated gene |
Function |
References |
|
rs2243250 |
IL-4 |
A pleiotropic cytokine; promotes the proliferation of B cells and T cell antibodies. |
[17,19] |
|
N/A |
ACE |
Converts angiotensin I into angiotensin II; catalyzes the bradykinin to kinin degradation products. |
[18,20] |
|
N/A |
eNOS |
A key enzyme in production of the vasodilator nitric oxide (NO). |
[21,22] |
|
rs1801133
|
MTHFR |
A key regulatory enzyme in folate and homocysteine metabolism. |
[23,24] |
|
rs1800629 |
TNF-α |
A multifunctional pro-inflammatory cytokine. |
[24,25] |
|
rs7975232 |
VDR |
A ligand-activated transcription factor, inducing genomic regulation of downstream targets. |
[24,26] |
|
rs16944 |
IL-1B |
A cytokine, acting on T and natural killer cells. |
[17,27] |
|
rs3184504 |
SH2B3 |
A key negative regulator of cytokine signaling; plays a critical role in hematopoiesis |
[28,29] |
|
rs7574865 rs7582694 |
STAT4 |
A transcription factor involved in the Th17 differentiation, monocyte activation and interferon-gamma production. |
[17,30] |
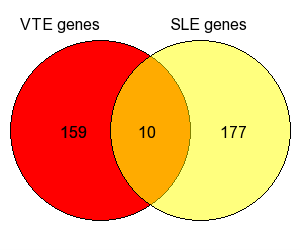
Figure 1. Venn diagram of SLE and VTE shared genes. (Core Graphic Module by Vijayaraj Nagarajan, and Web implementation by Mehdi Pirooznia. October 2006, usm.edu).
To summarize, we identify a small number of shared genetic factors of SLE and VTE, due to the fact that underlying molecular mechanisms leading to the development of VTE are still elusive. Moreover, according to the current knowledge, most VTE-risk factors are related to hemostatic system and inherited hypercoagulable states [10]. Specifically, they refer to variations in the genes responsible for blood coagulation and fibrinolytic factors, platelet membrane receptors as well as genes involved in erythrocytes biology [24]. However, a number of shared genetic factors related to inflammation, interferon signaling, metabolism and NF-κB pathways was identified in the present study. Therefore, we can assume that a “cross-talk” exists between the coagulation and inflammatory pathways, considering that gene polymorphisms involved in inflammation may also result in an increased susceptibility towards VTE. The link between inflammation and coagulation activation has been demonstrated in both animals and humans [19]. In this context, it has been suggested that cytokines primarily influence fibrinolysis besides tissue factor expression [19]. Although the genetic component does not seem to contribute substantially to the co-occurrence of the conditions under investigation, a further identification of more genetic factors is of emerging interest and may help to better delineate the mechanisms leading to their clinical association. These results will be important not only for future genetic and clinical studies but also for directing future function/structure studies of the molecular cause of VTE and SLE. Therefore, an in-depth study of the functional significance of the shared gene polymorphisms may explain how these genetic factors should be incorporated into clinical management of the patients.
A limitation of our study deals with the lack of information in the current literature about the role of the published gene polymorphisms in SLE and VTE patients of different ethnic and/or racial origin. This is a crucial point for further investigation given that different genes are involved in the development of these disorders according to the ancestry of the populations under examination. Moreover, the source of the inter-individual susceptibility to VTE or SLE does not lie exclusively in genetics, considering that epigenetics mechanisms are now widely believed to participate also to the susceptibility to these diseases. Thus, the role of potential shared epigenetic factors has to be investigated in future studies. Noteworthy, a new avenue refers to the detection of novel risk loci due to the information for rare risk variants gained by using WES and WGS technology, thus making a considerable contribution to the missing heritability of VTE and SLE [10].
The current decade promises to be a tipping point for genetic applications in personalized medicine. Despite the variety of genetic factors for VTE that have already been identified, less focus has been placed on ethnic diversity. Upon now, genetic studies have revealed clear disparities in the distribution of genetic factors for VTE in populations stratified by ethnicity worldwide [34]. As a consequence, it is essential for clinicians to establish different risk models that are suitable for each geographic region and ethnic group [42,43]. In the same framework, it has long been appreciated that SLE patients of different ancestral backgrounds have differences in clinical presentation and disease course. Understanding some of the molecular differences between populations, both at the genetic and protein expression levels, may suggest ways in which treatment could be personalized. Thus, selecting treatment based upon a patient's individual biology would be a major advance in SLE [44]. Furthermore, a natural development of the GWAS and the increasing number of detected variants will be the development and validation of gene risk scores (GRS) both for VTE and SLE. Thus, the combination of GRS with acquired risk factors will be useful for better risk assessment and tailored management of both diseases.
In conclusion, better functional characterization of the diseases-associated genes may lead to the detection of new therapeutic targets for pharmaceutical intervention and ultimately result in the better therapeutic management and diseases’ treatment.
Disclosure Statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Authors’ Contribution
M.I.Z. and G.N.G. designed the current study, searched the literature and drafted the manuscript. Both authors gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Acknowledgments
The authors apologize to all researchers whose original articles were not cited in the present article due to space limitations in these types of articles.
ORCID iD
George N Goulielmos https://orcid.org/0000-0002-9797-2310.
References
2. Li L, Lu N, Avina-Galindo AM, Zheng Y, Lacaille D, Esdaile JM, et al. The risk and trend of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a general population-based study. Rheumatology (Oxford). 2021 Jan 5;60(1):188-95.
3. Aviña-Zubieta JA, Chan J, De Vera M, Sayre EC, Choi H, Esdaile J. Risk of venous thromboembolism in ankylosing spondylitis: a general population-based study. Ann Rheum Dis. 2019 Apr;78(4):480-5.
4. Gazitt T, Pesachov J, Lavi I, Elias M, Haddad A, Feldhamer I, et al. The association between psoriatic arthritis and venous thromboembolism: a population-based cohort study. Arthritis Res Ther. 2022 Jan 7;24(1):16.
5. Zervou MI, Goulielmos GN. Correspondence on 'Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: a nationwide cohort study from Sweden'. Ann Rheum Dis. 2023 Apr;82(4):e87.
6. Goulielmos GN, Zervou MI. Risk of Venous Thromboembolism in Ankylosing Spondylitis and Rheumatoid Arthritis: Genetic Aspects. J Rheumatol. 2021 Sep;48(9):1492-3.
7. Zervou MI, Goulielmos GN. Co-occurrence of Psoriatic Arthritis with Venous Thromboembolism: Genetic Aspects. JSM Arthritis 2023;5(1):1032.
8. Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016 Jan;41(1):3-14.
9. Lowenstein CJ. GENETIC DETERMINANTS OF THROMBOSIS. Trans Am Clin Climatol Assoc. 2024;134:230-8.
10. Zöller B, Svensson PJ, Dahlbäck B, Lind-Hallden C, Hallden C, Elf J. Genetic risk factors for venous thromboembolism. Expert Rev Hematol. 2020 Sep;13(9):971-81.
11. Larsen TB, Sørensen HT, Skytthe A, Johnsen SP, Vaupel JW, Christensen K. Major genetic susceptibility for venous thromboembolism in men: a study of Danish twins. Epidemiology. 2003 May;14(3):328-32.
12. Kreidy R. Influence of acquired and genetic risk factors on the prevention, management, and treatment of thromboembolic disease. Int J Vasc Med. 2014;2014:859726.
13. Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014 Nov;34(11):2363-71.
14. Nossent JC, Keen HI, Preen DB, Inderjeeth CA. Long-term incidence, risk factors and complications for venous thromboembolism in patients with systemic lupus erythematosus. Lupus. 2024 Jul;33(8):787-96.
15. Tsokos GC. Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol 2020;21:605-14.
16. Tian J, Zhang D, Yao X, Huang Y, Lu Q. Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modelling study. Ann Rheum Dis. 2023 Mar;82(3):351-6.
17. Mohammadoo-Khorasani M, Salimi S, Tabatabai E, Sandoughi M, Zakeri Z, Farajian-Mashhadi F. Interleukin-1β (IL-1β) & IL-4 gene polymorphisms in patients with systemic lupus erythematosus (SLE) & their association with susceptibility to SLE. Indian J Med Res. 2016 May;143(5):591-6.
18. Gong AM, Li XY, Wang YQ, Yan HX, Xu ZX, Feng Z, et al. Association study of ACE polymorphisms and systemic lupus erythematosus in Northern Chinese Han population. Mol Biol Rep. 2012 Oct;39(10):9485-91.
19. Beckers MM, Ruven HJ, Haas FJ, Doevendans PA, ten Cate H, Prins MH, et al. Single nucleotide polymorphisms in inflammation-related genes are associated with venous thromboembolism. Eur J Intern Med. 2010 Aug;21(4):289-92.
20. Yesilkaya S, Karkucak M, Coban H, Ursavas A, Ture M, Yakut T. Angiotensin-Converting Enzyme Gene Insertion/Deletion Polymorphism in Patients with Pulmonary Thromboembolism. Int J Hum Genet. 2015 Dec 1;15(4):183-9.
21. Katkam SK, Indumathi B, Tasneem FSD, Rajasekhar L, Kutala VK. Impact of eNOS 27-bp VNTR (4b/a) gene polymorphism with the risk of Systemic Lupus Erythematosus in south Indian subjects. Gene. 2018 Jun 5;658:105-12.
22. Huang G, Deng X, Xu Y, Wang P, Li T, Hu P. Endothelial nitric oxide synthase polymorphism and venous thromboembolism: A meta-analysis of 9 studies involving 3993 subjects. Phlebology. 2021 Dec;36(10):797-808.
23. Zhou HY, Yuan M. MTHFR polymorphisms (rs1801133) and systemic lupus erythematosus risk: A meta-analysis. Medicine (Baltimore). 2020 Oct 2;99(40):e22614.
24. Lindström S, Wang L, Smith EN, Gordon W, van Hylckama Vlieg A, de Andrade M, et al. Genomic and transcriptomic association studies identify 16 novel susceptibility loci for venous thromboembolism. Blood. 2019 Nov 7;134(19):1645-57.
25. Chen L, Huang Z, Liao Y, Yang B, Zhang J. Association between tumor necrosis factor polymorphisms and rheumatoid arthritis as well as systemic lupus erythematosus: a meta-analysis. Braz J Med Biol Res. 2019 Mar 25;52(3):e7927.
26. Yang SK, Liu N, Zhang WJ, Song N, Yang JP, Zhang H, et al. Impact of Vitamin D Receptor Gene Polymorphism on Systemic Lupus Erythematosus Susceptibility: A Pooled Analysis. Genet Test Mol Biomarkers. 2022 Apr;26(4):228-38.
27. van Minkelen R, de Visser MC, Houwing-Duistermaat JJ, Vos HL, Bertina RM, Rosendaal FR. Haplotypes of IL1B, IL1RN, IL1R1, and IL1R2 and the risk of venous thrombosis. Arterioscler Thromb Vasc Biol. 2007 Jun;27(6):1486-91.
28. Crous-Bou M, De Vivo I, Camargo CA Jr, Varraso R, Grodstein F, Jensen MK, et al. Interactions of established risk factors and a GWAS-based genetic risk score on the risk of venous thromboembolism. Thromb Haemost. 2016 Sep 27;116(4):705-13.
29. Cunninghame Graham DS, Morris DL, Bhangale TR, Criswell LA, Syvänen AC, Rönnblom L, et al. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet. 2011 Oct;7(10):e1002341.
30. Celińska-Löwenhoff M, Iwaniec T, Dziedzina S, Kaczor M, Pastuszczak M, Dropiński J, et al. The association of STAT4 single nucleotide polymorphisms with thrombotic manifestations in a cohort of patients with autoimmune diseases and antiphospholipid antibodies. Research Square 2023; DOI: 10.21203/rs.3.rs-2858961/v1.
31. Zee RY, Glynn RJ, Cheng S, Steiner L, Rose L, Ridker PM. An evaluation of candidate genes of inflammation and thrombosis in relation to the risk of venous thromboembolism: The Women's Genome Health Study. Circ Cardiovasc Genet. 2009 Feb;2(1):57-62.
32. Srivastava S. Understanding Genetic Variations as Risk Factors for Development Venous Thrombo-Embolism (VTE). Adv Genet Eng 2016;5:143.
33. Klarin D, Emdin CA, Natarajan P, Conrad MF; INVENT Consortium; Kathiresan S. Genetic Analysis of Venous Thromboembolism in UK Biobank Identifies the ZFPM2 Locus and Implicates Obesity as a Causal Risk Factor. Circ Cardiovasc Genet. 2017 Apr;10(2):e001643.
34. Tang L, Hu Y. Ethnic diversity in the genetics of venous thromboembolism. Thromb Haemost. 2015 Nov;114(5):901-9.
35. Thibord F, Klarin D, Brody JA, Chen MH, Levin MG, Chasman DI, et al. Cross-Ancestry Investigation of Venous Thromboembolism Genomic Predictors. Circulation. 2022 Oct 18;146(16):1225-42.
36. Yin X, Kim K, Suetsugu H, Bang SY, Wen L, Koido M, et al. Meta-analysis of 208370 East Asians identifies 113 susceptibility loci for systemic lupus erythematosus. Ann Rheum Dis. 2021 May;80(5):632-40.
37. Remmers EF, Plenge RM, Lee AT, Graham RR, Hom G, Behrens TW, et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med. 2007 Sep 6;357(10):977-86.
38. Kwon YC, Chun S, Kim K, Mak A. Update on the Genetics of Systemic Lupus Erythematosus: Genome-Wide Association Studies and Beyond. Cells. 2019 Sep 30;8(10):1180.
39. Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. 2015 Dec;47(12):1457-64.
40. International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN); Harley JB, Alarcón-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008 Feb;40(2):204-10.
41. Morris DL, Sheng Y, Zhang Y, Wang YF, Zhu Z, Tombleson P, et al. Genome-wide association meta-analysis in Chinese and European individuals identifies ten new loci associated with systemic lupus erythematosus. Nat Genet. 2016 Aug;48(8):940-6.
42. van Hylckama Vlieg A, Flinterman LE, Bare LA, Cannegieter SC, Reitsma PH, Arellano AR, et al. Genetic variations associated with recurrent venous thrombosis. Circ Cardiovasc Genet. 2014 Dec;7(6):806-13.
43. de Haan HG, Bezemer ID, Doggen CJ, Le Cessie S, Reitsma PH, Arellano AR, et al. Multiple SNP testing improves risk prediction of first venous thrombosis. Blood. 2012 Jul 19;120(3):656-63.
44. Goulielmos GN, Zervou MI, Vazgiourakis VM, Ghodke-Puranik Y, Garyfallos A, Niewold TB. The genetics and molecular pathogenesis of systemic lupus erythematosus (SLE) in populations of different ancestry. Gene. 2018 Aug 20;668:59-72.
