Abstract
The role of CD146 and its soluble form have been intensely reviewed in the processes of cancer and inflammation. However, how CD146 is linked to coagulation and thrombotic events remains unclear. The aim of this paper is to shed light on this topic and clarify the contribution of CD146 as a procoagulant factor.
The CD146/Soluble CD146 Molecules
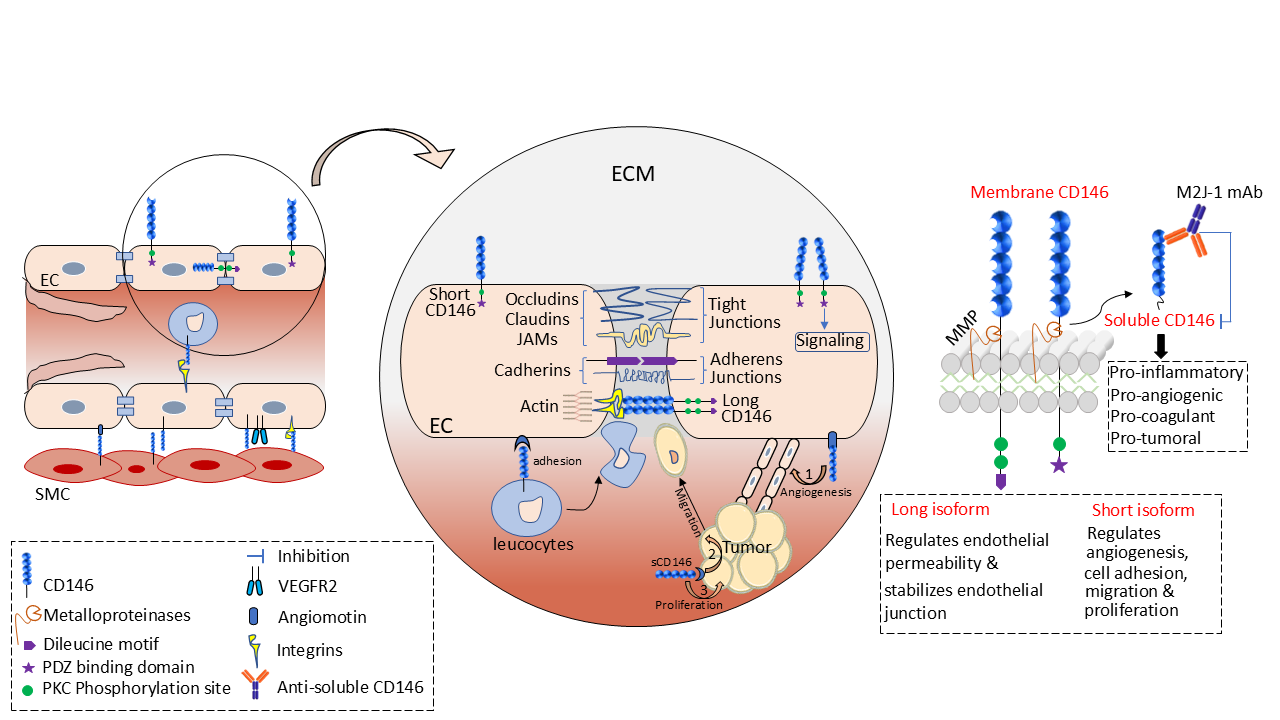
CD146, also known as melanoma cell adhesion molecule (Mel-CAM), MUC18, S-Endo1, A32 antigen, metastasis cell adhesion molecule (MET-CAM), and hemopoietic cell adhesion molecule (HEMCAM), is an integral membrane glycoprotein belonging to the immunoglobulin superfamily. It is constituted of two variable regions (V) and three constant regions (C2) V-VC2- C2-C2 that give rise to the extracellular domain of the receptor, a hydrophobic transmembrane region, and a short cytoplasmic tail [1,2]. Two isoforms of membrane CD146 have been described in literature, a long isoform and a short one, which are generated by alternative splicing of the transcript on exon 15, leading to a reading frame shift. These isoforms display similar extracellular and transmembrane domains with only differences in the intracellular domain. The short isoform displays a shorter cytoplasmic domain, with a phosphorylation site for protein kinase C (PKC) and an interaction site with proteins containing a PDZ domain, whilst the long isoform displays two domains for phosphorylation by PKC and an endocytosis signal sequence [3,4]. In addition to the membrane-anchored form, CD146 also exists as a soluble form that is generated by the ectodomain shedding of membrane CD146 in a calcium-induced, matrix metalloproteinase-dependent manner [5] (Figure 1). Theoretically, CD146 is estimated to have a molecular weight of 79 kDa based on the protein sequence derived from the coding region of the gene transcript. However, the actual molecular weight is around 110kDa, a shift that is caused by intensive glycosylation on the eight N-glycosylation sites present on the protein sequence, a common phenomenon occurring on mucins glycoproteins [6].
CD146 has been found to be conserved among species [7,8]. Homologous proteins with similar sequences have been described in mice, rats, chicken and zebrafish, hinting to its fundamental role in development.
Physiologically, CD146 is present on all the vascular tree. It is mainly expressed at the intercellular junction of endothelial cells and is involved in regulating cellcell cohesion, paracellular permeability, monocyte transmigration, and angiogenesis [9]. Aside from endothelial cells, CD146 is also found to be expressed on smooth muscle cells, trophoblasts, pericytes, myofibroblasts, and various immune cells such as TH17 cells where it plays an essential role in cell mobility [10].
Pathologically, CD146 is expressed by various tumors with a positive correlation between CD146 expression and cancer stage [11]. First identified on melanoma cells [1], CD146 was thereafter found to be also expressed on pancreatic, breast, prostate, ovarian, lung, kidney cancers, osteosarcoma, Kaposi sarcoma, angiosarcoma, Schwann cell tumours, and leiomyosarcoma [4,12]. Why and how cancer cells neo-express CD146 remains a matter of debate. In prostate cancer, Liu et al. showed that the hypermethylation of CD146 gene promoter potentiates its expression [13]. In parallel, other studies demonstrated a high increase in CD146 expression on renal carcinoma cells after becoming resistant to the tyrosine kinase inhibitor Sunitinib, with an overall increase in cell invasiveness and metastatic potential [14]. The adverse role of CD146 during inflammation and auto-immune diseases was also highlighted [15]. Different studies have shown that CD146 is a major player in multiple sclerosis and systemic sclerosis diseases, with the soluble form being strongly increased in the cerebrospinal fluid of patients as compared to healthy subjects [16,17]. All these studies led to the conclusion that soluble CD146 could constitute a biomarker for many diseases.
Up to now, no link had been described between CD146/ soluble CD146 and coagulation or thrombotic events. In their recent study in International Journal of Cancer, Stalin et al. [18] give new insights into the role of the CD146 molecule in cancer-associated thromboembolism and propose a therapeutic strategy to prevent thrombosis which is frequently associated with this pathology.
Role of Tissue Factor in Hemostasis and Thrombosis
Hemostasis is a process that allows blood to form a clot to prevent blood loss as a result of a vessel injury, followed by a repair [19]. During this process, platelets are recruited to the site of injury, where they become a major component of the developing thrombus [20]. Concomitantly, blood coagulation, after a cascade of proteolytic events, culminated in the generation of thrombin and fibrin [21]. When pathologic processes, as those found in cancers, overwhelm the regulatory mechanism of hemostasis, excessive quantities of thrombin form, initiating thrombosis. Both in physiological (hemostasis) and pathological (thrombosis) processes, an initiating factor plays a major role, the tissue factor (TF) [22].
Tissue factor, factor III, thromboplastin, or CD142 is a single-pass type I membrane glycoprotein constitutively expressed on sub-endothelial tissues as well as many nonvascular cells including various cancer cells depending upon their stage and the therapeutic approaches employed in the treatment [23,24]. It is also exposed at the surface of monocytes and endothelial cells upon stimulation [24]. This 47 kDa membrane protein consists of extracellular, transmembrane, and cytoplasmic domains. As a member of cytokine-receptor class II family, this receptor is activated by various inflammatory cytokines such as IFN-α, IFN-β, IFN-γ, TNF-α, interleukin-1 and -10 [25]. Its activation is also induced by endotoxin and the stimulation of T cells by PMA has been shown to potently induce TF expression [26]. It binds with high affinity to activated FVII, forming a surface complex that catalyses in a cofactorindependent mechanism a cascade of coagulation events, ultimately leading to fibrin clot. During the last decades, it has been clearly demonstrated that TF plays a major role in the etiology of cancer-related thrombosis [27]. Remarkably, the intracellular domain of TF is involved in signalling functions [28]. In addition, a soluble form of TF exists and is generated by alternative splicing of the mRNA transcript whereby exon 4 is spliced directly to exon 6, skipping exon 5 and liberating most of the extracellular domain. This alternatively spliced TF lacks important residues, reducing its procoagulant activity but playing a major role in cancer biology by promoting angiogenesis and proliferation of tumor cells [see below]. Of interest, Szotowski et al. have shown that the proinflammatory cytokine TNF-α is able to induce soluble TF in endothelial cells within 10 minutes of stimulation [29]. Finally, in addition to the effects of TF and sTF, it has been shown that cancer cells are able to generate microvesicles that are involved in thromboembolism. Thus, these microvesicles are able to induce thrombosis through different factors they convey at their surface, as TF, but also polyphosphates or phosphatidylethanolamine [30,31].
Role of Tissue Factor in Cancer Dissemination
In addition to its well-documented expression on cancer cells and the positive correlation existing between its expression density and cancer metastatic potential, a new role of TF has emerged as a potent inducer of cancer stem cell (CSC) phenotype in various cancer cell lines including breast cancer, squamous cell carcinoma (SCC) and pancreatic cancers [32,33]. This effect was demonstrated after treating SCC with TF neutralizing antibody and injecting cells into mouse tail which showed an impaired ability to develop tumors. In addition, the fact that TF expression on SCC was correlated with the expression of CD133, a cancer stem cell marker, further supports this idea. In breast cancer, it has been shown that cells expressing TF have higher ability to form spheres in vitro, and upregulate the expression of stemcell markers. Another study has shown that TF represents a therapeutic target for eradicating CSC in lung, breast, and ovarian cancer [32]. A positive correlation was demonstrated between CD133 and TF expressed in various cancer cells although the ratio TF over CD133 was not constant among the different cells. On the contrary, a study initiated by Clouston et al. has shown that colorectal cancer cells DLD-1, which intrinsically express higher TF level than SW620, have lower CSC activity as assessed by a decrease in colony-sphere forming efficiency and aldehyde dehydrogenase (ALDH) activity [34]. Moreover, they have shown that upon knocking down TF by shRNA, both SW620 and DLD-1 cell lines exhibit enhanced ability to form sphere colonies and ALDH activity was potentiated, indicating an overall increase in cancer stem cell phenotype. Hence, the therapeutic interest of targeting TF is highly dependent on the type of solid tumor as well as the stage of the tumor. In all cases, TF was found to be associated with enhanced cancer cell viability and proliferation. Likewise, soluble TF (sTF) was found to induce angiogenesis, survival and cell growth. In breast cancer patients, the plasma concentration of sTF was found to be correlated with the tumor growth and membrane TF and has been used as a biomarker for tumor prognosis, metastasis and patients’ overall survival [35].
CD146 and TF are Induced by the Same Molecules and Activate Common Signaling Pathways
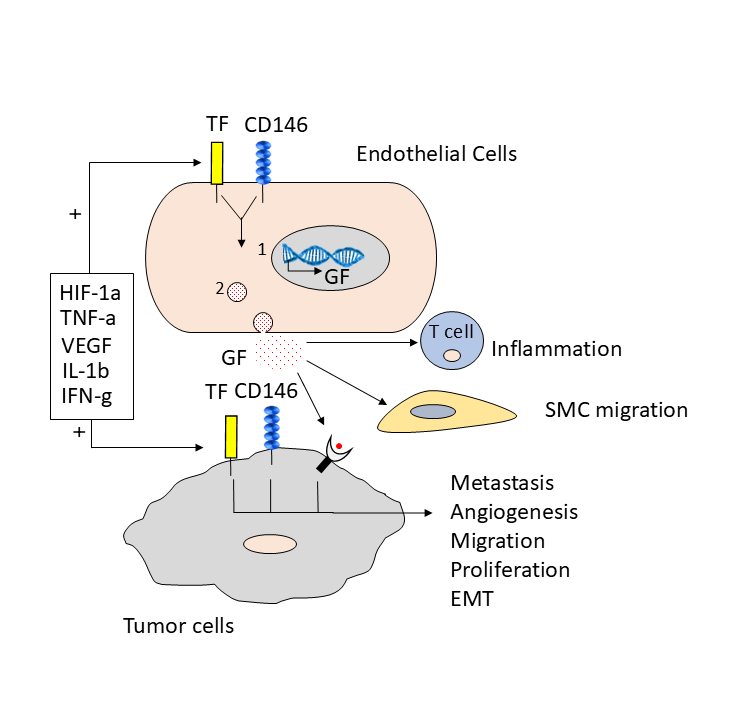
Recent studies have shown that hypoxia, proinflammatory cytokines and vascular endothelial growth factor potently induce TF expression on cancer cells [36-38]. Interestingly, these factors also induce CD146 expression via a common signalling pathway, the mitogen activated-protein kinase (MAPK), P38 signalling, PI3K, PKC, and AKT/PKB [39,40]. Luo et al. have shown that CD146 induces HIF1-α expression, while another study showed that HIF1-α induces TF overexpression on MDA-MB-231 cells. Alongside, Yu et al. validated that loss of P53 tumor suppressor enhanced TF expression, and another study by Jin et al. showed that overexpression of CD146 in mesenchymal stem cells (MSC) caused significant decrease in P53 marker.
Finally, both CD146 and TF expression were found to be correlated in surface expression and both receptors can induce tumor metastasis, invasion, and angiogenesis [18,28] (Figure 2). As mentioned earlier, CD146 and TF activate common signalling pathways but no relationship between these two molecules has been described before. Recently, a publication has suggested that soluble CD146 can induce effects on endothelial cells permeability through integrin beta 1 [41]. Likewise, soluble TF has been recognized to signal through integrin beta 1 on cancer cells, such as integrin alpha 6 beta 1, to induce cell proliferation, migration, and angiogenesis [42]. Besides, similar signalling pathways have been shown to be activated by both sCD146 and sTF, including the PKC, ERK1/2 and AKT signalling pathways (Figure 3).
Finally, thrombin-mediated activation of PAR1 receptor has been shown to upregulate CD146 on melanoma cells [43]. In view of these different results, it was of interest to hypothesize that CD146/sCD146 and TF/sTF share and activate common signalling pathways.
CD146/Soluble CD146 and Venous Thromboembolism: Therapeutic Interest of an Anti-Soluble CD146 Antibody
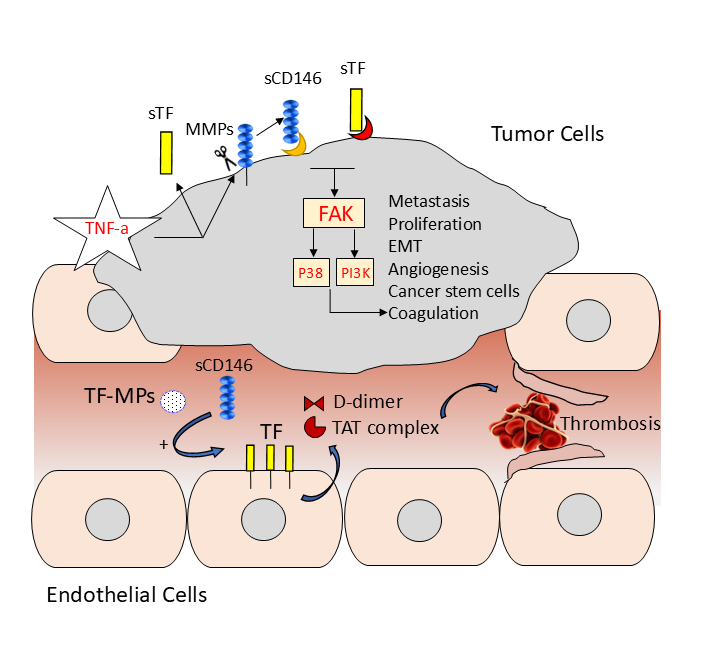
In the recent study published in International Journal of Cancer, Stalin et al [18] were the first to show the direct impact of CD146/soluble CD146 on TF expression and their interrelated consequences on tumor metastasis and venous thromboembolism. Using two highly metastatic CD146-positive cell lines, the ovarian HEY and melanoma A375, they showed that sCD146, which is secreted by the cells, was able to upregulate TF expression, increasing their procoagulant activity alongside with an enhanced epithelial to mesenchymal transition and overexpression of cancer stem cell genes. To this end, Stalin et al. used two experimental tumor models to demonstrate the in vivo effect of sCD146 in inducing cancer cell metastasis and pro-coagulant effects. In a first model, luciferaseexpressing cancer cells, HEY and A373, were injected subcutaneously before peritumoral treatment with recombinant sCD146 (rsCD146). In a second model, the same cells expressing luciferase were injected intracardially after in vitro treatment with rsCD146. At the endpoint, organs with potential tumor cell metastasis were isolated and bioluminescence was measured. They showed that rsCD146 treatment increased tumor cells metastasis, in particular to the liver and lungs, induced cancer cell growth, and drastically decreased mice overall survival as compared to control group. This effect was closely associated with an increase of the coagulation cascade and subsequent thrombosis. To support this hypothesis, they have studied the direct effect of sCD146 on TF expression by the cancer cell lines HEY and A375. Their results showed that sCD146 not only upregulated surface TF expression, but also induced a robust increase in soluble TF in the cell supernatants along with an increase in factor Xa, the substrate of membrane TF. Building on this, we can hypothesize that the tissue factor pathway will be activated to form a fibrin clot and trigger the subsequent thrombotic-mediated mouse death, explaining at least in part the lower survival rate of these mice. This mechanism appears to be very similar to that described for the tissue factor-bearing microparticles secreted by cancer cells [30] (Figure 3). In contrast, sCD146 failed to induce in vitro any proliferative, migrative, and invasive effect on the cancer cell line SW620, which was used as a negative control in this study as they minimally express membrane CD146. Authors also showed that the number of microparticles was significantly increased in the plasma of mice bearing CD146-positive tumors, especially in mice with metastases.
Of interest, in this article, Stalin et al. have introduced a therapeutic antibody (M2J-1 mAb) specifically targeting human sCD146 secreted by the cancer cells, but not the membrane CD146 which is present on cancer cells but also on the whole vascular system where it displays major physiological functions. Using RNA profiling analysis of cancer cells treated with the M2J-1 mAb as compared to control IgG, they showed that M2J-1 antibody potently neutralizes the adverse effect of sCD146, by decreasing the expression of genes implicated in EMT, cancer stem cells generation, and pro-oncogenes while increasing tumor suppressors. Also, they showed that M2J-1 mAb was able to reduce the expression of coagulation-related proteins such as TF, factor X, and thrombin receptor PAR1. Finally, they demonstrated that treatment with the M2J-1 mAb significantly reduced the number of cancer microparticles detected in the plasma of mice. This strongly emphasizes the interest of M2J-1 mAb as a therapeutic approach in treating cancers overexpressing CD146 due to its anti-metastatic and anti-thrombosis effects.
Conclusion
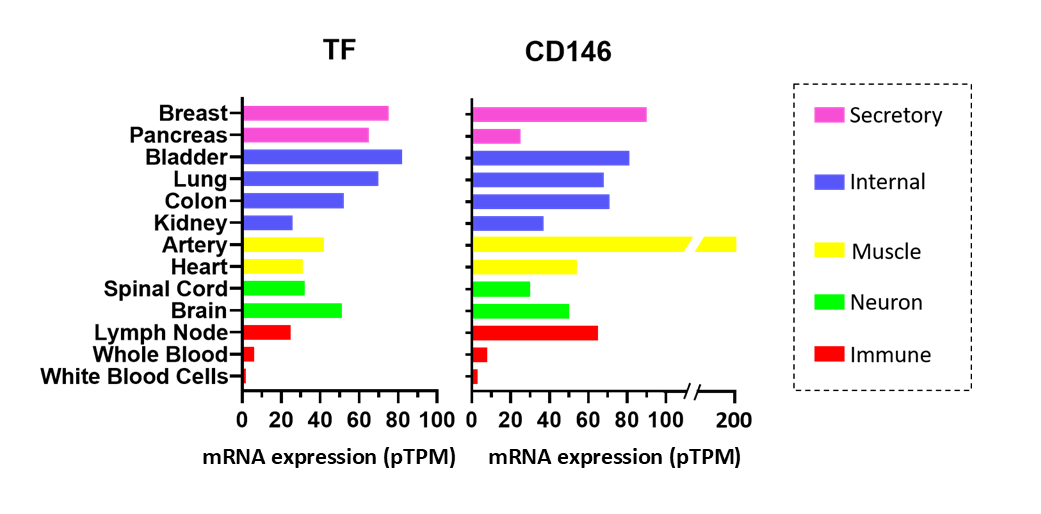
The leading cause of death in cancer patients is more and more described to be related to the adverse complications rather than the primary tumor itself. Multiple factors have been described as poor prognostic factors in cancer including the expression of CD146. Thrombosis has emerged as a critical issue that threatens health of cancer patients. The study of Stalin et al. shows for the first time a potential link between thrombotic events and the expression of CD146 on tumor cells. Interestingly, both CD146 and TF are expressed on the same tissues in human (Figure 4). In addition, Stalin et al. described a strong correlation between plasma levels of sCD146 and sTF in mice xenografted with CD146-positive cells. The fact that tumor-bearing mice treated with sCD146 led to an increase in coagulation-related proteins D-dimer, thrombin anti-thrombin complex (TAT), and TF supports the existence of a connection between these two molecules that orchestrate a series of events, leading not only to tumor dissemination, but also to venous thromboembolism. Of interest, the anti-soluble CD146 monoclonal antibody M2J-1 diminished both the number of circulating cancer microparticles and coagulation factors in mice bearing CD146-positive tumors, giving a new therapeutic opportunity for the treatment of this lethal side effect.
References
2. Lehmann JM, Holzmann B, Breitbart EW, Schmiegelow P, Riethmüller G, Johnson JP. Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer Research. 1987 Feb 1;47(3):841-5.
3. Guezguez B, Vigneron P, Alais S, Jaffredo T, Gavard J, Mège RM, et al. A dileucine motif targets MCAM-l cell adhesion molecule to the basolateral membrane in MDCK cells. FEBS Letters. 2006 Jun 26;580(15):3649-56.
4. Nollet M, Stalin J, Moyon A, Traboulsi W, Essaadi A, Robert S, et al. A novel anti-CD146 antibody specifically targets cancer cells by internalizing the molecule. Oncotarget. 2017 Dec 22;8(68):112283.
5. Bardin N, Frances V, Combes V, Sampol J, Dignat- George F. CD146: biosynthesis and production of a soluble form in human cultured endothelial cells. FEBS Letters. 1998 Jan 2;421(1):12-4.
6. Lei X, Guan CW, Song Y, Wang H. The multifaceted role of CD146/MCAM in the promotion of melanoma progression. Cancer Cell International. 2015 Dec 1;15(1):3.
7. Stalin J, Nollet M, Dignat-George F, Bardin N, Blot- Chabaud M. Therapeutic and diagnostic antibodies to CD146: thirty years of research on its potential for detection and treatment of tumors. Antibodies. 2017 Dec;6(4):17.
8. Yang H, Wang SW, Liu Z, Wu MW, McAlpine B, Ansel J, et al. Isolation and characterization of mouse MUC18 cDNA gene, and correlation of MUC18 expression in mouse melanoma cell lines with metastatic ability. Gene. 2001 Mar 7;265(1-2):133-45.
9. Leroyer AS, Blin MG, Bachelier R, Bardin N, Blot- Chabaud M, Dignat-George F. CD146 (Cluster of Differentiation 146) An Adhesion Molecule Involved in Vessel Homeostasis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019 Jun;39(6):1026-33.
10. Elshal MF, Khan SS, Takahashi Y, Solomon MA, McCoy Jr JP. CD146 (Mel-CAM), an adhesion marker of endothelial cells, is a novel marker of lymphocyte subset activation in normal peripheral blood. Blood. 2005 Oct 15;106(8):2923-4.
11. Targeting soluble CD146 with a neutralizing antibody inhibits vascularization, growth and survival of CD146-positive tumors | Oncogene [Internet]. [cited 2020 Mar 16].
12. Zeng P, Li H, Lu PH, Zhou LN, Tang M, Liu CY, et al. Prognostic value of CD146 in solid tumor: A Systematic Review and Meta-analysis. Scientific Reports. 2017 Jun 26;7(1):1-7.
13. Liu JW, Nagpal JK, Jeronimo C, Lee JE, Henrique R, Kim MS, et al. Hypermethylation of MCAM gene is associated with advanced tumor stage in prostate cancer. The Prostate. 2008 Mar 1;68(4):418-26.
14. Dufies M, Nollet M, Ambrosetti D, Traboulsi W, Viotti J, Borchiellini D, et al. Soluble CD146 is a predictive marker of pejorative evolution and of sunitinib efficacy in clear cell renal cell carcinoma. Theranostics. 2018;8(9):2447.
15. Guezguez B, Vigneron P, Lamerant N, Kieda C, Jaffredo T, Dunon D. Dual role of melanoma cell adhesion molecule (MCAM)/CD146 in lymphocyte endothelium interaction: MCAM/CD146 promotes rolling via microvilli induction in lymphocyte and is an endothelial adhesion receptor. The Journal of Immunology. 2007 Nov 15;179(10):6673-85.
16. Kaspi E, Heim X, Granel B, Guillet B, Stalin J, Nollet M, et al. Identification of CD146 as a novel molecular actor involved in systemic sclerosis. Journal of Allergy and Clinical Immunology. 2017 Nov 1;140(5):1448-51.
17. Gabsi A, Heim X, Dlala A, Gati A, Sakhri H, Abidi A, et al. TH17 cells expressing CD146 are significantly increased in patients with Systemic sclerosis. Scientific Reports. 2019 Nov 27;9(1):1-8.
18. Stalin J, Traboulsi W, Vivancos-Stalin L, Nollet M, Joshkon A, Bachelier R, et al. Therapeutic targeting of soluble CD146/MCAM with the M2J-1 monoclonal antibody prevents metastasis development and procoagulant activity in CD146-positive invasive tumors. International Journal of Cancer. 2020 Feb 5.
19. Balduini A, Balduini C. Haemostasis: General Pathways. In: eLS [Internet]. American Cancer Society; 2018 [cited 2020 Mar 16]. p. 1–11.
20. Kattula S, Byrnes JR, Wolberg AS. Fibrinogen and fibrin in hemostasis and thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2017 Mar;37(3):e13-21.
21. Gale AJ. Continuing education course# 2: current understanding of hemostasis. Toxicologic Pathology. 2011 Jan;39(1):273-80.
22. Furie B, Furie BC. Mechanisms of Thrombus Formation [Internet]. https://doi.org/10.1056/ NEJMra0801082. 2009 [cited 2020 Apr 1].
23. Cole M, Bromberg M. Tissue factor as a novel target for treatment of breast cancer. The Oncologist. 2013 Jan;18(1):14.
24. Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018 Apr;38(4):709-25.
25. van der Poll T, de Jonge E. Cytokines as regulators of coagulation. InMadame Curie Bioscience Database [Internet] 2013. Landes Bioscience.
26. De Palma R, Cirillo P, Ciccarelli G, Barra G, Conte S, Pellegrino G, Pasquale G, Nassa G, Pacifico F, Leonardi A, Insabato L. Expression of functional tissue factor in activated T-lymphocytes in vitro and in vivo: A possible contribution of immunity to thrombosis?. International Journal of Cardiology. 2016 Sep 1;218:188-95.
27. van den Berg YW, Osanto S, Reitsma PH, Versteeg HH. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood, The Journal of the American Society of Hematology. 2012 Jan 26;119(4):924-32.
28. Versteeg HH, Spek CA, Peppelenbosch MP, Richel DJ. Tissue factor and cancer metastasis: the role of intracellular and extracellular signaling pathways. Molecular Medicine. 2004 Jan;10(1):6-11.
29. Szotowski B, Antoniak S, Poller W, Schultheiss HP, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circulation Research. 2005 Jun 24;96(12):1233-9.
30. Lacroix R, Vallier L, Bonifay A, Simoncini S, Mege D, Aubert M, et al. Microvesicles and cancer associated thrombosis. InSeminars in thrombosis and hemostasis 2019 Aug 20. Thieme Medical Publishers.
31. Date K, Ettelaie C, Maraveyas A. Tissue factor-bearing microparticles and inflammation: a potential mechanism for the development of venous thromboembolism in cancer. Journal of Thrombosis and Haemostasis. 2017 Dec;15(12):2289-99.
32. Hu Z, Xu J, Cheng J, McMichael E, Yu L, Carson III WE. Targeting tissue factor as a novel therapeutic oncotarget for eradication of cancer stem cells isolated from tumor cell lines, tumor xenografts and patients of breast, lung and ovarian cancer. Oncotarget. 2017 Jan 3;8(1):1481.
33. Shaker H, Harrison H, Clarke R, Landberg G, Bundred NJ, Versteeg HH, et al. Tissue Factor promotes breast cancer stem cell activity in vitro. Oncotarget. 2017 Apr 18;8(16):25915.
34. Clouston HW, Rees PA, Lamb R, Duff SE, Kirwan CC. Effect of tissue factor on colorectal cancer stem cells. Anticancer Research. 2018 May 1;38(5):2635-42.
35. Ueno T, Toi M, Koike M, Nakamura S, Tominaga T. Tissue factor expression in breast cancer tissues: its correlation with prognosis and plasma concentration. British Journal of Cancer. 2000 Jul;83(2):164-70.
36. Bluff JE, Brown NJ, Reed MW, Staton CA. Tissue factor, angiogenesis and tumour progression. Breast Cancer Research. 2008 Apr 1;10(2):204.
37. Poon RT, Lau CP, Ho JW, Yu WC, Fan ST, Wong J. Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clinical Cancer Research. 2003 Nov 1;9(14):5339-45.
38. Hu Z, Cheng J, Xu J, Ruf W, Lockwood CJ. Tissue factor is an angiogenic-specific receptor for factor VIItargeted immunotherapy and photodynamic therapy. Angiogenesis. 2017 Feb 1;20(1):85-96.
39. Luo Y, Teng X, Zhang L, Chen J, Liu Z, Chen X, et al. CD146-HIF-1a hypoxic reprogramming drives vascular remodeling and pulmonary arterial hypertension. Nature Communications. 2019 Aug 7;10(1):1-7.
40. Yoshioka S, Fujiwara H, Higuchi T, Yamada S, Maeda M, Fujii S. Melanoma cell adhesion molecule (MCAM/CD146) is expressed on human luteinizing granulosa cells: enhancement of its expression by hCG, interleukin-1 and tumour necrosis factor-a. Molecular Human Reproduction. 2003 Jun 1;9(6):311-9.
41. Wang D, Duan H, Feng J, Xiang J, Feng L, Liu D, et al. Soluble CD146, a cerebrospinal fluid marker for neuroinflammation, promotes blood-brain barrier dysfunction. Theranostics. 2020;10(1):231.
42. Van den Berg YW, Van Den Hengel LG, Myers HR, Ayachi O, Jordanova E, Ruf W, et al. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proceedings of the National Academy of Sciences. 2009 Nov 17;106(46):19497-502.
43. Melnikova VO, Balasubramanian K, Villares GJ, Dobroff AS, Zigler M, Wang H, et al. Crosstalk between protease-activated receptor 1 and platelet-activating factor receptor regulates melanoma cell adhesion molecule (MCAM/MUC18) expression and melanoma metastasis. Journal of Biological Chemistry. 2009 Oct 16;284(42):28845-55.