Abstract
Aim: To evaluate the real-world safety and effectiveness of risankizumab in patients with inflammatory bowel disease (IBD), using clinical outcomes and faecal calprotectin (FCP) as markers of response.
Methods: A retrospective observational study was conducted at East Lancashire Hospitals NHS Trust. Patients with a confirmed diagnosis of IBD who were initiated on risankizumab were included. Clinical outcomes, FCP levels, and treatment tolerability were assessed. As FCP values were non-normally distributed (Shapiro–Wilk p < 0.05), the Wilcoxon signed-rank test was used to compare pre-and post-treatment values.
Results: Thirty-two patients who were started on risankizumab treatment were analysed. Paired FCP data were available for 14 patients. Median FCP levels decreased from 1368.5 µg/g (range 146–2100) before treatment to 368 µg/g (range 52–2100) after treatment. The Wilcoxon signed-rank test demonstrated a statistically significant reduction in FCP following risankizumab therapy (Z=–2.201, p=0.028). Overall, 87.5% of patients reported clinical improvement and continued treatment. No adverse effects were reported while on therapy.
Conclusions: From this study, we conclude that risankizumab appears to be an effective and well-tolerated treatment option for patients with IBD.
Keywords
Risankizumab, Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis, Biologic therapy, Real-world study, Monoclonal antibody, Gastroenterology
Introduction
Idiopathic Inflammatory bowel disease (IBD) is a common presentation in medical practice and carries a high disease burden both in hospital and the community. Two clinically distinct subtypes are recognised—Crohn's disease and Ulcerative colitis.
Patients with IBD often require maintenance immunosuppressive therapy in order to avoid repeated flare ups, steroid exposure, and the long-term complications of inflammatory intestinal damage. The options for medical therapy have expanded in recent years, driven by the expansion of classical biologic therapies and the emergence of so-called small molecule drugs.
Risankizumab is a monoclonal antibody which binds selectively to the p19 subunit of interleukin 23. It is one example of a new generation of IL-23 inhibitors which are purported to exhibit greater disease specificity through selective targeting of the p19 subunit. Risankizumab was approved by the National Institute for Health and Care Excellence for use in moderate to severely active Crohn's disease in May 2023 [1], and for use in moderate to severely active Ulcerative colitis in August 2024 [2].
The dosing for Crohn's disease is initially with 600 mg every 4 weeks for the first three doses, followed by a maintenance of 360 mg every 8 weeks [3]. The dosing for Ulcerative colitis is initially with 1.2 gm every 4 weeks for the first three doses, followed by a maintenance of 180 mg every 8 weeks or alternatively 360 mg every 8 weeks if inadequate disease improvement after induction regimen [3].
The advice from NICE is to discontinue treatment if there is no response after 24 weeks. The aim of this study was to evaluate the real-world safety and effectiveness of risankizumab in patients with inflammatory bowel disease (IBD), using clinical outcomes and faecal calprotectin (FCP) as markers of response.
Materials and Methods
This was a retrospective observational study conducted at East Lancashire Hospitals NHS trust. Data were collected from both the electronic health records and clinic note of patients with a diagnosis of inflammatory bowel disease, including Crohn’s and ulcerative colitis. Data were collected between May 2023 and June 2025.
Inclusion/exclusion criteria
Patients with a confirmed diagnosis of IBD who were initiated on standard risankizumab regime were included in the study and no additional exclusion criteria were applied.
Outcomes and follow up
The main outcome was treatment effectiveness, assessed by measurement of faecal calprotectin levels before and after initiation of Risankizumab. FCP measures were obtained at varying time points during routine clinical assessment. Clinical outcomes were determined based on these results as well as physician assessment after starting treatment.
Safety
Adverse effects were identified via review of electronic records and follow up clinic documentations.
Statistical analysis
Faecal calprotectin (FCP) values before and after risankizumab therapy were analysed as paired observations. Normality of the data was assessed using the Shapiro–Wilk test, which demonstrated non-normal distribution for both pre-treatment (p=0.019) and post-treatment values (p=0.003). As assumptions for parametric testing were not met, the Wilcoxon signed-rank test was used to evaluate within-patient changes in FCP. Descriptive statistics were reported as medians and ranges where appropriate. Statistical analyses were performed using IBM SPSS Statistics (version 31.0.1.0). A significance threshold of p<0.05 was applied. No power calculation was performed due to the retrospective nature of the study.
Results
Safety profile
No adverse events were reported during the study, indicating a favourable safety profile for risankizumab in this cohort.
|
Characteristic |
Category |
n |
% |
|
Total Patients |
- |
32 |
100 |
|
Gender |
Male |
15 |
46.9 |
|
|
Female |
17 |
53.1 |
|
Age (year) |
<20 |
1 |
3.1 |
|
|
20–29 |
9 |
28.1 |
|
|
30–39 |
7 |
21.9 |
|
|
40–49 |
4 |
12.5 |
|
|
50–59 |
3 |
9.4 |
|
|
60–69 |
4 |
12.5 |
|
|
70–79 |
2 |
6.3 |
|
|
80–89 |
2 |
6.3 |
|
Locality |
Central/East Lancashire |
18 |
56.3 |
|
|
North Lancashire |
3 |
9.4 |
|
|
South-East Lancashire |
11 |
34.4 |
|
Prior Biologic exposure |
≥2 biologics |
22 |
68.8 |
|
|
<2 biologics |
10 |
31.2 |
|
Patients with both pre- and post-treatment FCP |
Yes |
14 |
43.8 |
Treatment response and outcomes
Among the analysed 32 patients, we collated pre and post treatment FCP levels for 14 of them; the rest are currently still mid induction/maintenance treatment and so a post treatment FCP has not been applicable— a small minority have completed treatment but did not have a post treatment blood sample due to administrative issues.
Faecal calprotectin response
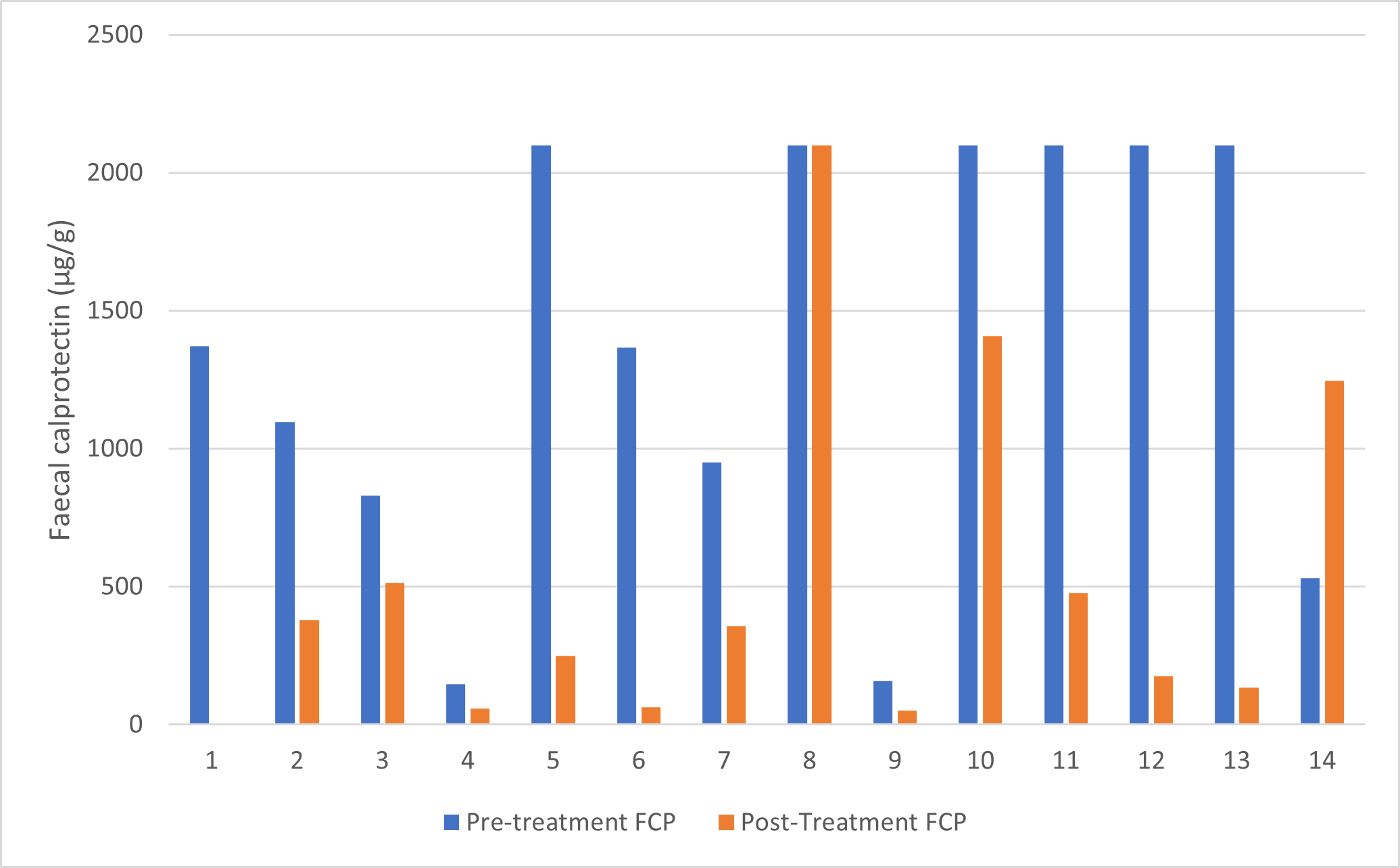
Paired pre- and post-treatment faecal calprotectin (FCP) values were available for 14 patients. Pre-treatment FCP values were highly skewed, ranging from 146 to 2100 µg/g, with a median of 1368.5 µg/g. Post-treatment values ranged from 52 to 2100 µg/g, with a median of 368 µg/g, demonstrating a substantial reduction following risankizumab therapy.
The Shapiro–Wilk test confirmed that both pre- and post-treatment FCP values were non-normally distributed (p<0.05). Therefore, the Wilcoxon signed-rank test was used to compare paired values. There was a statistically significant reduction in FCP levels after treatment (Z =–2.201, p=0.028).
A total of 11 out of 14 patients (79%) demonstrated a reduction in FCP (negative ranks), two showed an increase, and one had no change. This pattern was supported by histogram distributions, which showed a clear leftward shift toward lower post-treatment FCP values.
|
Patient |
Pre-Treatment FCP |
Post-Treatment FCP |
|
1 |
1371 |
>2100 |
|
2 |
1097 |
380 |
|
3 |
831 |
514 |
|
4 |
146 |
59 |
|
5 |
2100 |
250 |
|
6 |
1366 |
64 |
|
7 |
949 |
356 |
|
8 |
2100 |
2100 |
|
9 |
159 |
52 |
|
10 |
2100 |
1408 |
|
11 |
2100 |
478 |
|
12 |
2100 |
177 |
|
13 |
2100 |
134 |
|
14 |
530 |
1246 |
Figure 1. Graphical representation of pre and post treatment faecal calprotectin levels (µg/g) n=14.
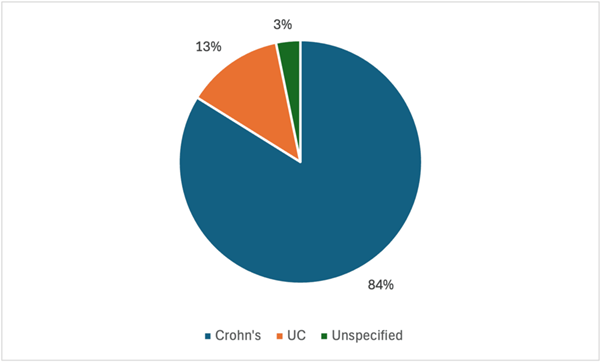
Figure 2. Distribution of inflammatory bowel disease by aetiology amongst patient cohort (n=32).
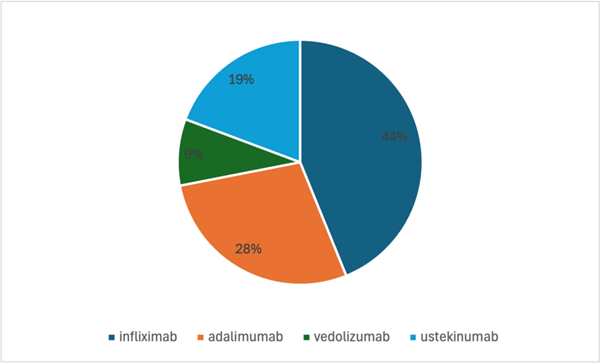
Figure 3. Frequency of biologic and immunomodulator treatments prior to being started on risankizumab (n=32).
Discussion
This single-centre retrospective observational study provides real-world evidence supporting the clinical utility of risankizumab (Ris) in a diverse cohort of patients, most of whom presented with mild to moderate Crohn’s disease. Notably, the majority of participants had previously exhibited inadequate response to multiple advanced therapies, often receiving combinations of up to four biologic or immunomodulatory agents without achieving remission [4,5]. This highlights the refractory nature of the cohort and underscores the need for effective alternative treatments. The primary objective was to evaluate the clinical and biochemical outcomes associated with risankizumab as an add-on therapy in routine clinical practice. Across the cohort, faecal calprotectin (FCP) levels, a well-established biomarker of intestinal inflammation [6], declined significantly in most patients. Specifically, 11 out of 14 patients (79%) demonstrated a reduction in faecal calprotectin following treatment, which was consistent with reported symptomatic improvement. This concordance aligns with prior studies demonstrating that reductions in FCP reliably predict clinical remission and mucosal healing in Crohn’s disease [7,8].
Importantly, no mandatory washout period was enforced between discontinuing prior biologic therapies and initiating risankizumab. This approach reflects real-world clinical practice, where delaying effective treatment can exacerbate disease activity and adversely impact outcomes [9]. Recent evidence supports the safety and efficacy of immediate switching between biologics in refractory patients, without compromising treatment response or increasing adverse events [10,11]. Our findings are consistent with these observations, as the absence of a formal washout period did not negatively influence outcomes.
The study also included three biologic-naïve patients, two of whom had pre- and post-treatment FCP data showing at least a threefold decrease following risankizumab initiation. All three reported symptomatic relief. These results counter concerns that risankizumab’ s efficacy depends on prior biologic exposure or synergistic effects and instead support its potential as an effective first-line biologic agent in appropriate clinical contexts [12,13].
All patients received the standard induction regimen of 600 mg IV every four weeks for three doses, as recommended by regulatory guidelines [14]. No serious adverse events were reported, corroborating risankizumab’ s favourable safety profile documented in both clinical trials and real-world studies [15,16].
Risankizumab has demonstrated robust efficacy and safety in moderate-to-severe Crohn’s disease across multiple pivotal trials. Phase 2 and 3 studies (ADVANCE, MOTIVATE, and FORTIFY) reported significant clinical and endoscopic remission rates, with up to 71% of patients maintaining remission at one year [17,18]. The SEQUENCE head-to-head trial found risankizumab superior to Ustekinumab in achieving endoscopic and steroid-free remission at 48 weeks [19]. Real-world data from the French GETAID cohort supported these findings, showing nearly half of a highly refractory population attained long-term steroid-free remission on risankizumab [20].
Together, these findings consolidate risankizumab’ s position as a promising therapy for patients with active Crohn’s disease, including those who have cycled through multiple biologics without success. Its efficacy in both biologic-experienced and biologic-naïve populations, combined with a manageable safety profile, supports its role within increasingly complex treatment paradigms [21].
Key insights and study limitations
The major strength of this study is its reflection of real-world clinical practice, providing insight beyond the controlled settings of randomized trials. The observed high rate of symptomatic and biochemical improvement underscores risankizumab’ s therapeutic potential in refractory Crohn’s disease.
However, limitations inherent to this retrospective, single-centre study include the small sample size, which may limit generalizability. Additionally, the lack of standardized endoscopic assessment and heterogeneity in baseline disease characteristics, previous treatments, and comorbidities introduce potential biases [22]. Despite these limitations, our findings align with broader clinical trial and registry data, reinforcing risankizumab’ s clinical utility.
Conclusion
This retrospective study suggests that risankizumab is an effective and well-tolerated treatment modality for patients with inflammatory bowel disease in a real-world setting. The majority of patients demonstrated clinical improvement in symptoms, accompanied by significant reductions in faecal calprotectin levels. No adverse events were reported in this cohort, which indicates a good safety profile of the drug. These findings support risankizumab as a valuable treatment option for complex IBD cases; however further multi-centered, larger studies are warranted to confirm the long-term efficacy and safety of risankizumab in the treatment of IBD.
Conflict of Interest
Nothing to declare.
Funding Statement
Nothing to declare.
Acknowledgements
Kit Patel – Pharmacist, East Lancashire Hospitals NHS Trust.
References
2. National Institute for Health and Care Excellence (NICE). Risankizumab for treating moderately to severely active ulcerative colitis | Guidance | NICE [Internet]. NICE; 2024 [cited 2025 Nov 1]. Available from: https://www.nice.org.uk/guidance/ta998.
3. Joint Formulary Committee. British National Formulary: risankizumab [Internet]. London: BMJ Group and Pharmaceutical Press; [cited 2025 Nov 1]. Available from: https://bnf.nice.org.uk/drugs/risankizumab/.
4. Barberio B, Gracie DJ, Black CJ, Ford AC. Efficacy of biological therapies and small molecules in induction and maintenance of remission in luminal Crohn’s disease: systematic review and network meta-analysis. Gut. 2023 Feb 1;72(2):264–74.
5. Rispo A, Calabrese G, Testa A, Imperatore N, Patturelli M, Allocca M, et al. Hocus pocus: the role of hand-held ultrasonography in predicting disease extension and endoscopic activity in ulcerative colitis. Journal of Crohn's and Colitis. 2023 Jul 1;17(7):1089–96.
6. Gaya DR, Lyon TD, Duncan A, Neilly JB, Han S, Howell J, et al. Faecal calprotectin in the assessment of Crohn's disease activity. Qjm. 2005 Jun 1;98(6):435–41.
7. Takeda T, Nishimata N, Fujioka S, Tsuruoka N, Furuta Y, Takahashi H, et al. Fecal Calprotectin is a Useful Biomarker for Defining Small Bowel Endoscopic Remission in Crohn’s Disease Without Active Colonic Lesions: A Prospective Multicenter Study from the IBD-Quality Team. Inflammatory Bowel Diseases. 2025 Jun 30: izaf138.
8. Jain AV, Gopal S, Shetty AJ, Shenoy S, Tantry BV, Unnikrishnan B, et al. Predictive accuracy of fecal calprotectin in assessing clinical activity and disease severity in patients with Ulcerative Colitis and Crohn’s disease. BMC Gastroenterology. 2025 Jun 4;25(1):429.
9. Lv H, Li HY, Zhang HN, Liu Y. Delayed diagnosis in inflammatory bowel disease: Time to consider solutions. World Journal of Gastroenterology. 2024 Sep 21;30(35):3954.
10. Bressler B. Is there an optimal sequence of biologic therapies for inflammatory bowel disease? Therapeutic Advances in Gastroenterology. 2023 Apr; 16:17562848231159452.
11. Taylor F, Bartalucci G, Gleave C, Dobson L, Bodger K, Dodd S, et al. Real-world outcomes from the UK IBD Registry on second-line biologic therapy following anti–TNF exposure in Crohn’s disease: results from the BISCUITS study. Therapeutic Advances in Gastroenterology. 2025 Jul; 18:17562848251352446.
12. Lusetti F, D'Amico F, Allocca M, Furfaro F, Zilli A, Fiorino G, et al. Positioning risankizumab in the treatment algorithm of moderate-to-severe Crohn's disease. Immunotherapy. 2024 May 14;16(9):581–95.
13. Johnson AM, Loftus Jr EV. Risankizumab to treat moderately to severely active Crohn’s disease in adults: an evaluation of trials and data. Expert Review of Gastroenterology & Hepatology. 2023 Dec 2;17(12):1169–83.
14. Feagan BG, Panaccione R, Schreiber S, Loftus Jr EV, Peyrin-Biroulet L, Arai T, et al. Effect of Risankizumab Induction and Maintenance Therapy on the Rate of Hospitalization in Patients with Crohn’s Disease. Gastro Hep Advances. 2025 Jan 1;4(4):100603.
15. Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. The Lancet. 2018 Aug 25;392(10148):650–61.
16. Clement B, De Felice K, Afzali A. Indications and safety of newer IBD treatments in the older patient. Current Gastroenterology Reports. 2023 Jul;25(7):160–8.
17. Atreya R, Ferrante M, Panaccione R, Feagan B, Shchukina O, Jairath V, et al. Risankizumab Is Associated With Normalization of Biomarkers in Patients With Crohn’s Disease: Results From the Phase 3 ADVANCE, MOTIVATE, and FORTIFY Studies. Journal of Crohn's and Colitis. 2025 Apr;19(4): jjae164.
18. Colombel JF, Schreiber S, D’Haens G, Rizzo J, Kligys K, Griffith J, et al. Risankizumab induction therapy achieves early symptom improvements that are associated with future clinical and endoscopic outcomes in Crohn’s disease: post hoc analysis of the ADVANCE, MOTIVATE, and FORTIFY phase 3 studies. Journal of Crohn's and Colitis. 2024 Jun 1;18(6):818–27.
19. Peyrin-Biroulet L, Chapman JC, Colombel JF, Caprioli F, D’Haens G, Ferrante M, et al. Risankizumab versus ustekinumab for moderate-to-severe Crohn’s disease. New England Journal of Medicine. 2024 Jul 18;391(3):213–23.
20. Fumery M, Defrance A, Roblin X, Altwegg R, Caron B, Hébuterne X, et al. Effectiveness and safety of risankizumab induction therapy for 100 patients with Crohn's disease: A GETAID multicentre cohort study. Alimentary Pharmacology & Therapeutics. 2023 Feb;57(4):426–34.
21. Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. ECCO guidelines on therapeutics in Crohn's disease: medical treatment. Journal of Crohn's and Colitis. 2020 Jan 1;14(1):4–22.
22. Fantini MC, Fiorino G, Colli A, Laharie D, Armuzzi A, Caprioli FA, et al. Pragmatic trial design to compare real-world effectiveness of different treatments for inflammatory bowel diseases: the PRACTICE-IBD European consensus. Journal of Crohn's and Colitis. 2024 Aug;18(8):1222–31.