Abstract
Background: Inflammatory bowel disease is a chronic relapsing and remitting inflammation of the bowel. Tumour necrosis factor α - antagonists are safe and effective in the treatment of inflammatory bowel disease. Indications and outcomes with consecutive anti-tumour necrosis factor agents, although often used, are not clear. Since data for this treatment choice is scarce, we set out to evaluate the use of consecutive anti-tumour necrosis factor agents in patients with inflammatory bowel disease.
Method: A national registry established by The South African Gastroenterology Society was used for retrospective data extraction in patients with consecutive anti-tumour necrosis factor agent use. Demographic, clinical details, treatment outcomes and adverse events were documented.
Results: Eight-six (7.5%) of 1150 patients received consecutive tumour necrosis factor-antagonists. There were 41 (48%) patients with Crohn’s disease and 45 (52%) with ulcerative colitis. Gender distribution was equal with 45 (52%) male and 41 (48%) female patients. Patients failed the first anti-tumour necrosis factor agent over 30 months, but remission rates improved with second agent. Immunomodulator therapy had no effect of anti-tumour necrosis agent discontinuation rates. Adalimumab treatment had higher rate of dose escalation/switching as well as adverse events compared to infliximab. Most patients remained in clinical remission except a few with CD who required surgery.
Conclusion: Using a second anti-tumour necrosis factor agent when the first agent failed is often necessary in inflammatory bowel disease. Although cost-effective, this strategy lacks clarity. Patient selection is crucial and therapeutic drug monitoring should be central in that decision. Adalimumab is associated with higher rates of dose escalation and a worse side-effect profile. Patients with UC switched earlier compared to CD.
Keywords
Crohn's disease, Ulcerative colitis, TNF-antagonists
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are chronic inflammatory diseases of the intestine with a relapsing and remitting course. Maintenance therapy in the form of immunomodulators and aminosalicylates are often required to prevent disease flares. Despite adequate doses of maintenance therapy, patients still experience flares requiring escalation of treatment with biological therapy [1,2].
Following failure of conventional therapy in inflammatory bowel disease (IBD), most treatment guidelines still recommend TNFα-antagonists as first line biologic therapy, with exceptions. The efficacy of anti-TNFα agents in IBD are well established and with the advent of biosimilars, they offer significant cost-savings [3,4].
TNFα-antagonists (infliximab, adalimumab, golimumab and certolizumab) were the first biological drugs on the market and have been extensively used for the treatment of IBD over the last 2 decades. Their mechanism of action is by blocking the binding of TNFα with its receptor, TNFR, and thus preventing the downstream inflammatory cascade [5]. Although the efficacy of anti-TNFα agents is well established, outcome data longer than 5 years with this treatment in lacking. A recent retrospective study on long-term anti-TNFα treatment persistence, showed that 52% of patients remain on therapy after 2 years and only 18% after 10 years [6]. Furthermore, it is not clear whether the different anti-TNFα agents have similar clinical efficacy, as they differ in molecular constructs, the dose used, and the route of administration differs. It also appears that the mechanism of action of anti-TNFα agents is not solely through TNFa neutralisation, but through a Fc mediated anti-inflammatory mechanism [7] .
Treatment with anti-TNFα agents is often discontinued for 2 main reasons among others: loss of response (LOR) and adverse events. Primary non-response (PNR) is reported to be as high as 20-40% in clinical trials and 10-20% in real life cohorts; and secondary loss of response (SLR) due to immunogenicity and increased anti-TNF clearance in 13-26% of patients over a 12 month period [8]. Adverse event rates vary widely among the many anti-TNFα studies. Adverse events leading to discontinuation of therapy was up to 20% for CD and 7% for UC in a recent meta-analysis [9]. Adverse event rates for adults with IBD were higher with adalimumab treatment compared to infliximab in some studies, but not in others [10,11].
The practical management of PNR is not straightforward. Data suggests that if the anti-TNF drug level is low following induction failure, it may be worthwhile to rapidly escalate the dose of the anti-TNFα agent to achieve clinical remission. This strategy is supported by data from the CLASSIC-1 and ULTRA-2 studies [12,13]. However, if the anti-TNFα drug level is adequate, there is no good scientific evidence to simply escalate the dose and switching to a different anti-TNF agent may be useful, although controlled studies are lacking and overall response rates to this strategy are generally within the region of 50-60% [14].
SLR is defined as the recrudescence of IBD symptoms with documented evidence of active disease. As immunogenicity is regarded an important reason for SLR, current recommendation is that an immunomodulator be co-prescribed with TNFa-antagonist treatment [15]. As the majority (70%) of patients with SLR are due to subtherapeutic drug trough concentration levels, therapeutic drug monitoring (TDM) should be a normal part of clinical practice [16]. Good evidence exists for both proactive and reactive TDM to ensure clinical remission and extend the lifespan of the anti-TNF agent [17].
Current treatment guidelines recommend that if a patient on treatment with a TNFα-antagonist experiences SLR with a normal therapeutic drug level, the patient should be switched to another class of biologic. If the patient has a subtherapeutic drug level however, treatment with the same anti-TNF can be optimised by increasing the dose or reducing the dosing frequency. Furthermore, with the arrival of anti-TNFα biosimilars at the end of 2020 in South Africa, the treatment cost has reduced by 20-30%, making tandem TNFα-antagonists treatments the preferred option.
Treatment with biologics in sub-Saharan Africa is not a straightforward decision and more often dependant on drug availability, cost, patient preference, and funder rules. Newer classes of biologics are simply not available everywhere [18]. Often, TNFa-antagonists are the only available biologic agents, legitimising consecutive anti-TNF agent use.
Adverse event rates and intolerance is another important reason for switching or discontinuing anti-TNF therapy [19]. Quoted adverse event rates, including serious infections, leading to discontinuation of therapy ranges from 19 – 25% in published literature [6,9,20].
We conducted a retrospective study from a national IBD registry on the use of a second TNFα- antagonist in a real world setting. The primary endpoint was the length of treatment with the first anti-TNFα agent and the reason(s) for switching to a second anti-TNFα agent. Comparisons were made between CD and UC, the anti-TNFα drugs used, the duration of treatment as well as concomitant IBD therapies used. Adverse events rates were reported.
Methods
Ethical issues
The South African Gastroenterology Society (SAGES) has established an IBD registry in 2016. The objective was to create a national database of all patients commenced on a biologic for IBD. A dedicated research questionnaire was drafted to allow for uniformity and includes pertinent aspects of IBD care. Patient details as well as the treatment progression and adverse events are recorded in this registry. SAGES obtained ethics clearance (SAGES BIOL 001) from PharmaEthics to run this registry. The treating gastroenterologist as well as the patient signed informed consent to participate in the registry. This study was approved by the Human Ethics Research Committee (HREC) of PharmaEthics. All data has been de-identified.
Patient details
Using the registry, all patient with CD and UC who received a second anti-TNFα agent and who were eligible for inclusion into the study were identified. Data was retrieved from the database from August 2016 to December 2021, but patients who started on anti-TNF prior to 2016 were also included. Data collected included: demographics details including age and gender; smoking history; disease location and extent; disease duration; concomitant and prior medication history; previous bowel surgery; indication for anti-TNF commencement and reason for subsequent discontinuation/switching as well as all adverse events. Specific laboratory parameters as well as disease activity scores (Mayo scores for UC and CDAI for Crohn’s disease) were recorded. All patient outcome were documented.
TNFα-antagonists
The anti-TNF agents used in this study include infliximab, adalimumab and golimumab. As biosimilars were generally unavailable in the country during the study period, they are not included. Standard maintenance doses implied in this study are infliximab 5 mg/kg every 8 weeks, adalimumab 40 mg every other week and golimumab 50 – 100 mg every 4 weeks. Dose escalation was defined as infliximab 10 mg/kg every 4-6 weeks, adalimumab 40 mg weekly and golimumab 100 mg every other week.
Treatment
The decision to initiate/escalate/switch between anti-TNFα agents and the choice of the agent used was solely that of the treating physician. Generally, initiating/escalating biologic therapy followed a step-up approach. Anti—TNFα therapy was initiated once patients failed conventional therapy. All patients were commenced on standard induction doses. Patients who experienced loss of response on an anti-TNFα agent despite adequate drug levels were switched to another anti-TNFα agent, while those with inadequate drug levels were dose optimised with increasing the dose of the same agent. Failure to respond was defined as having (i) persistent symptoms – increased or worsening diarrhoea, abdominal cramping and blood/mucous in the stool (UC); (ii) raised faecal calprotectin above normal threshold of 250 mcg/mg and (iii) high disease activity score – a Mayo score ≥ 8 for UC and CDAI ≥ 220 in CD.
Statistical analysis
Categorical variables are represented as percentages. Non-categorical data were calculated as median and interquartile range. Statistical comparisons were examined with Chi-square test (or Fisher-Freeman-Halton Exact tests), Student’s T-test and one-way analysis of variance (ANOVA). Cox regression analysis was used for univariate and multivariate analysis to identify risk factors of significance. Pearson’s Chi-square goodness-of-fit test was utilized to compare hypothetical assumptions to the dataset. Odds ratios were calculated to compare treatment groups. The results were considered significantly different at p<0.05. Statistical analysis was performed using IBM SPSS statistical package.
Results
The total number of 1150 patients were in the registry - 764 (66.4%) with CD and 387 (33.6%) with UC. The gender distribution in the total population was equal (51% female, 49% male).
Eighty six (7.5%) patients received a second ant-TNFα agent. There was equal distribution between CD (41) and UC (45) in the study population. The gender distribution in the study population was equal with 45 (52.3%) male and 41 (47.6%) female patients.
Demographic details
Demographic and clinical disease characteristics are presented in Table 1 with a further breakdown into CD and UC. The age and gender rates were equal between the groups. The majority, two-thirds of patients never smoked with a further 13% giving up smoking after diagnosis. A small minority (15%) continued to smoke with no difference in the rate of smokers between CD and UC. The smoking status of 1 patient with UC was unknown. Thirty-one percent of patients with CD had previous surgery: colectomy with end-ileostomy in 4 (9.7%) patients and permanent colostomy in 3 (7.3%). One patient with UC had a total colectomy and ileo-pouch anal anastomosis. Immunomodulator therapy was generally low in the entire cohort with only a third of patients on it. However, IM use was highest in CD at 62%, while combination therapy of 5-ASA and IM was highest in UC at 51%. A third of patients was on oral corticosteroids at the time of starting anti-TNF therapy; patients with UC twice as many as those with CD.
| Total n=86 |
CD n=41 |
UC n=45 |
|
|---|---|---|---|
| Male/Female | 45/41 | 24/17 | 21/24 |
| Median age (in years) | 42.4 (IQR 30-53) | 42.5 (IQR 32-51) | 45 (IQR 29-53) |
| Median age at diagnosis (in years) | 32.8 (IQR 19-44) | 31.3 (IQR 18-40) | 34.2 (IQR 23-45) |
| Median time from diagnosis to starting first biologic (in months) | 36 (IQR 12-48) | 36 (IQR 12-84) | 37 (IQR 15-74) |
| Smoking: | |||
|
59 (69%) | 27 (66%) | 32 (71%) |
| 11 (13%) | 6 (15%) | 5 (11%) | |
| 14 (16%) | 7 (17%) | 7 (16%) | |
| Disease duration (in years) | 6 (IQR 4-12) | 8 (IQR 5-14) | 6 (IQR 4-9) |
| Disease characteristics: | |||
| Age distribution: | |||
|
9 (22%) | ||
| 22 (54%) | |||
| 7 (17%) | |||
| 3 (7%) | |||
| Location: | |||
|
12 (29%) | ||
| 11 (27%) | |||
| 18 (44%) | |||
| Behaviour: | |||
|
19 (46%) | ||
| 7 (17%) | |||
| 4 (10%) | |||
| Perianal disease | 11 (27%) | ||
| Fistula | 13 (32%) | ||
| Previous surgery | 12 (29%) | ||
| Disease extent: | |||
|
0 | ||
| 16 (42%) | |||
| 21 (55%) | |||
| Concomitant medication | |||
|
27 (31%) | 20 (49%) | 7 (16%) |
| 14 (16%) | 2 (5%) | 12 (27%) | |
| 34 (40%) | 11 (27%) | 23 (51%) | |
| Corticosteroids at start of anti-TNF | 28 (33%) | 10 (24%) | 18 (40%) |
TNFα-antagonist use
Table 2 gives representation of the distribution of TNFα-antagonists used as first or second agent in this study. Forty-four (51.1%) patients started infliximab as the first anti-TNFα agent and 40 (46.5%) were started on adalimumab. Only 2 (2.3%) patients with UC started golimumab as first line anti-TNFα agent.
| Infliximab | Adalimumab | Golimumab | ||
|---|---|---|---|---|
| First anti-TNF agent | CD 41 | 22 | 19 | 0 |
| UC 45 | 22 | 21 | 2 | |
| Total 86 | 44 (51%) | 40 (47%) | 2 (2%) | |
| Second anti-TNF agent | CD 41 | 19 | 22 | 0 |
| UC 45 | 19 | 21 | 5 | |
| Total 86 | 38 (44%) | 43 (50.0%) | 5 (6%) |
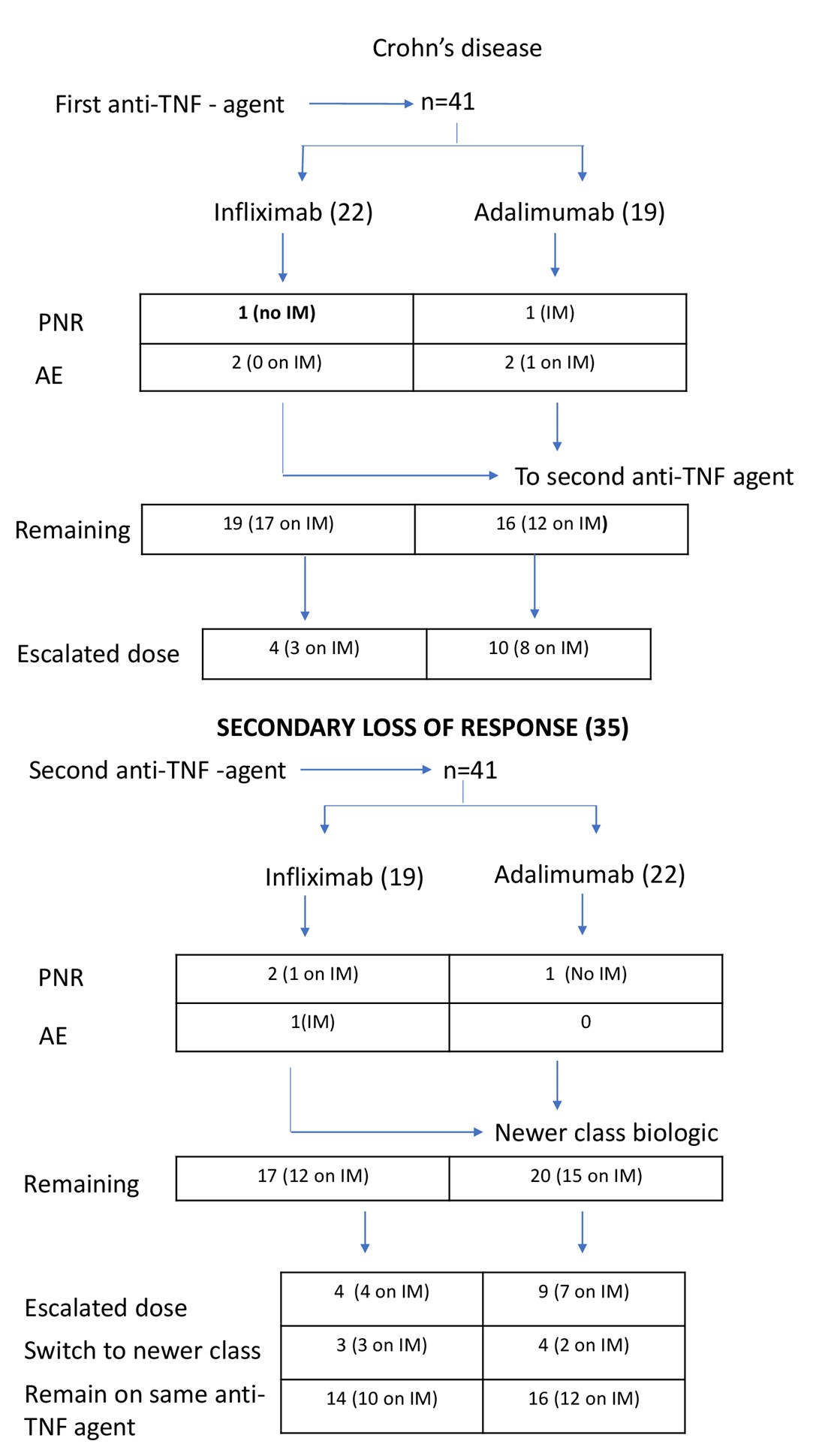
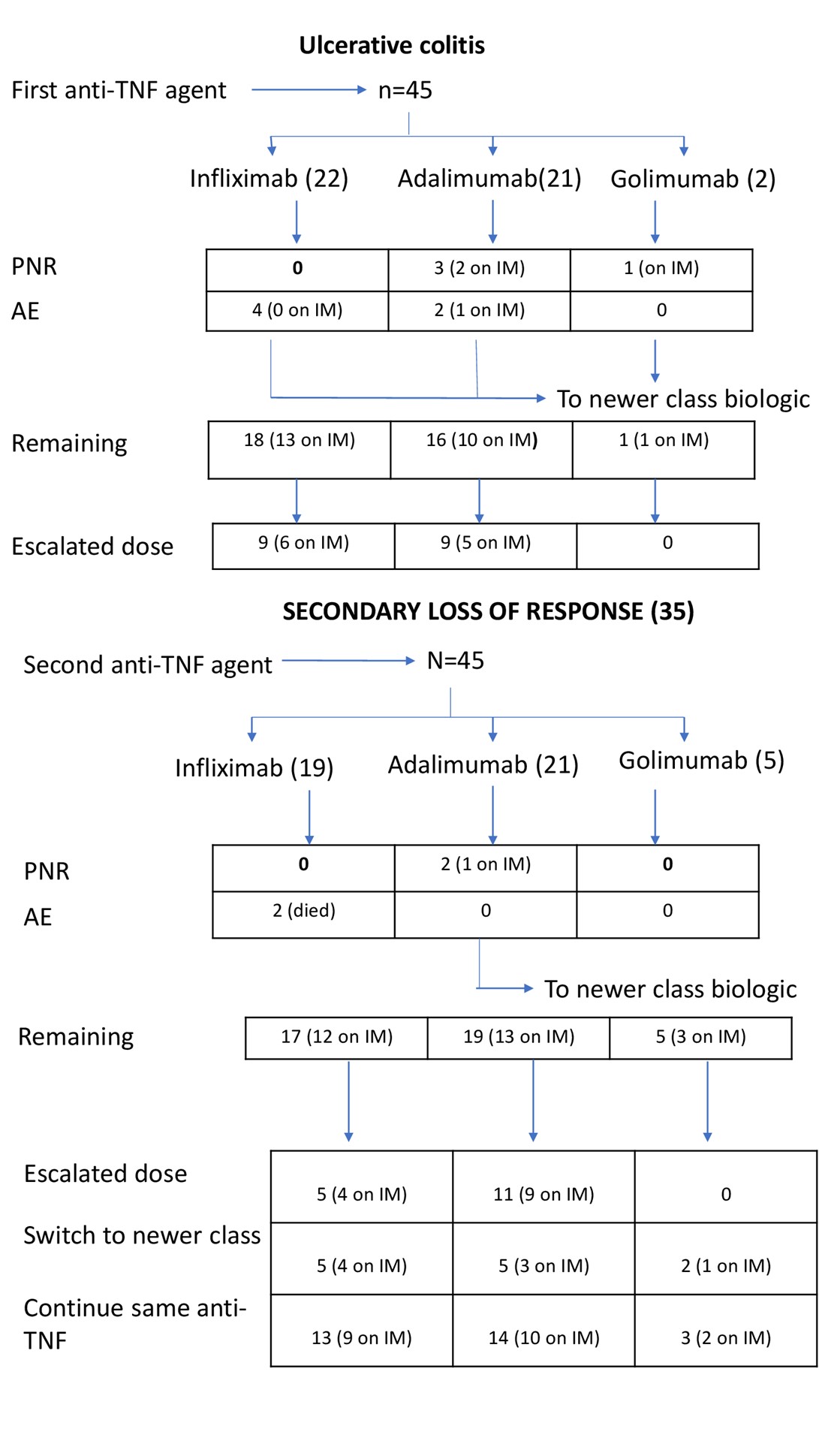
Infliximab was used as second anti-TNF agent in 38 (44.2%) patients, adalimumab in 43 (50.0%) patients and golimumab in 5 (5.8%) patients (Table 2). The was no difference in the proportional distribution of infliximab and adalimumab as first, or second line agent between CD and UC. Figures 1 and 2 give further breakdown of the treatment algorithms followed in this study.
The time from diagnosis to commencement of first anti-TNF agent for the entire group was 36 months and was similar between CD and UC (36 vs 37 months). Patients remained on the first TNF antagonist for a median of 24 months.
Primary non-response
First anti-TNF agent: Six (7.0%) patients failed induction therapy, 4 patients on adalimumab and 1 each on infliximab and golimumab. Being on an IM had no influence on PNR rate as 4 patients were on an IM.
Second anti-TNF agent: Five (5.8%) experienced SLR, 3 on adalimumab and 2 on infliximab. Three patients were not on an IM.
PNR for the entire cohort was 12.8% with no difference between disease entity or anti-TNF agent used. None of the patients who failed the first anti-TNF agent experienced PNR with the second anti-TNF agent.
Figures 1 & 2 give representations of the patients started on treatment, their PNR, SLR, adverse events rates, IM therapy and treatment withdrawal.
Secondary loss of response
First anti-TNF agent: Seventy (81.4%) patients remained on the first anti-TNF agent for a median duration of 21 months until they switched to the second anti-TNF agent. The number of patients in each disease category was equally matched with 35 patient each in CD and UC. Thirty seven (52.9%) were on infliximab, 32 (45.7%) were on adalimumab and 1 (1.4%) on golimumab.
The majority [29 of 35 patients (82.8%)] of patients with CD were on an IM, while only 24 (68.5%) of the 35 patients with UC were on IM therapy. Of the 13 (35.1%) patients on infliximab requiring dose escalation, 9 were on IM therapy. Nineteen (59.4%) of the 32 patients on adalimumab required dose escalation of which 13 were on combination therapy with an IM. Of all the patients requiring dose escalation, only 10 (30.3%) of the 33 patients were not on an IM.
SLR was the main reason for switching to a second anti-TNF agent. Drug and antibody testing were unavailable at the inception of the registry and was measured in only 40 of the 70 patients. Generally, 3 trends were observed during these measurements: (i) low to undetectable drug level (0.5 – 5.0) with high (>25.0) drug antibody level in the majority (57.5%), (ii) normal to high (>7.54) drug level with no measurable (<2.50) antibody levels (27.5%) and (iii) subtherapeutic (5.0 – 7.54) drug levels with no measurable antibody levels in the minority (15.0%). Three patients with CD developed new complications while on the first anti-TNF agent: one developed spontaneous colonic perforation, one required small bowel resection and one developed new perianal fistula.
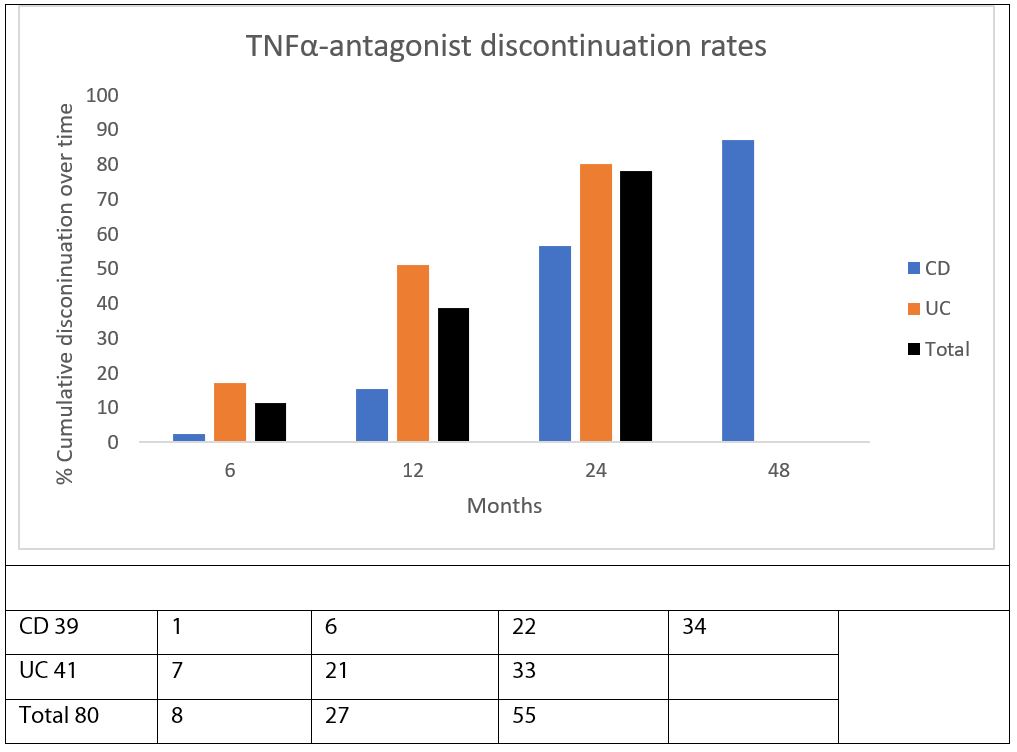
The switch rate to the second anti-TNF agent between the two disease entities were markedly different as seen in Figure 3. By 24 months 80% of UC patients were switched while for patients with CD the 80% switched rate was only reached at 48 months. All patients with UC had switched to the second anti-TNF agent by 31.1 months but a very small proportion of CD patients (14.3%) remained on the first anti-TNF for a period of 56.6 months.
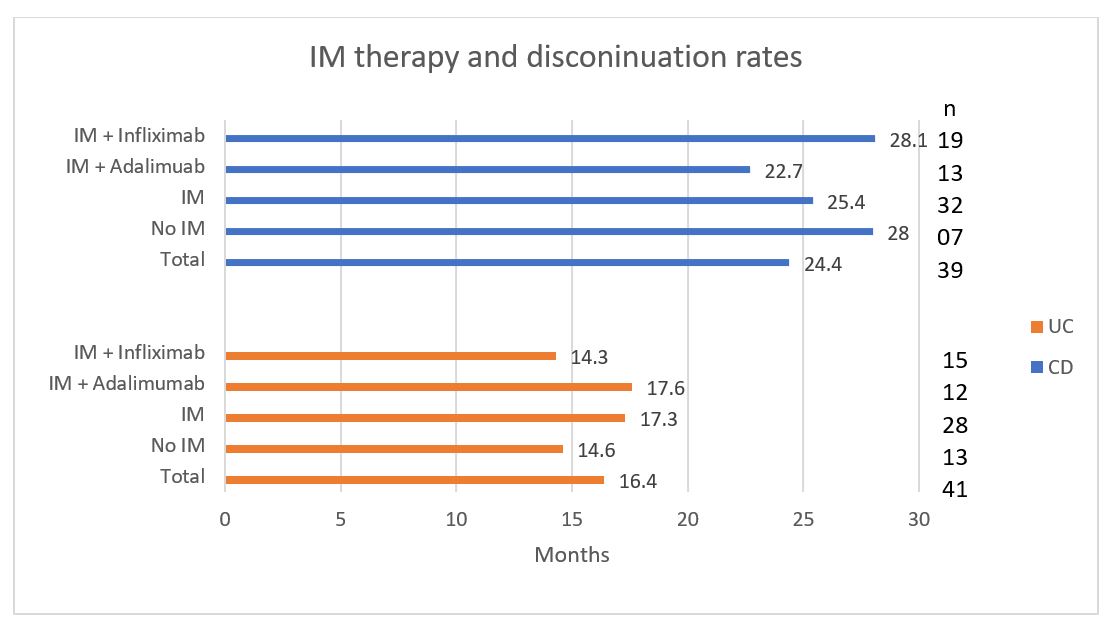
Second anti-TNF agent: Seventy-eight (90.6%) patients remained on the second anti-TNF agent. Time to switch from the first to the second anti-TNF was a median of 16.4 months for UC, while it was 24.4 months for CD (Figure 4).
From Figure 4 it is clear that IM therapy had no significant impact on withdrawal rates from an anti-TNF agent for CD (p=0.078), or for the UC (p=0.346). Similarly, there was no statistically significant difference in the switch rates between infliximab (p=0.481) and adalimumab (p=0190) on IM therapy.
The number of patients in each disease category was equal with 37 CD and 41 UC. Thirty-four (43.6%) patients were on infliximab, 39 (50%) on adalimumab and 5 (6.4%) on golimumab. There was no statistically significant difference for IM therapy use between CD (72.9%) and UC (68.3%). Similarly, no significant difference was noted with IM use comparing infliximab and adalimumab. Of the 9 (26.4%) patients on infliximab requiring dose escalation, 8 were on IM therapy. For adalimumab, 20 (51.3%) patients required dose escalation of which 16 were on IM therapy. Of the 29 patients in total who required dose escalation, 24 (82.8%) patients were on an IM.
For the entire cohort, 50 (64.1%) patients remained on the second anti-TNF agent until the end of the study, 17 (8.9%) patients switched to newer class biological agents and 11 (14.1%) were lost to follow-up. Drug and antibody testing was mainly performed in patients who failed the second anti-TNF and switched to newer class agents. Most of these patients (73.7%) had low/undetectable drug levels (<2.50), while the rest had therapeutic drug levels (>8.0). A further breakdown in disease specific categories showed 30 (81.1%) of CD patients remaining in the study to the end of the study for a mean of 24.0 months, while only 7 (18.9%) were switched (after mean 24.0 months) to newer class biologic. For UC, 19 (46.3%) patients remained on the study to the end for a mean period of 20.4 months, while 12 (29.3%) patients switched to a newer biological (after a mean period of 14.5 months) and 11 (26.8%) were lost to follow-up.
Figures 1 & 2 give representations of the patients started on treatment, their PNR, SLR, adverse events rates, IM therapy and treatment withdrawal.
The use of infliximab or adalimumab as a first agent of choice and associated risk of dose escalation was also independently assessed. The risk of dose escalation was similar for adalimumab or infliximab (27% vs 22%) as a first anti-TNF agent in patients with UC {OR=1.60, 95% CI [0.48, 5.34], p>0.05}. However, once switched to a second anti-TNF, patients on adalimumab had a 3-fold (27% vs 9%) risk of dose escalation compared to infliximab {OR=5.33, 95% CI [1.32, 21.53], p<0.05}.
Patients with CD started on adalimumab as first anti-TNF agent had a 3-fold (55% vs 17%) increased risk to escalate therapy compared to infliximab {OR=5.28, 95% CI [1.29, 21.51], p<0.05}. Once switched to a second anti-TNF agent, patients on adalimumab had a 2-fold risk (43% vs 27%) of dose escalation compared to infliximab {OR=4.25, 95% CI [1.09, 16.61], p<0.05}.
Adverse events
First anti-TNF agent: Ten (11.6%) patients experienced adverse events significant to switch to a different anti-TNFα agent. For the 6 patients who were on infliximab, none were on IM therapy. Two of the 4 patients on adalimumab were on IM therapy. Adverse events include pulmonary TB, pneumonitis, intractable arthralgia, skin eruption, peripheral neuropathy, serious infusion reactions and intolerance.
Second anti-TNF agent: Only 1 patient with CD on infliximab and IM therapy developed severe pneumonitis requiring treatment withdrawal. Two patients with UC on infliximab and IM therapy died during the study period: 1 from a myocardial infarction and 1 from complications of a haematological malignancy. This was not considered a drug-related serious adverse event.
The total adverse event rate was 12.8%. All patients were in clinical remission at time of switching although the median drug levels (2.54) were very low with high to very high (>25.0) antibody levels. No adverse events were reported for golimumab, but this is believed to reflect the low number of patients on this agent.
Risk factor prediction
These are patients with aggressive disease at high risk of disease progression and need for escalation of therapy. We assessed the usual risk factors (age, gender, smoking status, disease extent and concomitant medication use) associated with worse outcome in IBD for significance of response to anti-TNF therapy. Using Fisher-Freeman-Halton Exact tests, ANOVA, and Cox regression analysis, no single risk-factor was identified that was associated with a correlation to failing a specific anti-TNF agent, in either UC or CD.
Pearson’s Chi-squared goodness-of-fit tests, with a Yate’s correction, was run to assess the impact of escalating maintenance doses to achieve clinical remission. While the p-values show that escalation of the maintenance dose does not have an impact on the patient’s likelihood to move on to another biologic, the numbers do suggest that further data should be collected. As we discuss below, a major limitation of this study is the relatively small sample size.
The same goodness-of-fit tests were also run to evaluate likelihood to achieve remission on a consecutive anti-TNF agent. The analysis suggests that switching treatments has a statistically significant impact (p<0.001) on a patient’s likelihood to maintain therapy and remain in clinical remission.
Discussion
Switching from one anti-TNF agent to another when the first agent fails in patients with IBD appears counterintuitive. However, this was common practice in the era before the availability of newer classes biological drugs, and even today to a lesser extent. In developing countries, this is often a reality for patients with IBD and limited healthcare funding. Even so, the use of a second TNFa-antagonist was rare and occurred in only 8% of all patients in this study.
PNR is a reality for about 30% of IBD patients starting on an anti-TNF agent for the first time [21]. Furthermore, patients with PNR to the first anti-TNF agent is less likely to respond to a second anti-TNF agent [22]. In this registry study, PNR was reported in 6.9% of the entire IBD population, which is lower than shown in previous studies. There is no ready explanation for this low rate, but it may be that, because of cost and accessibility, selection bias could be a factor. Gisbert et al. showed that a second anti-TNF agent following PNR with first anti-TNF agent may be a reasonable option, although the efficacy of the second anti-TNF agent may be short-lived [23]. However, in this study we found that all these patients responded favourably to retreatment with another anti-TNF agent. The reasons for PNR are often multifactorial and generally not linked to immunogenicity as is the case with secondary loss of response and as such a trial with a second anti-TNF is not unreasonable. It is noteworthy that none of the UC patients who failed induction therapy was on infliximab.
Only 5 patients experienced PNR with the second anti-TNF agent, none of which experienced PNR with first anti-TNF agent. Most (4 of 5) patients were on IM therapy and both patients with UC who failed induction were on adalimumab. Limited data seems to suggest that for UC, infliximab is superior to adalimumab in inducing clinical remission, although maintenance of remission is equal [10,24]. Although the numbers are small, data from this study corroborate the above and we suggest that infliximab may be a better choice as induction agent in patients with UC. For the entire cohort, PNR was still low at 15.1%, but in keeping with established real world data.
Secondary loss of response is a common cause for the discontinuation of biologic therapies as a group, and TNFα-antagonists may be more prone to this than other classes of biologics. As immunogenicity is often a cause, the combined use of IM therapy is strongly recommended together with anti-TNFα agents in the maintenance phase in IBD. Although recent communication suggests that pharmacodynamics may play a bigger role over immunogenicity in SLR [25]. Secondary loss of response (SLR) is estimated as high as 50% over a 12 month period [21]. A recent meta-analysis and systematic review calculated the annual risk of SLR at 20.3% and 13.3% per patient year, respectively [8]. In this study, the rate of withdrawal from the first anti-TNF agent was very different for CD and UC with a much rapid withdrawal for UC. By the end of 12 months, only 15.4% in the CD group had switched to the second anti-TNF agent, while 51.2% had switched in the UC group. By 24 months, 80.1% of UC patients had already switched while 87.2% in the CD group had switched by 48 months. This data is in keeping with results recently published by Blesl et al. in a long-term persistence study [6]. Treatment persistence was longer at 39 months for CD compared to only 13 months for UC. They further noted that males with CD had longer treatment persistence than females but showed no gender difference in UC regarding persistence of treatment. Our study showed no gender predominance with respect to length of treatment or withdrawal of treatment for either UC or CD.
To prevent immunogenicity and subsequent secondary loss of response, combination therapy with an IM is usually recommended together with an anti-TNF agent. Concurrent IM therapy in this setting has shown to improve pharmacokinetics of the anti-TNF agent, reduce anti-drug antibody formation and increasing the serum concentration of the anti-TNF agent leading to higher rates of clinical remission [26]. However, the concomitant use of IM therapy had no significant influence on discontinuation rates in either CD or UC. Furthermore, it had no material effect on the anti-TNF agent used in this study. We, therefore, agree with Alsoud et al. that drug pharmacology may be more important in anti-TNF failure than appreciated until recently. We still strongly believe that combination therapy with an IM will be part of the current treatment regimen together with an anti-TNF agent as immunogenicity is a significant driver of adverse events.
Adverse event rates and intolerance to anti-TNF therapy was another reason for switching or discontinuation of anti-TNF therapy. Our study had a discontinuation rate of 12.8% due to serious adverse events. For all adverse events reported during the study, the standout feature was that the majority (7 out of 10) were not on an IM. Six of the 10 patients were female, but this was not statistically significant. Quoted adverse event rates leading to discontinuation of therapy ranges from 19 – 25% in published literature [6,9,20,27]. Adverse events are primarily driven by immunogenicity, however there is also an increased risk of serious infection [28]. With tuberculosis (TB) being endemic in South Africa, we were surprised that only 1 patient developed tuberculosis while on anti-TNFα therapy. A possible explanation for this TB rate may be that these patients were of higher socio-economic strata and could afford private healthcare. All patients are screened for tuberculosis prior to starting biological therapy, but they are not routinely offered prophylactic treatment. Although not directly related to adverse events, eleven patients with UC were lost to follow-up which implies that they were no longer receiving any biological therapy; they may have died, gave up medical insurance, sought alternative medical care or undergone surgery.
In CD, patients started on adalimumab as first anti-TNF agent had a significant risk of dose escalation compared to infliximab. Similarly, patients have a statistically significant risk of dose escalation if switched to adalimumab as a second anti-TNF agent, compared to infliximab.
There was no significantly increased risk of dose escalation for patients with UC started on either adalimumab or infliximab as a first anti-TNF agent. However, patients switched to adalimumab as second anti-TNF in UC, had a significant increased risk of dose escalation compared to infliximab. Although not the smoking gun, a possible explanation for this may be that many patients with UC on adalimumab were not on IM therapy compared to CD.
This study confirmed that all anti-TNF agents eventually failed over time, and it seems this happens earlier in patients with UC compared to CD. Patients with UC treated with adalimumab had increased risk of hospitalization and serious adverse events compared to infliximab according to a study by Singh et al. [10]. Our study has also shown, albeit to a smaller size, that adalimumab is associated with more serious adverse events compared to infliximab. Moreover, patients on adalimumab also had a greater risk of dose escalation before loss of response.
The biggest limitation of this study is its retrospective nature which has inherent shortcomings. We have controlled for recall bias, but could not control for missing data and loss to follow-up. Although a dedicated research questionnaire was drafted for the data entry, we had little control over treatment choices, decision making and outcome assessments. As stated before, not all patients had all data reported, especially so for drug and antibody levels. However, the benefit of this is that it allows evaluation of real-life decision making. The small number of patients in this study makes meaningful interrogation of the data difficult, but patient input onto the database is ongoing and results can be updated in future.
There are some published data that support a second anti-TNF agent when the first agent failed, as shown in this study. A study by Penaccione et al. showed that patients with Crohn’s disease who developed loss of response to infliximab, did well and remain in clinical remission with adalimumab treatment for over 96 weeks [29]. A recent study from Casanova et al. further showed that patients do respond to a second and third anti-TNF agent when the first agent failed, although to a lesser extent and higher subsequent failure rate [30].
Conclusion
Escalation of biologic treatment in resource constraint settings in IBD is fraught with difficulty. The use of consecutive TNFa-antagonists in this setting have increased despite inadequate evidence. Of late, the appropriate use of a second anti-TNF agent after the first one failed has become clearer, especially with regard to clinical and other criteria as shown in this study. With the arrival of biosimilars in sub-Saharan Africa, drug availability and cost to these drugs have improved greatly. Therapeutic drug monitoring remain an essential component of this decision-making process, although not universally available. Therefore, in resource constraint settings, the use of a second anti-TNF agent after failure of the first agent, is reasonable considering cost in the appropriate setting. However, close follow-up of these patients is mandatory and will create opportunity for further studies to address outstanding issues.
Funding
No funding was required for this study.
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Acknowledgement
The corresponding author takes responsibility for the integrity of this study from its inception to completion. Author contributions are stated in the submitted manuscript. All authors have read and approved the manuscript.
Data availability
Once accepted for publication, the data will be uploaded on a cloud-based repository.
References
2. Yeshi K, Ruscher R, Hunter L, Daly NL, Loukas A, Wangchuk P. Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products. J Clin Med. 2020 Apr 28;9(5):E1273.
3. Lichtenstein GR. Comprehensive review: antitumor necrosis factor agents in inflammatory bowel disease and factors implicated in treatment response. Therap Adv Gastroenterol. 2013 Jul;6(4):269-93.
4. Cohen HP, Blauvelt A, Rifkin RM, Danese S, Gokhale SB, Woollett G. Switching Reference Medicines to Biosimilars: A Systematic Literature Review of Clinical Outcomes. Drugs. 2018 Mar;78(4):463-78.
5. Zhang C, Shu W, Zhou G, Lin J, Chu F, Wu H, et al. Anti-TNF-α Therapy Suppresses Proinflammatory Activities of Mucosal Neutrophils in Inflammatory Bowel Disease. Mediators Inflamm. 2018;2018:3021863.
6. Blesl A, Binder L, Högenauer C, Wenzl H, Borenich A, Pregartner G, et al. Limited long-term treatment persistence of first anti-TNF therapy in 538 patients with inflammatory bowel diseases: a 20-year real-world study. Aliment Pharmacol Ther. 2021 Sep;54(5):667–77.
7. Billmeier U, Dieterich W, Neurath MF, Atreya R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J Gastroenterol. 2016 Nov 14;22(42):9300-13.
8. Fine S, Papamichael K, Cheifetz AS. Etiology and Management of Lack or Loss of Response to Anti-Tumor Necrosis Factor Therapy in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2019 Dec;15(12):656-65.
9. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015 Apr;41(7):613–23.
10. Singh S, Andersen NN, Andersson M, Loftus EV, Jess T. Comparison of Infliximab and Adalimumab in Biologic-Naive Patients With Ulcerative Colitis: A Nationwide Danish Cohort Study. Clin Gastroenterol Hepatol. 2017 Aug;15(8):1218-1225.e7.
11. Narula N, Kainz S, Petritsch W, Haas T, Feichtenschlager T, Novacek G, et al. The efficacy and safety of either infliximab or adalimumab in 362 patients with anti-TNF-α naïve Crohn’s disease. Aliment Pharmacol Ther. 2016 Jul;44(2):170–80.
12. Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006 Feb;130(2):323–33; quiz 591.
13. Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D'Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012 Feb;142(2):257-265.e1-3.
14. Roda G, Jharap B, Neeraj N, Colombel JF. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin Transl Gastroenterol. 2016 Jan 7;7:e135.
15. Targownik LE, Benchimol EI, Bernstein CN, Singh H, Tennakoon A, Zubieta AA, et al. Combined Biologic and Immunomodulatory Therapy is Superior to Monotherapy for Decreasing the Risk of Inflammatory Bowel Disease-Related Complications. J Crohns Colitis. 2020 Oct 5;14(10):1354-63.
16. Vande Casteele N, Herfarth H, Katz J, Falck-Ytter Y, Singh S. American Gastroenterological Association Institute Technical Review on the Role of Therapeutic Drug Monitoring in the Management of Inflammatory Bowel Diseases. Gastroenterology. 2017 Sep;153(3):835-857.e6.
17. Papamichael K, Cheifetz AS, Melmed GY, Irving PM, Vande Casteele N, Kozuch PL, et al. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2019 Aug;17(9):1655-1668.e3.
18. Watermeyer G, Awuku Y, Fredericks E, Epstein D, Setshedi M, Devani S, et al. Challenges in the management of inflammatory bowel disease in sub-Saharan Africa. Lancet Gastroenterol Hepatol. 2022 Oct;7(10):962-72.
19. Shivaji UN, Sharratt CL, Thomas T, Smith SCL, Iacucci M, Moran GW, et al. Review article: managing the adverse events caused by anti-TNF therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2019 Mar;49(6):664-80.
20. Lew D, Yoon SM, Yan X, Robbins L, Haritunians T, Liu Z, et al. Genetic associations with adverse events from anti-tumor necrosis factor therapy in inflammatory bowel disease patients. World J Gastroenterol. 2017 Oct 28;23(40):7265-73.
21. Papamichael K, Gils A, Rutgeerts P, Levesque BG, Vermeire S, Sandborn WJ, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015 Jan;21(1):182-97.
22. Atreya R, Neurath MF, Siegmund B. Personalizing Treatment in IBD: Hype or Reality in 2020? Can We Predict Response to Anti-TNFα Front Med (Lausanne). 2020;7:517.
23. Gisbert JP, Chaparro M. Primary Failure to an Anti-TNF Agent in Inflammatory Bowel Disease: Switch (to a Second Anti-TNF Agent) or Swap (for Another Mechanism of Action)? J Clin Med. 2021 Nov 15;10(22):5318.
24. Danese S, Fiorino G, Peyrin-Biroulet L, Lucenteforte E, Virgili G, Moja L, et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med. 2014 May 20;160(10):704-11.
25. Alsoud D, Verstockt B, Vermeire S. Letter: immunogenicity is not the root cause for loss of response to anti-TNF agents in patients with IBD in TDM era. Aliment Pharmacol Ther. 2022 Apr;55(7):885-6.
26. Vermeire S, Gils A, Accossato P, Lula S, Marren A. Immunogenicity of biologics in inflammatory bowel disease. Therap Adv Gastroenterol. 2018;11:1756283X17750355.
27. Colombel JF, Sandborn WJ, Reinisch W, Peyrin-Biroulet L, Panaccione R, Rutgeerts P, et al. Long-term safety of adalimumab in clinical trials in adult patients with Crohn's disease or ulcerative colitis. Aliment Pharmacol Ther. 2018 Jan;47(2):219-28.
28. Adegbola SO, Sahnan K, Warusavitarne J, Hart A, Tozer P. Anti-TNF Therapy in Crohn's Disease. Int J Mol Sci. 2018 Jul 31;19(8):E2244.
29. Panaccione R, Sandborn WJ, D’Haens G, Wolf DC, Berg S, Maa JF, et al. Clinical Benefit of Long-Term Adalimumab Treatment in Patients With Crohn’s Disease Following Loss of Response or Intolerance to Infliximab: 96-Week Efficacy Data From GAIN/ADHERE Trials. J Crohns Colitis. 2018 Jul 30;12(8):930–8.
30. Casanova MJ, Chaparro M, Mínguez M, Ricart E, Taxonera C, García-López S, et al. Effectiveness and Safety of the Sequential Use of a Second and Third Anti-TNF Agent in Patients With Inflammatory Bowel Disease: Results From the Eneida Registry. Inflamm Bowel Dis. 2020 Mar 4;26(4):606–16.