Abstract
Most gastro-oesophageal reflux events are triggered by transient lower oesophageal sphincter relaxations (TLOSRs). Acid-related reflux disease with typical heartburn and reflux oesophagitis generally responds well to acid suppression with proton pump inhibitors (PPI), but treating non-acid and mixed gaseous reflux has proven more challenging. Unfortunately, the majority of refractory gastrooesophageal reflux disease (GORD) and laryngopharyngeal reflux (LPR) is linked to functional gut disease or proximal weakly acid reflux, which is more associated with upper GI dysmotility. Although considered to be our gold standard, detecting proximal oesophageal and airway reflux with oesophageal pH impedance has serious limitations. Oesophageal mucosal impedance and reflux scintigraphy have the potential to assess proximal reflux more accurately with the latter able to accurately detect even minute amounts of airway contamination of the larynx, lungs, sinuses, and ears. Our current treatment algorithm completely ignores the effect of downstream colonic faecal and/or gaseous distension, which may be occult or associated with irritable bowel syndrome. Colonic distension has not only been shown to trigger TLOSR and increase reflux events, but also induce upper GI dysmotility associated with LPR and functional upper gut disease. Simple patient screening with abdominal x-rays and treatment with laxative therapy to reverse motility reflexes provides an easy way to optimise outcomes in refractory GORD or LPR. Refractory GORD and LPR do not respond well to even high dose PPI therapy because acid suppression does not address the underlying pathophysiology.
Keywords
Refractory gastro-oesophageal reflux disease, Laryngopharyngeal reflux, Reflux scintigraphy, Oesophageal pH impedance monitoring, Oesophageal mucosal impedance, Colonic distension reflexes, Proton pump inhibitors, Nizatidine, Prucalopride
Introduction
The pathophysiology of typical gastro-oesophageal reflux disease (GORD) symptoms and reflux oesophagitis is associated with excess acid reflux, but both refractory GORD and laryngopharyngeal reflux (LPR) have strong links with functional gut disorders [1-3]. Oesophageal pH impedance monitoring, our accepted gold standard for diagnosing GORD, has significant shortcomings when assessing proximal oesophageal and in particular pharyngeal reflux [4]. In addition, identifying potential contamination of other parts of the respiratory tract such as lungs or sinuses is not possible. The association between irritable bowel syndrome (IBS) and both refractory GORD and LPR suggests a common pathogenesis. IBS subjects are known to have increased sensitivity to colonic distension causing pain and increased contractility [5], but colonic distension has also been found to affect upper gut motility [6-9] and increase reflux events in physiological studies [10]. Treating GORD and LPR symptoms refractory to proton pump inhibitor (PPI) therapy remains challenging, but the effect of downstream colonic distension or occult constipation on treating GORD and LPR to date has been largely ignored. Hence in our study, we hypothesised that reducing colonic distension mainly with simple osmotic laxative therapy would not only improve colonic symptoms but also LPR, refractory GORD and functional upper gut symptoms.
Pathophysiology of GORD
It is well accepted that post-prandial gastric fundal distension is the main trigger inducing transient lower oesophageal sphincter relaxations (TLOSRs) causing reflux events [11] and the majority of TLOSRs occur after meals [12]. Other factors such as large hiatus hernias or lax lower oesophageal sphincters may predispose to more supine and prolonged acid reflux, reflux oesophagitis and Barrett’s oesophagus [13]. Although treated predominantly with acid suppression, GORD is essentially a motility disorder and not a disorder of increased acid production. Those who experience typical heartburn or develop reflux oesophagitis do not necessarily have more reflux events, but simply have more acid in their reflux than controls [14].
Many patients with apparent refractory reflux symptoms not responding to PPI therapy have functional heartburn (FH) rather than true GORD [1]. They are also more likely to have concurrent functional dyspepsia (FD) [2] or IBS [2,15]. FH also has strong ties to FD [16] confirming the strong underlying functional component associated with refractory GORD. Not only is IBS a major risk factor for refractory GORD, but patients presenting with LPR or chronic cough are more likely to have IBS than typical GORD symptoms [17,18]. Slower gastric emptying, which occurs commonly in these patients, results in more proximal weakly acid reflux [19], which is a frequent finding in both refractory GORD [20] and LPR [21]. Patients with FH are known to have increased sensitivity to oesophageal balloon distension [22] and are significantly more symptomatic when they have gas in their reflux [23], suggesting that oesophageal luminal distension with gas is important in its pathogenesis. LPR is a disorder associated with mainly mixed gaseous and weakly acid reflux [3,24]. Although LPR may be associated with aberrant upper oesophageal sphincter (UOS) function [25,26], mixed reflux is known to elicit reflex relaxation of the UOS [27] making the laryngopharynx more susceptible to contamination with tiny amounts of gastric juice without the subject being aware of heartburn or frank reflux.
However, many patients with typical GORD experience symptoms in the late evening or waking them overnight often more than 4-6 hours after their main evening meal, when in the absence of significant gastroparesis, their stomachs should be empty. Hence there must be an alternative mechanism triggering TLOSRs and causing these symptoms. Studies in dogs show that rectal distension induces TLOSRs to the same extent as gastric distension [10]. Infusing fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) into the right colon increases TLOSRs and associated acid reflux events in normals. In GORD patients, consuming increased dietary FODMAPs over a week without causing significant IBS symptoms also increases TLOSRs and typical reflux symptoms with both a post-prandial and late peak 4-5 hours later when FODMAPs are fermenting in the proximal colon [28,29]. Although short chain fatty acids produced from colonic fermentation of FODMAPs have been shown to increase TLOSRs possibly via inducing proximal gastric relaxation [30], it is very likely based on physiological principles from balloon studies that colonic distension induced by FODMAPs triggers reflux events. Hence colonic distension may need to be addressed by medical therapy to optimise treatment outcomes in GORD patients.
Diagnosis
One of the main limitations in diagnosing reflux is that oesophageal pH impedance monitoring has poor reliability and inter-observer reproducibility in detecting reflux events above the distal oesophagus and trying to assess pharyngeal reflux is fraught with technical problems [4]. In addition, no reflux pattern identified on pH impedance predicts response to PPI therapy. Clinical or endoscopic indicators including non-erosive disease and associated FD or IBS better predict failed therapy [2]. The diagnosis and treatment of LPR until recently has been suboptimal with both the limitations of oesophageal pH impedance testing and multiple studies showing minimal improvement over placebo with PPI therapy [31]. Recent studies assessing oesophageal mucosal impedance (MI), which can be performed quickly and easily via a probe at the time of endoscopy [32], reflect oesophageal injury from long-term mucosal exposure to noxious agents associated with duodeno-gastro-oesophageal reflux and may be a more reliable measure of chronic reflux. A graded reduction in MI was demonstrated from erosive disease to NERD with positive pH studies to NERD with negative pH studies in patients with LPR [33]. Distal oesophageal MI may be useful to help identify patients with refractory GORD who respond well to acid suppression therapy if their distal oesophageal acid exposure time is equivocal between 4-6% [34]. LPR patients have been found to have lower proximal oesophageal MI values and lower proximal to distal MI ratios compared with NERD patients without LPR symptoms acting as controls. Those with more laryngeal inflammation also had lower proximal MI levels suggesting a correlation between proximal oesophageal reflux and reflux-related laryngeal irritation [35]. Hence although LPR is not necessarily associated with excess acid reflux, there is still evidence of low-grade reflux of noxious material on MI.
Reflux scintigraphy performed with the correct protocol has been shown by our group to more accurately detect upper airway reflux and lung aspiration with good response to anti-reflux surgery in selected patients [36,37]. In LPR patients with symptoms refractory to high dose PPI therapy undergoing combined oesophageal and scintigraphy studies, a high incidence of non-acid reflux is the rule often with reduced lower oesophageal sphincter tone.
Impaired oesophageal motility on manometry and poor oesophageal bolus clearance on impedance are associated with increased lung contamination by isotope [38]. We found that up to 50% of patients with functional colonic symptoms have symptoms consistent with LPR affecting the nasal or laryngeal region and reflux scintigraphy has good sensitivity in diagnosing LPR with positive scans in 98% correlating nicely with their ear, nose, throat or lung disease in most cases [18]. Delayed gastric emptying of liquids was common and correlated with increased severity of LPR symptoms (Pearson Correlation Coefficient (PCC) 0.41, p=0.01). Although not quite reaching statistical significance (PCC 0.27, p=0.09), there was a trend for increased volume of scintigraphic reflux correlating with the degree of laryngeal inflammation and there was a strong positive correlation between improvement of laryngeal inflammation with treatment and volume of reflux (PCC 0.58, p<0.005) [18]. In addition, a group of controls with neither LPR symptoms nor evidence of GORD were shown to have physiological distal oesophageal reflux, but no airway reflux on scintigraphy as well as consistently normal gastric emptying times (in press). However, as many subjects with significant oesophageal acid reflux remain asymptomatic, it is likely that airway reflux may also occur without causing symptoms.
Limitations of PPI Therapy for GORD and LPR
Although non-erosive reflux disease (NERD) has been associated with a poorer response to PPI therapy [2], the majority of those with both erosive reflux disease (ERD) and NERD where increased acid exposure has been documented on pH monitoring respond well to acid suppression therapy [39] with equivalent relief of heartburn [40]. So logically where excess acid reflux has been confirmed, acid suppression therapy with PPIs generally works well. However, PPIs do not reduce reflux events, but simply increase the pH of the refluxate [41]. Mixed gaseous and liquid reflux events still outnumber acid reflux even in GORD patients [42] and non-acid reflux accounts for around one third of symptomatic reflux events in PPI-refractory patients [43]. In these PPIrefractory patients around half those with typical GORD symptoms have a negative symptom index for reflux events on oesophageal pH impedance, increasing to around 80% for those with atypical GORD/LPR symptoms [43]. Effective therapy to reduce weakly and non-acid reflux events appears to be lacking.
PPI therapy is often commenced for any reflux-like or dyspeptic symptoms in patients many of whom have delayed gastric emptying without increased acid reflux and hence their efficacy in this situation is limited. As PPIs may worsen gastric emptying [44], this may potentially increase LPR in this cohort and predispose patients to aspiration hence explaining the increased risk of pneumonitis in the first few weeks of therapy. Chronic cough related to LPR was also found to be an independent risk factor for both Barrett’s oesophagus and oesophageal adenocarcinoma (OAC) [45,46]. LPR appears to be a functional disorder associated with impaired GI motility [18,47], not an aciddriven disorder. Barrett’s patients, particularly those with complicated disease, are also known to have both increased bile reflux consistent with impaired upper GI motility [48,49] and ineffective oesophageal motility [50] making them more prone to LPR. Combined reflux of both gastric and duodenal juice causes more severe oesophageal mucosal damage than acid alone [51]. Multiple rat studies have demonstrated that duodeno-gastro-oesophageal reflux exposing the oesophageal mucosa to bile and pancreatic juice increases the risk of developing dysplasia in Barrett’s and associated adenocarcinoma more than acid reflux alone [52-54]. Hence improved upper GI motility and clearance of duodenal fluid may potentially reduce the risk of OAC. Unfortunately, neither PPI therapy nor antireflux surgery appear to achieve this objective as neither have been shown to reduce the incidence of OAC [55].
Optimising Management of Refractory GORD and LPR
In our prospective cohort study, forty subjects with both functional colonic and LPR symptoms consistently demonstrated excess colonic faecal loading on abdominal x-ray (AXR) (18) equivalent to IBS patients in Raahave’s study [56]. Treatment of IBS symptoms predominantly with osmotic laxatives helped to improve or resolve refractory GORD symptoms in all cases (p<0.005) and significantly improved LPR symptoms (p<0.01). More importantly, improvement in LPR (PCC 0.70, p<0.001) and functional upper GI symptoms (PCC 0.59, p<0.005) correlated strongly with reduction in functional colonic symptoms [18]. Although the study was only small from a single centre, the strongly positive correlations between improvement in colonic, upper gut and LPR symptoms with treatment to reduce colonic distension is of greater theoretical importance. IBS patients have previously been documented with more redundant colons with slower colonic transit and increased colonic faecal loading on AXR compared with controls, where functional colonic symptoms including bloating and lower abdominal pain correlate with proximal and distal colonic faecal loading respectively [56]. The key principle documented by our study is that more effective colonic clearance improves all upstream symptoms extending right up to the airway including some patients whose rhinosinusitis resolved. It also suggests that LPR probably does play a dominant role in triggering upper airway symptoms and disease in many cases, but treatment must address underlying GI dysmotility. Our other unpublished data with larger numbers confirms that refractory GORD and functional heartburn rarely occur if the colon is emptying more effectively and that the treatment response in LPR is generally suboptimal unless the colon (in particular the proximal colon) is emptying more efficiently. Although only observational at this stage, improvement or resolution of chronic cough, laryngitis, rhinosinusitis, late onset asthma and bronchiectasis has been commonplace with appropriate treatment which improves colonic and upper GI motility. A recent GORD review suggested that in the absence of confirmed acid reflux, GORD is rarely the dominant cause of upper airway symptoms, which are likely to be secondary to multiple other cofactors. However, this was based on studies using pH impedance as the only validated screening test and the poor response to PPI therapy in this patient group [13]. As most refractory GORD and LPR is either functional or not due to excess acid reflux in the first place, it is hardly surprising that acid suppression is ineffective.
A paediatric study has confirmed the close association between constipation and GORD, where children presenting with constipation had the same degree of acid reflux on 24 hours oesophageal pH monitoring as a control group presenting with reflux symptoms [57]. Treatment of occult constipation in children also helped to resolve refractory GORD symptoms in another study [58]. In a randomised controlled trial (RCT) simple treatment with psyllium seeds was as effective for GORD symptoms as double dose PPI therapy in subjects with functional constipation with less recurrence 3 months after cessation of therapy [59].
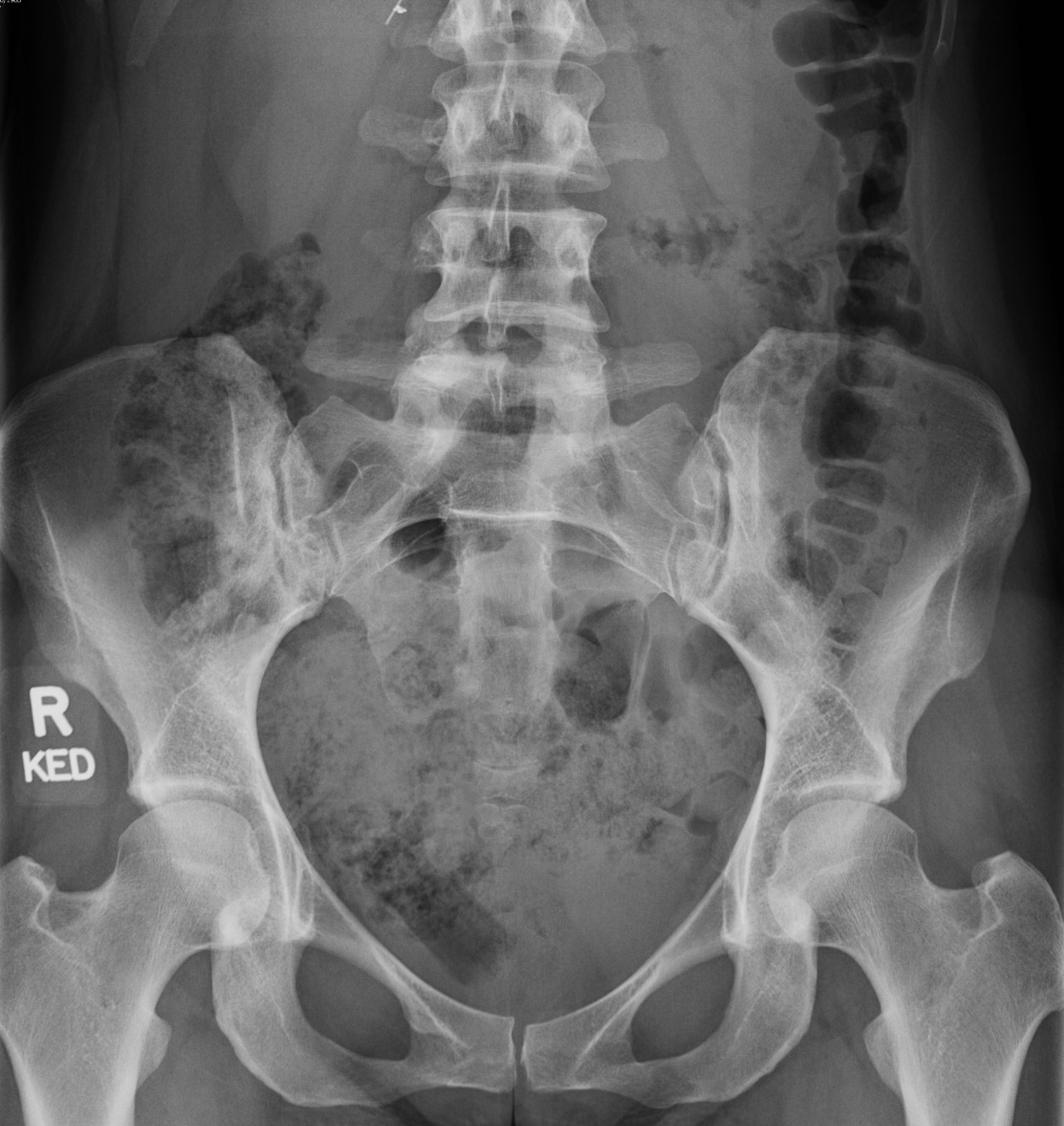
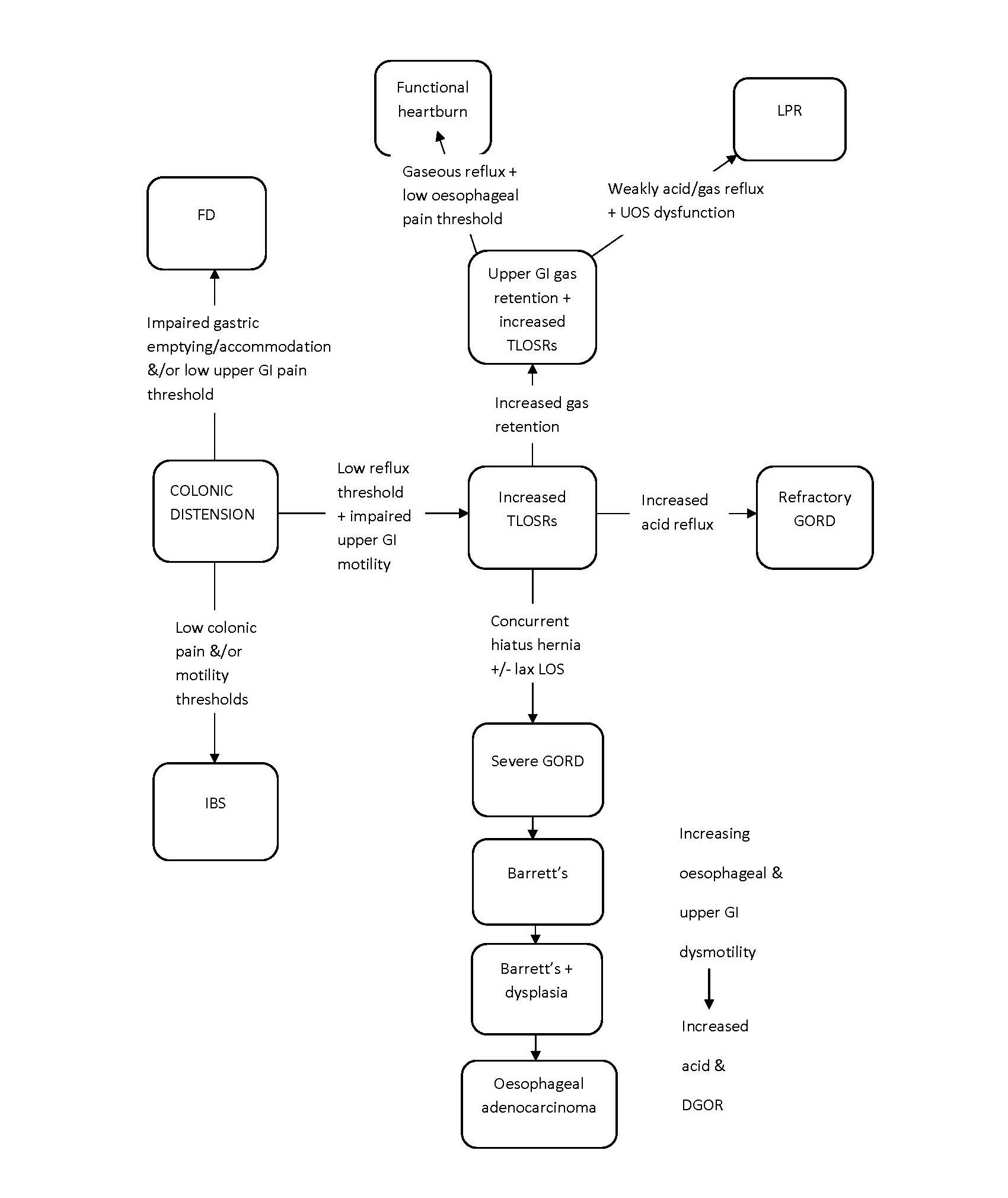
With a normal pattern of bowel habit, where most people move their bowels predominantly in the first half of the day, the colon is typically fullest when their evening meal (usually their largest meal) starts to fill their colon 2-3 hours later. Even with normal diets up to 20% of dietary starch is not digested in the small intestine [60]. As the proximal colon distends with faeces followed later by some additional gas as undigested carbohydrates ferment, this then triggers reflux in the late evening or overnight. The degree of right colonic distension overnight may be exacerbated further by a decrease in colonic tone by up to 50% during sleep [61]. Performing simple AXRs on these patients usually confirms that their proximal and often their entire colon is already faecally loaded regardless of how regular their bowels may be [18] (refer Figure 1). However, the degree of colonic distension is a dynamic process depending on both the individual’s food input and pattern of their bowel movements. Hence AXRs need to be examined and interpreted in the context of the patient’s symptoms taking the above factors into account. Although MRI scans have also been used to assess colonic volume more accurately [62], they are obviously not practical in the everyday situation. Subjects with confirmed slow transit constipation often have stool frequency within the normal range [63], but may present with concurrent dyspeptic, reflux or LPR symptoms. Rectal or colonic barostat balloon distension has also been shown to trigger colonic pain and increased motility [5], reduce oesophageal, gastric and small intestinal motility [6,7] and impair gastric accommodation [8,9]. Patients more sensitive to rectal balloon distension have been found to have higher indigestion, reflux, abdominal pain, constipation and IBS scores, which highlights the multiple different colonic reflexes that may be triggered by distension generating both upper and lower GI symptoms [64]. Hence AXRs to assess the degree of colonic distension are essential in Figure 2). In many cases, adding simple laxative therapy screening patients with refractory GORD and LPR (refer is enough to improve or resolve reflux-related symptoms. In more severe or refractory cases where the proximal colon remains full on AXR despite laxative therapy, adding prucalopride may prove invaluable as it has been shown to preferentially improve proximal colonic motility [65] in addition to improving gastric emptying and reducing oesophageal acid exposure and proximal oesophageal reflux in healthy subjects [66], all of which are beneficial in controlling both refractory GORD and LPR.
Figure 1. Patient with mild bloating, but regular bowel habit with likely functional heartburn and confirmed LPR. Note quite marked faecal loading and gas retention throughout the colon.
Figure 2. Effect of colonic distension on functional GI disease, refractory gord & LPR. DGOR: Duodeno-Gastro-Oesophageal Reflux; FD: Functional Dyspepsia; GI: Gastrointestinal; GORD: Gastro-Oesophageal Reflux Disease; IBS: Irritable Bowel Syndrome; LOS: Lower Oesophageal Sphincter; LPR: Laryngopharyngeal Reflux; TLOSRs: Transient Lower Oesophageal Sphincter Relaxations; UOS: Upper Oesophageal Sphincter.
Nizatidine is a more attractive drug for treating weakly acid reflux than PPI therapy, but not due to its acidsuppressing effect. Nizatidine has cholinergic activity and hence improves gastric emptying, but has also been found to increase basal lower oesophageal sphincter pressure, reduce post-prandial TLOSRs and reduce the rate of acid reflux during TLOSRs in healthy subjects [67]. Using H2 antagonists for either acid suppression or nocturnal acid suppression in combination with PPI therapy results in rapid tachyphylaxis [68,69], but using nizatidine in a split dose before food helps gastric emptying and reduces reflux events. The author has observed long-term improvement of LPR symptoms on nizatidine therapy, which recur off therapy and settle again on recommencement, suggesting that tachyphylaxis does not occur in regard to its cholinergic effect. Although higher volume supine reflux only tends to occur in more severe cases of typical GORD [70], supine low-grade reflux to the laryngopharynx occurs in most cases of LPR including those with only mild symptoms as confirmed by scintigraphy [18]. Using a simple alginate-containing antacid before bedtime may be useful to both reduce nocturnal reflux symptoms as well as cough or throat irritation, which is often triggered by supine posture.
To my knowledge, not one paper addressing the management of refractory GORD raises the issue of assessing and reducing the degree of colonic distension to reduce reflux symptoms. Although we can reduce the size of our meals to reduce gastric distension to some extent, adequate nutrition must be maintained, so postprandial TLOSRs are unavoidable. However, it is relatively simple to reduce colonic distension with medications that stimulate colonic transit such as osmotic and stimulant laxatives or prucalopride. It is common for refractory GORD or LPR patients to have prompt improvement in their heartburn, cough or throat irritation following a bowel prep, which decompresses their colon. On the other hand, acute distension of the right colon from the high osmotic load induced by the first half of the bowel prep has also been observed by the author to precipitate acute onset of cough, wheeze and dyspnoea more than likely from LPR. A patient’s response to bowel prep and subsequent laxative therapy can be both therapeutic and informative.
The current approach to investigating refractory GORD after endoscopy and a failed trial of PPI therapy involves further time-consuming, expensive and often poorly accessible investigations including oesophageal manometry and 24 hours oesophageal pH impedance monitoring, but a simple AXR and trial of high dose polyethylene glycol (PEG) solution 1 litre followed by osmotic +/- stimulant laxative therapy is both cost-effective and clinically invaluable. As our study demonstrated a strong correlation between improvement of functional colonic and upper gut, refractory GORD and LPR symptoms with treatment as the colon decompresses [18], prompt improvement in cough, reflux or dyspeptic symptoms with bowel cleansing suggests that a good longterm response on maintenance therapy is achievable. Not all patients presenting with LPR or refractory GORD have colonic symptoms, but if not, their AXRs often reveal occult constipation or excess colonic faecal loading at least affecting the proximal colon. As colonic distension may trigger multiple upper GI reflexes, improvement or resolution of other functional upper and lower gut symptoms including functional heartburn is also common with laxative therapy. The concept is simple - a full colon acts as a functional obstruction, which may cause reflux upstream as high as the middle ears as demonstrated by reflux scintigraphy.
Conclusion
PPI therapy is invaluable for acid-related disease, but the pathophysiology of reflux or upper gut-related symptoms must be adequately addressed by treatment to be effective. Refractory GORD and LPR often have a functional component or are associated with weakly or non-acid +/- gaseous reflux, which responds poorly to acid suppression. Oesophageal pH impedance monitoring has significant limitations apart from its role prior to considering anti-reflux surgery, but oesophageal mucosal impedance potentially performed at the time of endoscopy and reflux scintigraphy with the correct protocol may be more practical and clinically useful options for the everyday gastroenterologist to assess for LPR. Reflux scintigraphy using the correct protocol appears to have good sensitivity and specificity in detecting LPR and aids in medical management by providing data on upper gut motility. Occult constipation or colonic distension appears to play an important role in the pathogenesis of reflux and functional upper gut symptoms. A simple trial of initial high dose followed by maintenance laxative therapy (usually PEG/macrogol) is quick, simple, safe and cost-effective for assessing and treating refractory GORD and LPR with very little to lose. Addressing the pathophysiology of GORD more effectively may also improve upper GI motility and potentially reduce the incidence of oesophageal adenocarcinoma.
References
2. Zerbib F, Belhocine K, Simon M, Capdepont M, Mion F, des Varannes SB, et al. Clinical, but not oesophageal pH-impedance, profiles predict response to proton pump inhibitors in gastro-oesophageal reflux disease. Gut. 2012 Apr 1;61(4):501-6.
3. de Bortoli N, Nacci A, Savarino E, Martinucci I, Bellini M, Fattori B, et al. How many cases of laryngopharyngeal reflux suspected by laryngoscopy are gastroesophageal reflux disease-related?. World Journal of Gastroenterology: WJG. 2012 Aug 28;18(32):4363-70.
4. Zerbib F, Roman S, Des Varannes SB, Gourcerol G, Coffin B, Ropert A, et al. Normal values of pharyngeal and esophageal 24-hour pH impedance in individuals on and off therapy and interobserver reproducibility. Clinical Gastroenterology and Hepatology. 2013 Apr 1;11(4):366- 72.
5. Kanazawa M, Palsson OS, Thiwan SI, Turner MJ, Van Tilburg MA, Gangarosa LM, et al. Contributions of pain sensitivity and colonic motility to IBS symptom severity and predominant bowel habits. The American Journal of Gastroenterology. 2008 Oct;103(10):2550-61.
6. Shafik A, El-Sibai O. Esophageal and gastric motile response to rectal distension with identification of a rectoesophagogastric reflex. International Journal of Surgical Investigation. 2000;1(5):373-9.
7. Youle MS, Read NW. Effect of painless rectal distension on gastrointestinal transit of solid meal. Digestive Diseases and Sciences. 1984 Oct 1;29(10):902-6.
8. Lei Y, Zhu H, Xing J, Chen JD. Rectal distension modulates canine gastric tone and accommodation. Digestive Diseases and Sciences. 2005 Nov 1;50(11):2134- 40.
9. Uchida M, Iwamoto C. Effect of colonic distension on gastric adaptive relaxation in rats: barostatic evaluation using an orally introduced gastric balloon. Journal of Smooth Muscle Research. 2014;50:78-84.
10. Graça J, Neves J, Mota B, Lopes L, Goiana S, Bezerra M, et al. Rectal distension increases transient lower esophageal sphincter relaxations in dogs: investigation of mechanisms of a putative rectoesophageal reflex: 10. Neurogastroenterology & Motility. 2010 Aug;22:3-4.
11. Tack J, Pandolfino JE. Pathophysiology of gastroesophageal reflux disease. Gastroenterology. 2018 Jan 1;154(2):277-88.
12. Blonski W, Vela MF, Castell DO. Comparison of reflux frequency during prolonged multichannel intraluminal impedance and pH monitoring on and off acid suppression therapy. Journal of Clinical Gastroenterology. 2009 Oct 1;43(9):816-20.
13. Katzka DA, Pandolfino JE, Kahrilas PJ. Phenotypes of gastroesophageal reflux disease: Where rome, lyon, and montreal meet. Clinical Gastroenterology and Hepatology. 2020 Apr 1;18(4):767-76.
14. Sifrim D, Holloway R, Silny J, Tack J, Lerut A, Janssens J. Composition of the postprandial refluxate in patients with gastroesophageal reflux disease. The American Journal of Gastroenterology. 2001 Mar 1;96(3):647-55.
15. De Bortoli N, Frazzoni L, Savarino EV, Frazzoni M, Martinucci I, Jania A, et al. Functional heartburn overlaps with irritable bowel syndrome more often than GERD. American Journal of Gastroenterology. 2016 Dec 1;111(12):1711-7.
16. Savarino E, Pohl D, Zentilin P, Dulbecco P, Sammito G, Sconfienza L, et al. Functional heartburn has more in common with functional dyspepsia than with non-erosive reflux disease. Gut. 2009 Sep 1;58(9):1185-91.
17. Ford AC, Forman D, Moayyedi P, Morice AH. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax. 2006 Nov 1;61(11):975-9.
18. Simpson SB, Qian KY, Sacks R, Novakovic D, Simpson R, Van der Wall H. Observational cohort study: Getting to the bottom of laryngopharyngeal reflux—It’sa motility disorder and not just about the acid. GastroHep. 2019 Sep;1(5):223-35.
19. Emerenziani S, Sifrim D. Gastroesophageal reflux and gastric emptying, revisited. Current Gastroenterology Reports. 2005 May 1;7(3):190-5.
20. Tutuian R, Vela MF, Hill EG, Mainie I, Agrawal A, Castell DO. Characteristics of symptomatic reflux episodes on acid suppressive therapy. American Journal of Gastroenterology. 2008 May 1;103(5):1090-6.
21. Oelschlager BK, Quiroga E, Isch JA, Cuenca-Abente F. Gastroesophageal and pharyngeal reflux detection using impedance and 24-hour pH monitoring in asymptomatic subjects: defining the normal environment. Journal of Gastrointestinal Surgery. 2006 Jan 1;10(1):54-62.
22. Trimble KC, Pryde A, Heading RC. Lowered oesophageal sensory thresholds in patients with symptomatic but not excess gastro-oesophageal reflux: evidence for a spectrum of visceral sensitivity in GORD. Gut. 1995 Jul 1;37(1):7-12.
23. Emerenziani S, Sifrim D, Habib FI, Ribolsi M, Guarino MP, Rizzi M, et al. Presence of gas in the refluxate enhances reflux perception in non-erosive patients with physiological acid exposure of the oesophagus. Gut. 2008 Apr 1;57(4):443-7.
24. Kawamura O, Aslam M, Rittmann T, Hofmann C, Shaker R. Physical and pH properties of gastroesophagopharyngeal refluxate: a 24-hour simultaneous ambulatory impedance and pH monitoring study. The American Journal of Gastroenterology. 2004 Jun;99(6):1000-10.
25. Babaei A, Venu M, Naini SR, Gonzaga J, Lang IM, Massey BT, et al. Impaired upper esophageal sphincter reflexes in patients with supraesophageal reflux disease. Gastroenterology. 2015 Nov 1;149(6):1381-91.
26. Szczesniak MM, Williams RB, Brake HM, Maclean JC, Cole IE, Cook IJ. Upregulation of the esophago-UES relaxation response: a possible pathophysiological mechanism in suspected reflux laryngitis. Neurogastroenterology & Motility. 2010 Apr;22(4):381-e89.
27. Babaei A, Bhargava V, Mittal RK. Upper esophageal sphincter during transient lower esophageal sphincter relaxation: effects of reflux content and posture. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2010 May;298(5):G601-7.
28. Piche T, Zerbib F, Varannes SB, Cherbut C, Anini Y, Roze C, et al. Modulation by colonic fermentation of LES function in humans. American Journal of Physiology- Gastrointestinal and Liver Physiology. 2000 Apr 1;278(4):G578-84.
29. Piche T, des Varannes SB, Sacher-Huvelin S, Holst JJ, Cuber JC, Galmiche JP. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterology. 2003 Apr 1;124(4):894- 902.
30. Ropert A, Cherbut C, Roze C, Le Quellec A, Holst JJ, Fu-Cheng X, et al. Colonic fermentation and proximal gastric tone in humans. Gastroenterology. 1996 Aug 1;111(2):289-96.
31. Reimer C, Bytzer P. Management of laryngopharyngeal reflux with proton pump inhibitors. Therapeutics and clinical risk management. 2008 Feb;4(1):225-33.
32. Vaezi MF, Choksi Y. Mucosal impedance: a new way to diagnose reflux disease and how it could change your practice. American Journal of Gastroenterology. 2017 Jan 1;112(1):4-7.
33. Kavitt RT, Lal P, Yuksel ES, Ates F, Slaughter JC, Garrett CG, et al. Esophageal mucosal impedance pattern is distinct in patients with extraesophageal reflux symptoms and pathologic acid reflux. Journal of Voice. 2017 May 1;31(3):347-51.
34. Rengarajan A, Savarino E, Della Coletta M, Ghisa M, Patel A, Gyawali CP. Mean nocturnal baseline impedance correlates with symptom outcome when acid exposure time is inconclusive on esophageal reflux monitoring. Clinical Gastroenterology and Hepatology. 2020 Mar 1;18(3):589-95.
35. Sakin YS, Vardar R, Sezgin B, Cetin ZE, Alev Y, Yildirim E, et al. The diagnostic value of 24-hour ambulatory intraesophageal pH-impedance in patients with laryngopharyngeal reflux symptoms comparable with typical symptoms. United European Gastroenterology Journal. 2017 Aug;5(5):632-40.
36. Burton L, Falk GL, Parsons S, Cusi M, Van Der Wall H. Benchmarking of a simple scintigraphic test for gastrooesophageal reflux disease that assesses oesophageal disease and its pulmonary complications. Molecular Imaging and Radionuclide Therapy. 2018 Oct;27(3):113- 20.
37. Falk GL, Beattie J, Ing A, Falk SE, Magee M, Burton L, et al. Scintigraphy in laryngopharyngeal and gastroesophageal reflux disease: a definitive diagnostic test?. World Journal of Gastroenterology: WJG. 2015 Mar 28;21(12):3619-27.
38. Burton L, Falk GL, Baumgart K, Beattie J, Simpson S, Van der Wall H. Esophageal Clearance in Laryngopharyngeal Reflux Disease: Correlation of Reflux Scintigraphy and 24-hour Impedance/pH in a Cohort of Refractory Symptomatic Patients. Molecular Imaging and Radionuclide Therapy. 2020 Feb;29(1):7-16.
39. Wang AJ, Wang H, Xu L, Lv NH, He XX, Hong JB, et al. Predictors of clinical response of acid suppression in Chinese patients with gastroesophageal reflux disease. Digestive and Liver Disease. 2013 Apr 1;45(4):296-300.
40. Weijenborg PW, Cremonini F, Smout AJ, Bredenoord AJ. PPI therapy is equally effective in well-defined non-erosive reflux disease and in reflux esophagitis: a meta-analysis. Neurogastroenterology & Motility. 2012 Aug;24(8):747-57, e350.
41. Vela MF, Camacho-Lobato L, Srinivasan R, Tutuian R, Katz PO, Castell DO. Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: effect of omeprazole. Gastroenterology. 2001 Jun 1;120(7):1599-606.
42. Sifrim D, Holloway R, Silny J, Xin Z, Tack J, Lerut A, et al. Acid, nonacid, and gas reflux in patients with gastroesophageal reflux disease during ambulatory 24- hour pH-impedance recordings. Gastroenterology. 2001 Jun 1;120(7):1588-98.
43. Mainie I, Tutuian R, Shay S, Vela M, Zhang X, Sifrim D, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedancepH monitoring. Gut. 2006 Oct 1;55(10):1398-402.
44. Sanaka M, Yamamoto T, Kuyama Y. Effects of proton pump inhibitors on gastric emptying: a systematic review. Digestive Diseases and Sciences. 2010 Sep 1;55(9):2431- 40.
45. Nason KS, Murphy T, Schindler J, Schipper PH, Hoppo T, Diggs BS, et al. A Cross-sectional Analysis of the Prevalence of Barrett’s Esophagus in Otolaryngology Patients with Laryngeal Symptoms. Journal of Clinical Gastroenterology. 2013 Oct;47(9):762-8.
46. Reavis KM, Morris CD, Gopal DV, Hunter JG, Jobe BA. Laryngopharyngeal reflux symptoms better predict the presence of esophageal adenocarcinoma than typical gastroesophageal reflux symptoms. Annals of Surgery. 2004 Jun;239(6):849-56.
47. Fouad YM, Katz PO, Hatlebakk JG, Castell DO. Ineffective esophageal motility: the most common motility abnormality in patients with GERD-associated respiratory symptoms. The American Journal of Gastroenterology. 1999 Jun 1;94(6):1464-7.
48. Caldwell MT, Lawlor P, Byrne PJ, Walsh TN, Hennessy TP. Ambulatory oesophageal bile reflux monitoring in Barrett’s oesophagus. British Journal of Surgery. 1995 May;82(5):657-60.
49. Vaezi MF, Richter JE. Role of acid and duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology. 1996 Nov 1;111(5):1192- 9.
50. Bazin C, Benezech A, Alessandrini M, Grimaud JC, Vitton V. Esophageal Motor Disorders Are a Strong and Independant Associated Factor of Barrett’s Esophagus. Journal of Neurogastroenterology and Motility. 2018 Apr;24(2):216-25.
51. Kauer WK, Peters JH, DeMeester TR, Ireland AP, Bremner CG, Hagen JA. Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy reemphasized. Annals of Surgery. 1995 Oct;222(4):525-31.
52. Cheng P, Li JS, Gong J, Zhang LF, Chen RZ. Effects of refluxate pH values on duodenogastroesophageal refluxinduced esophageal adenocarcinoma. World Journal of Gastroenterology: WJG. 2011 Jul 7;17(25):3060-5.
53. Nishijima K, Miwa K, Miyashita T, Kinami S, Ninomiya I, Fushida S, et al. Impact of the biliary diversion procedure on carcinogenesis in Barrett’s esophagus surgically induced by duodenoesophageal reflux in rats. Annals of Surgery. 2004 Jul;240(1):57-67.
54. Pera M, Trastek VF, Carpenter HA, Fernandez PL, Cardesa A, Mohr U, et al. Influence of pancreatic and biliary reflux on the development of esophageal carcinoma. The Annals of Thoracic Surgery. 1993 Jun 1;55(6):1386-93.
55. Spechler SJ, Lee E, Ahnen D, Goyal RK, Hirano I, Ramirez F, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. Jama. 2001 May 9;285(18):2331-8.
56. Raahave D, Christensen E, Loud FB, Knudsen LL. Correlation of bowel symptoms with colonic transit, length, and faecal load in functional faecal retention. Dan Med Bull. 2009 May 2;56(2):83-8.
57. Baran M, Özgenç F, Arikan Ç, Cakir M, Ecevit ÇÖ, Aydogdu S, et al. Gastroesophageal reflux in children with functional constipation. The Turkish journal of gastroenterology: the official journal of Turkish Society of Gastroenterology. 2012 Jan 1;23(6):634-8.
58. Borowitz SM, Sutphen JL. Recurrent vomiting and persistent gastroesophageal reflux caused by unrecognized constipation. Clinical Pediatrics. 2004 Jun;43(5):461-6.
59. Hosseini M, Salari R, Akbari Rad M, Salehi M, Birjandi B, Salari M. Comparing the Effect of Psyllium Seed on Gastroesophageal Reflux Disease With Oral Omeprazole in Patients With Functional Constipation. Journal of Evidence-Based Integrative Medicine. 2018 Mar 27;23:2515690X18763294.
60. Stephen AM, Haddad AC, Phillips SF. Passage of carbohydrate into the colon: direct measurements in humans. Gastroenterology. 1983 Sep 1;85(3):589-95.
61. VASSALLO MJ, Camilleri M, PHILLIPS SF, STEADMAN CJ, TALLEY NJ, HANSON RB, et al. Colonic tone and motility in patients with irritable bowel syndrome. InMayo Clinic Proceedings 1992 Aug 1 (Vol. 67, No. 8, pp. 725-731).
62. Lam C, Chaddock G, Marciani L, Costigan C, Paul J, Cox E, et al. Colonic response to laxative ingestion as assessed by MRI differs in constipated irritable bowel syndrome compared to functional constipation. Neurogastroenterology & Motility. 2016 Jun;28(6):861- 70.
63. Dinning PG, Hunt L, Lubowski DZ, Kalantar JS, Cook IJ, Jones MP. The impact of laxative use upon symptoms in patients with proven slow transit constipation. BMC gastroenterology. 2011 Dec 1;11(1):121.
64. Ludidi S, Mujagic Z, Jonkers D, Keszthelyi D, Hesselink M, Kruimel J, et al. Markers for visceral hypersensitivity in patients with irritable bowel syndrome. Neurogastroenterology & Motility. 2014 Aug;26(8):1104- 11.
65. Bouras EP, Camilleri M, Burton DD, McKinzie S. Selective stimulation of colonic transit by the benzofuran 5HT4 agonist, prucalopride, in healthy humans. Gut. 1999 May 1;44(5):682-6.
66. Kessing BF, Smout AJ, Bennink RJ, Kraaijpoel N, Oors JM, Bredenoord AJ. Prucalopride decreases esophageal acid exposure and accelerates gastric emptying in healthy subjects. Neurogastroenterology & Motility. 2014 Aug;26(8):1079-86.
67. Iwakiri K, Kawami N, Sano H, Tanaka Y, Umezawa M, Futagami S, et al. The effects of nizatidine on transient lower esophageal sphincter relaxations (TLESRs) and acid reflux in healthy subjects. Journal of Smooth Muscle Research. 2011;47(6):157-66.
68. Miner Jr PB, Allgood LD, Grender JM. Comparison of gastric pH with omeprazole magnesium 20.6 mg (Prilosec OTC) om famotidine 10 mg (Pepcid AC) bd and famotidine 20 mg bd over 14 days of treatment. Alimentary Pharmacology & Therapeutics. 2007 Jan;25(1):103-9.
69. Fackler WK, Ours TM, Vaezi MF, Richter JE. Longterm effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology. 2002 Mar 1;122(3):625- 32.
70. Ouatu-Lascar R, Lin OS, Fitzgerald RC, Triadafilopoulos G. Upright versus supine reflux in gastroesophageal reflux disease. Journal of Gastroenterology and Hepatology. 2001 Nov;16(11):1184-90.