Abstract
We published a manuscript entitled “A Novel, Non-opioid Treatment for Chronic Pelvic Pain in Women with Previously Treated Endometriosis Utilizing Pelvic-Floor Musculature Trigger-Point Injections and Peripheral Nerve Hydrodissection”. There is little consensus in the literature on the underlying etiology of endometriosis. There is even less evidence on effective treatment options for endometriosis and its associated chronic pelvic pain. Although there is no cure, traditionally endometriosis symptoms are managed with a combination of hormonal and surgical treatments. This manuscript is a commentary on a unique outpatient neuromusculoskeletal protocol to add to the traditional hormonal and surgical approaches to help improve pain and function in patients with endometriosis. This commentary takes a gastrointestinal and colorectal slant as to how the complex disease process of endometriosis can affect these organ systems and the symptoms that arise when this happens.
Keywords
Endometriosis, Endometriosis pain, Pelvic pain, Chronic pelvic pain syndrome, Pelvic floor muscle dysfunction, Pelvic floor dyssynergia, Pelvic floor hypertonia, Nonrelaxing pelvic floor, Levator ani syndrome, Pelvic floor muscle spasm, Dyschezia, Peripheral sensitization, Central sensitization
Introduction
Endometriosis is a chronic, hormone-dependent, inflammatory disease, characterized by the presence and growth of endometrial tissue outside the uterine cavity and it is associated with chronic pelvic pain and infertility [1,2]. Worldwide, approximately 176 million women between the ages of 15 and 49 are affected by endometriosis [3]. Endometriosis is a complex disease that induces a chronic inflammatory process and can be challenging to treat [4]. Chronic pelvic pain syndrome (CPPS) is defined as pelvic pain lasting greater than three to six months that is not solely related to menstruation, sexual activity or bowel movements [5]. The symptoms of CPPS include abdominal, lumbosacral, buttock, vulvovaginal, perineal and rectal pain, urinary and bowel symptoms, and pain associated with intercourse. Patients with endometriosis often suffer for many years and see multiple physicians and medical providers before receiving a diagnosis. In patients aged 18- 45, the average delay in diagnosis is 6.7 years [6]. This may be secondary to the fact that endometriosis is a diagnosis of exclusion. The gold standard for the diagnosis of endometriosis has been visual inspection by laparoscopy, preferably with histological confirmation [7]. Because there is lack of a noninvasive test for endometriosis, there is often a significant delay in diagnosis of this disease. One caveat is transvaginal ultrasonography, transrectal ultrasound, and MRI, have the potential to facilitate the diagnosis of certain types of endometriosis, particularly an endometrioma or deep infiltrating endometriosis [8]. No serum marker has been found to diagnose endometriosis with adequate sensitivity and specificity [9]. Compounding the complexity of diagnosis, patients can often have recurrence of the disease even after a surgical excision of endometriosis. The overall recurrence rates range between 6% to 67% [4].
Currently there is no definitive cure for endometriosis however, therapy has three main objectives: (1) to reduce pain; (2) to increase the possibility of pregnancy; (3) to delay recurrence for as long as possible. [10]. The disease treatment options can be divided into surgical and hormonal, and most often they are combined. This is known as a medico-surgical approach [11]. Almost all the available hormonal treatment options suppress ovarian function and are not curative [12]. On example of a hormonal treatment option is Elagolix (Orilissa). Elagolix is an oral, nonpeptide, gonadotropin-releasing hormone (GnRH) antagonist used to treat moderate to severe pain related to endometriosis. Inhibition of GnRH leads to estrogen suppression and subsequent dose-dependent inhibition of endometriotic proliferation [13]. Surgical resection can be accomplished through therapeutic laparoscopic techniques using scissors, electrosurgical instruments, lasers, suture, and staplers [14]. One study showed that laparoscopic excision of endometriosis was more effective than placebo at decreasing pain and improving quality of life for endometriosis patients of all stages [15]. We propose a non-operative neuromusculoskeletal approach in addition to the hormonal and surgical combination, to help reduce pelvic pain symptoms associated with endometriosis.
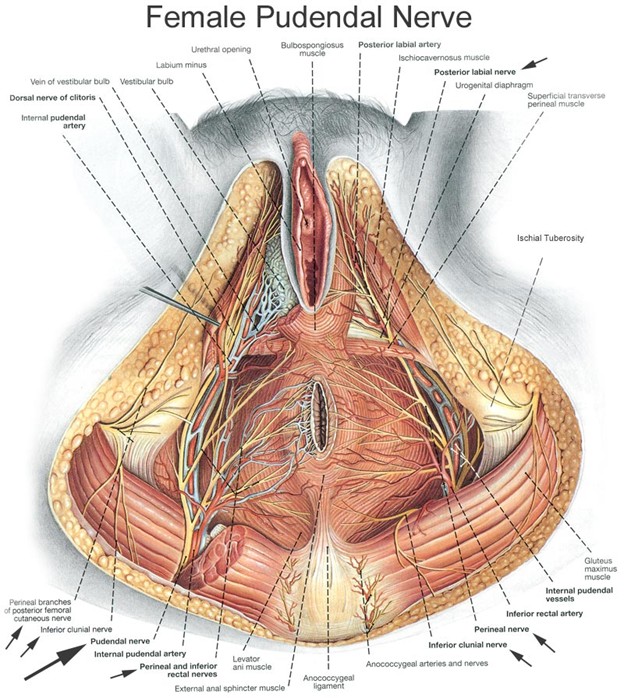
In 2019, we published a retrospective chart review on treating women with Chronic Pelvic Pain Syndrome (CPPS) and a pathological diagnosis of Endometriosis. The objective of this study was to assess treating the pain associated with endometriosis with a series of pelvic floor musculature trigger point injections and peripheral nerve hydrodissection with a goal of decreasing pain and improving function. In this paper sixteen female patients with biopsy-confirmed endometriosis underwent a series of external ultrasound guided trigger point injections to the iliococcygeus, pubococcygeus and puborectalis in combination with peripheral nerve hydrodissection along both the pudendal nerve at Alcocks canal and the posterior femoral nerve at the Obturator canal (Figure 1). These treatments were once weekly for six weeks. Pelvic pain intensity as measured pretreatment and post treatment by the 0 to 10 Visual Analogue Scale (VAS) and the Functional Pelvic Pain Scale (FPPS). The FPPS rates pelvic function in eight categories: bladder, bowel, intercourse, walking, sleeping, working, running, and lifting. The patient rates each category from 0 to 4, with 0 being normal function and 4 being severe debilitation. Thus, each patient can be given a total score from 0 to 32. The mean VAS score decreased from 6.0 (SD 2.7) to 2.9 (SD 2.6) post treatment. The mean total FPPS score decreased from 14.4 (SD 5.2) to 9.1 (SD 5.8) post treatment. The analysis suggested that the treatment was effective at relieving pain and improving overall pelvic function related to endometriosis [16].
Figure 1: Female pudendal nerve. (Source - Clemente CD. Anatomy: A Regional Atlas of the Human Body. 4th Edition. Williams & Wilkins. 1997).
Discussion
The most common location for extragenital endometriosis is the bowel [17]. Common symptoms for endometriosis patients are abdominal pain, rectal pain, bloating, constipation, difficulty evacuating stool, straining with bowel movement, splinting the posterior vagina, anal digitation, incomplete evacuation, sense of anal blockage during defecation, dyschezia, and bowel frequency. In addition, some endometriosis patients complain about blood in their stool around their menstrual cycle. Given the predominance in gastroenterology symptoms in endometriosis patients, the first specialists many of our patients seek help from is a gastrointestinal specialist. It is very common for our endometriosis patients to come see us having had both an upper and lower endoscopy with a diagnosis of irritable bowel syndrome. Endometriosis can cause gastro-intestinal symptoms via several mechanisms; direct innervation of pelvic nerves, direct innervation of the bowel itself, stimulating a hypertonic pelvic floor, cross-sensitization, peripheral, and central sensitization.
Endometriosis can directly innervate pelvic nerves, particularly the pudendal nerve [18,19]. This contributes to pudendal neuralgia symptoms of anorectal pain and pain with bowel movements. Innervation of the pudendal nerve also contributes to increased bowel frequency. Endometriosis can also directly invade the bowel itself contributing to bowel symptoms of constipation and pain with bowel movement [17].
The presence of endometriosis in the pelvis can cause a secondary chronic guarding of pelvic floor musculature [20]. This chronic guarding state leads to nonrelaxing pelvic floor dysfunction and myofascial trigger points (MTrPs). A nonrelaxing pelvic floor, will cause symptoms of difficulty evacuating stool, straining with bowel movements, a sense of incomplete evacuation, bloating, and constipation. The pelvic floor muscles in nonrelaxing pelvic floor dysfunction are short, spastic, weaker and poorly coordinated. This leads to dyssynergic defecation. Myofascial trigger points (MTrPs) are short contracted taut bands of skeletal muscle that often co-exist with nonrelaxing pelvic floor dysfunction [21]. One study of eighteen patients with pathology confirmed endometriosis, published in 2015 by the National Institute of Health showed that 94% of women with endometriosis and pelvic pain had myofascial trigger points on exam [20]. Once formed, MTrPs can become a self-sustaining source of pain even after the endometriosis has been excised. Active MTrPs serve as a source of ongoing nociception; they can decrease pain thresholds, upregulate visceral and referred pain patterns, and sensitize the nervous system contributing to both peripheral and central sensitization. [21] Therefore, it is important to treat a hypertonic nonrelaxing pelvic floor and associated MTrPs in endometriosis patients.
Cross-sensitization in the pelvis can contribute to endometriosis upregulating surrounding pelvic structures such as the bowel, pelvic floor musculature and nerves [22]. Cross sensitization in the pelvis refers to the transmission of noxious stimuli from a diseased pelvic organ to a normal adjacent structure. This occurs through shared neural pathways at prespinal, spinal and supraspinal levels. The result is functional changes of sensitization of the adjacent normal structure with increased membrane excitability, increased synaptic recruitment and a decreased threshold for peripheral nociceptors to fire [22]. Upregulation of the bowel by adjacent endometriosis plaques may contribute to the abnormal pain perception or visceral hypersensitivity (VH) considered to be an important mechanism underlying symptoms of irritable bowel syndrome [23,24]. Crosssensitization of pelvic floor muscles will contribute to the chronic guarding state and a nonrelaxing pelvic floor. With ultimate, constipation, straining on the toilet, and a sensation of incomplete evacuation. Cross-sensitization of the pudendal nerve will contribute to pudendal neuralgia symptoms of anorectal pain, pain with bowel movement and bowel frequency.
Peripheral and central sensitization are seen in endometriosis and need to be addressed as they can lead to gastrointestinal symptoms [25,26]. Peripheral sensitization is seen in endometriosis as it is a proinflammatory state due to both the endometrial plaques and the concomitant chronic guarding of the pelvic floor. Endometrial plaques contribute to the release of proinflammatory cytokines such as IL 1-beta, IL-6, IL-8, macrophages, Nerve Growth Factor (NGF) and TNF-alpha [27]. This ultimately increases neurogenic inflammation and peripheral sensitization. With chronic guarding, the pelvic floor MTrP squeezes the local nerve, causing a neural ischemia. This restriction in blood flow, can alter the local pH to a more acidic environment, which in turn trigger’s the inflammatory cascade and the release of proinflammatory cytokines around the pelvic nerves. This inflammation around the nerves known as neurogenic inflammation can contribute to and maintain peripheral sensitization and central sensitization [28]. Peripheral sensitization of the pudendal nerve in endometriosis patients can lead to symptoms of pudendal nerve dysfunction such as anorectal pain, pain with bowel movement, increased bowel frequency, and pelvic floor muscle dyssynergia. Central sensitization results from an increase in membrane excitability and synaptic efficacy. The process of central sensitization results in neuroplasticity with long-term potentiation of neuronal synapses in the anterior cingulate cortex. There is altered sensory processing in the brain, loss of descending pain inhibition, and increased synaptic recruitment in pain pathways. [29] Central sensitization in endometriosis patients can lead to anxiety [30] and subsequent subconscious tensing of pelvic floor musculature which facilitates pelvic floor hypertonia. This subconscious holding pattern of the pelvic floor will contribute to the bowel symptoms mentioned above of constipation, straining and the toilet, difficulty evacuating stool, bloating and ultimately will make it difficult for patients to leave the chronic guarding pelvic pain cycle that occurs. Several studies have shown the connection between the supplemental motor area (SMA) and the pelvic floor musculature [31]. One study demonstrated the effects of central sensitization in pelvic pain patients particularly on the supplemental motor area (SMA) via fMRI of the brain. The SMA territory central changes that occur with central sensitization may contribute to the maintenance of the pelvic floor hypertonic state in CPPS patients. [32]
Our protocol aims to concomitantly reverse the pelvic floor myofascial pain and dysfunction, peripheral sensitization and central sensitization that exists in endometriosis patients. The approach is threefold. First, we aim to reduce spontaneous ectopic activity of peripheral nociceptors in the pudendal and posterior femoral cutaneous nerves with repetitive exposure to lidocaine 1% [33]. With this process, we are resetting and desensitizing the Nav1.7 channels involved in the aberrant firing of peripheral nociceptors [34]. Second, we aim to increase blood flow to the peripheral nerves by releasing connective tissue and fascial restrictions with hydro-dissection [35]. Neurogenic inflammation is treated both with reversing the neural ischemia and using the medication dexamethasone one time on each side [28]. Third, we aim to reset short, spastic and weak muscle spindles with trigger point injections to each muscle in the levator ani sling [36,37].
Conceptually, creating space and increasing blood flow via lysis of fascial restrictions and release of constricting hypertonic muscular spasms [38] creates a better environment for the pelvic nerves to then heal themselves. Decreasing myofascial spasm and neurogenic inflammation, will ultimately reverse peripheral sensitization and subsequently decrease central sensitization as central pain processing is maintained by afferent nociceptive input [39].
Much of the benefit of the injection protocol is mechanical in nature. However, the repetitive exposure to the anesthetic Lidocaine 1% is also crucial to reset hyperactive peripheral nociceptors and decrease the mast cell release of histamine [40]. We have realized that using dexamethasone one time on each side is sufficient to treat the neurogenic inflammation. Therefore, we have transitioned to using normal saline with lidocaine instead of using the homeopathic medication traumeel [16] with lidocaine for the subsequent treatments.
Additionally, one potential theory as to why patients are responding to the injection protocol is that we are creating space along the course of both the pudendal and posterior femoral cutaneous nerves by hydro-dissecting both Alcock’s and Obturator canal using a supine and prone approach. There is significant overlap in terms of innervation with the pudendal and posterior femoral cutaneous nerve [41]. Given their proximity and the crosssensitization that occurs in the pelvis, the pudendal nerve and the posterior femoral cutaneous nerve upregulate one another. Therefore, it is important to treat both nerves simultaneously.
Endometriosis patients are often underdiagnosed, undertreated and suffer for many years before receiving a diagnosis. Ultimately, the chronicity of symptoms is the most challenging aspect of treatment as the longer the pain and functional impairments are present the more challenging it is to reverse due to changes in mRNA and recruitment of silent nociceptors. Imprinting in the mRNA in the dorsal horn of the spinal cord is noted particularly for the pain pathway neuropeptides dynorphin, enkephalin and substance P [42]. With chronic pain and inflammation there is upregulation of silent nociceptors. One study found that silent nociceptors account for 50% of all nociceptors in visceral organs and deep somatic tissues and are sensitized by the inflammatory mediator NGF seen in endometriosis peritoneal fluid [27]. This suggests that the upregulation of silent nociceptors significantly contributes to inflammation-induced mechanical hyperalgesia and can be challenging to reverse [43]. Therefore, raising awareness about endometriosis and initiating treatment for prevalent underlying musculoskeletal disorders [44] and sensitization [45] seen in endometriosis may assist in decreasing the amount of time patients are suffering. In order to prevent the neuroplastic changes in the nervous system described above, we suggest initiating our protocol if six weeks of pelvic floor physical therapy alone does not resolve symptoms as our protocol has been shown to be extremely safe in that we use a 27G needle, no anesthesia is required, patients go straight to work and it speeds up the healing process in that it can help decrease pain and improve function.
We have retrospectively analyzed a larger cohort of seventy female patients with CPPS and Endometriosis using VAS and FPPS (Functional Pelvic Pain Scale) for outcome measurement and the results continue to be shown. Demonstrating that treatment with external ultrasound guided trigger point injections to the levator ani sling with peripheral nerve hydrodissection continues to decrease pain and improve function for these patients on a larger scale.
Conclusion
In treating endometriosis patients, we believe it is important to look at the integration of the organ systems, with the peripheral and central nervous system, muscles, and fascia. As there is no “silver bullet” or known cure for endometriosis, a multimodal, interdisciplinary medicosurgical approach to a complex multi-faceted disease process may help endometriosis patients not only decrease pain but also improve function.
Patient’s endometriosis symptoms can be explained and treated with safe, effective, non-opioid therapies. We encourage gastrointestinal physicians who see women with persistent gastrointestinal symptoms despite a negative work up and appropriate medical treatment to consider endometriosis in their differential. We propose a functional, restorative approach to ameliorate the pelvic pain symptoms associated with endometriosis. Targeting peripheral mechanisms in endometriosis-associated inflammatory pain may lead to improved treatment.
Continued research in this area is required, to help improve the quality of life for these patients. Conceptually, understanding the importance of resetting the peripheral and central nervous system and treating persisting pelvic floor muscle dysfunction that co-exists in endometriosis patients can help improve their pain and function.
Conflict of Interest Statement
There are no conflicts of interest to report.
Funding
There is no funding to report for this study.
References
2. Laux-Biehlmann A, d’Hooghe T, Zollner TM. Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends in Pharmacological Sciences. 2015 May 1;36(5):270-6.
3. Taylor RN, Hummelshoj L, Stratton P, Vercellini P. Pain and endometriosis: Etiology, impact, and therapeutics. Middle East Fertility Society Journal. 2012 Dec 1;17(4):221-5.
4. Selçuk I, Bozdag G. Recurrence of endometriosis; risk factors, mechanisms and biomarkers; review of the literature. Journal of the Turkish German Gynecological Association. 2013;14(2):98-103.
5. Allaire C, Williams C, Bodmer-Roy S, Zhu S, Arion K, Ambacher K, et al. Chronic pelvic pain in an interdisciplinary setting: 1-year prospective cohort. American Journal of Obstetrics and Gynecology. 2018 Jan 1;218(1):114-e1-12.
6. Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco Nardone F, de Cicco Nardone C, Jenkinson C, Kennedy SH, Zondervan KT, Study WE. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertility and Sterility. 2011 Aug 1;96(2):366-73.
7. Hsu AL, Khachikyan I, Stratton P. Invasive and noninvasive methods for the diagnosis of endometriosis. Clinical Obstetrics and Gynecology. 2010 Jun;53(2):413- 419.
8. Goncalves MO, Dias Jr JA, Podgaec S, Averbach M, Abrão MS. Transvaginal ultrasound for diagnosis of deeply infiltrating endometriosis. International Journal of Gynecology & Obstetrics. 2009 Feb 1;104(2):156-60.
9. Brosens J, Timmerman D, Starzinski-Powitz A, Brosens I. Noninvasive diagnosis of endometriosis: the role of imaging and markers. Obstetrics and Gynecology Clinics. 2003 Mar 1;30(1):95-114.
10. Donnez J, Squifflet J, Pirard C, Jadoul P, Wyns C, Smets M. The efficacy of medical and surgical treatment of endometriosis-associated infertility and pelvic pain. Gynecologic and Obstetric Investigation. 2002;54(Suppl. 1):2-10.
11. Donnez J, Chantraine F, Nisolle M. The efficacy of medical and surgical treatment of endometriosisassociated infertility: arguments in favour of a medicosurgical aproach. Human Reproduction Update. 2002 Jan 1;8(1):89-94.
12. Ferrero S, Evangelisti G, Barra F. Current and emerging treatment options for endometriosis. Expert Opinion on Pharmacotherapy. 2018 Jul 3;19(10):1109-25.
13. Ford B. Elagolix (Orilissa) for Endometriosis Pain. American Family Physician. 2019 Oct 15;100(8):502-4.
14. Adamson GD, Nelson HP. Surgical treatment of endometriosis. Obstetrics and Gynecology Clinics of North America. 1997 Jun 1;24(2):375-409.
15. Abbott J, Hawe J, Hunter D, Holmes M, Finn P, Garry R. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertility and Sterility. 2004 Oct 1;82(4):878-84.
16. Plavnik K, Tenaglia A, Hill C, Ahmed T, Shrikhande A. A Novel, Non-opioid Treatment for Chronic Pelvic Pain in Women with Previously Treated Endometriosis Utilizing Pelvic-Floor Musculature Trigger-Point Injections and Peripheral Nerve Hydrodissection. PM&R. 2019 Oct 4.
17. Nezhat C, Li A, Falik R, Copeland D, Razavi G, Shakib A, et al. Bowel endometriosis: diagnosis and management. American journal of Obstetrics and Gynecology. 2018 Jun 1;218(6):549-62.
18. Anaf V, Simon P, El Nakadi I, Fayt I, Simonart T, Buxant F, et al. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Human Reproduction. 2002 Jul 1;17(7):1895-900.
19. Ceccaroni M, Clarizia R, Roviglione G, Bruni F, Ruffo G, Peters I, et al. Deep rectal and parametrial infiltrating endometriosis with monolateral pudendal nerve involvement: case report and laparoscopic nerve-sparing approach. European Journal of Obstetrics and Gynecology and Reproductive Biology. 2010 Dec 1;153(2):227-9.
20. Stratton P, Khachikyan I, Sinaii N, Ortiz R, Shah J. Association of chronic pelvic pain and endometriosis with signs of sensitization and myofascial pain. Obstetrics and gynecology. 2015 Mar;125(3):719-728.
21. Aredo JV, Heyrana KJ, Karp BI, Shah JP, Stratton P. Relating chronic pelvic pain and endometriosis to signs of sensitization and myofascial pain and dysfunction. InSeminars in Reproductive Medicine 2017 Jan; 35(1):88- 97.
22. Malykhina AP. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience. 2007 Nov 9;149(3):660- 72.
23. Van Wanrooij SJ, Wouters MM, Van Oudenhove L, Vanbrabant W, Mondelaers S, Kollmann P, et al. Sensitivity testing in irritable bowel syndrome with rectal capsaicin stimulations: role of TRPV1 upregulation and sensitization in visceral hypersensitivity?. American Journal of Gastroenterology. 2014 Jan 1;109(1):99-109.
24. Farmer AD, Aziz Q. Gut pain & visceral hypersensitivity. British Journal of Pain. 2013 Feb;7(1):39-47.
25. Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L. Endometriosis is associated with central sensitization: a psychophysical controlled study. The Journal of Pain. 2003 Sep 1;4(7):372-80.
26. As-Sanie S, Harris RE, Harte SE, Tu FF, Neshewat G, Clauw DJ. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstetrics and Gynecology. 2013 Nov;122(5):1047-55.
27. Kyama CM, Mihalyi A, Simsa P, Falconer H, Fulop V, Mwenda JM, et al. Role of cytokines in the endometrialperitoneal cross-talk and development of endometriosis. Frontiers in Bioscience (Elite edition). 2009;1:444-54.
28. Matsuda M, Huh Y, Ji RR. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. Journal of Anesthesia. 2019 Feb 20;33(1):131-9.
29. Levesque A, Riant T, Ploteau S, Rigaud J, Labat JJ. Clinical criteria of central sensitization in chronic pelvic and perineal pain (convergences pp criteria): elaboration of a clinical evaluation tool based on formal expert consensus. Pain Medicine. 2018 Oct 1;19(10):2009-15.
30. Schlereth T, Heiland A, Breimhorst M, Fechir M, Kern U, Magerl W, Birklein F. Association between pain, central sensitization and anxiety in postherpetic neuralgia. European Journal of Pain. 2015 Feb;19(2):193-201.
31. Kuhtz-Buschbeck JP, Van der Horst C, Wolff S, Filippow N, Nabavi A, Jansen O, Braun PM. Activation of the supplementary motor area (SMA) during voluntary pelvic floor muscle contractions—an fMRI study. Neuroimage. 2007 Apr 1;35(2):449-57.
32. Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, Farmer MA, Apkarian AV, Mackey S, Martucci KT, Clauw DJ. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. The Journal of Urology. 2014 Sep;192(3):947-55.
33. Tu FF, Hellman KM, Backonja MM. Gynecologic management of neuropathic pain. American Journal of Obstetrics and Gynecology. 2011 Nov 1;205(5):435-43.
34. Dick IE, Brochu RM, Purohit Y, Kaczorowski GJ, Martin WJ, Priest BT. Sodium channel blockade may contribute to the analgesic efficacy of antidepressants. The Journal of Pain. 2007 Apr 1;8(4):315-24.
35. Trescot A, Brown M. Peripheral nerve entrapment, hydrodissection, and neural regenerative strategies. Techniques in Regional Anesthesia and Pain Management. 2015 Jan 1;19(1-2):85-93.
36. Tadros NN, Shah AB, Shoskes DA. Utility of trigger point injection as an adjunct to physical therapy in men with chronic prostatitis/chronic pelvic pain syndrome. Translational andrology and urology. 2017 Jun;6(3):534- 537.
37. Bartley J, Han E, Gupta P, Gaines N, Killinger KA, Boura JA, et al. Transvaginal trigger point injections improve pain scores in women with pelvic floor hypertonicity and pelvic pain conditions. Female Pelvic Medicine & Reconstructive Surgery. 2019 Sep 1;25(5):392- 6.
38. Prendergast SA, Weiss JM. Screening for musculoskeletal causes of pelvic pain. Clinical obstetrics and gynecology. 2003 Dec 1;46(4):773-82.
39. Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. PAIN®. 2009 Sep 1;145(1-2):96-104.
40. Yanagi H, Sankawa H, Saito H, Iikura Y. Effect of lidocaine on histamine release and Ca2+ mobilization from mast cells and basophils. Acta Anaesthesiologica Scandinavica. 1996 Oct;40(9):1138-44.
41. Murinova N, Krashin D, Trescot AM. Posterior femoral cutaneous nerve entrapment: Low back. InPeripheral Nerve Entrapments 2016 (pp. 605-613). Springer, Cham.
42. Delander GE, Schött E, Brodin E, Fredholm BB. Temporal changes in spinal cord expression of mRNA for substance P, dynorphin and enkephalin in a model of chronic pain. Acta Physiologica Scandinavica. 1997 Nov;161(4):509-16.
43. Prato V, Taberner FJ, Hockley JR, Callejo G, Arcourt A, Tazir B, et al. Functional and molecular characterization of mechanoinsensitive “silent” nociceptors. Cell reports. 2017 Dec 12;21(11):3102-15.
44. Tu FF, As-Sanie S, Steege JF. Prevalence of pelvic musculoskeletal disorders in a female chronic pelvic pain clinic. The Journal of Reproductive Medicine. 2006 Mar;51(3):185-9.
45. Levesque A, Riant T, Ploteau S, Rigaud J, Labat JJ. Clinical criteria of central sensitization in chronic pelvic and perineal pain (convergences pp criteria): elaboration of a clinical evaluation tool based on formal expert consensus. Pain Medicine. 2018 Oct 1;19(10):2009-15.