Abstract
Primary central nervous system post-transplant lymphoproliferative disorders (PCNS-PTLD) are rare complications of transplantation. Due to PCNS-PTLD’s scarcity, optimal treatments, risk factors, and outcomes are poorly characterized. By retrospective analysis of 20 patients treated at the University of Wisconsin between the years 2000–2022, we aimed to describe patient/disease characteristics, therapies received, and survival outcomes of PCNS-PTLD. Three separate clinical and pathological databases were reviewed to identify cases, with all patients having at least 2 years of long-term follow-up. Kaplan Meier survival curves were generated for cohorts treated from 2000–2010 (Cohort 1) and 2011–2022 (Cohort 2), with univariate and multivariable analysis performed for baseline variables associated with progression-free (PFS) and overall survival (OS). The median PFS was 59.2 months (95% confidence interval [CI], 36.0–78.9 months) and the median OS was 71.2 months (95% CI, 47.6–91.6 months). Univariate analysis of patient sex, age, time to PTLD diagnosis, transplant type, transplant indication, time to treatment, therapy type, and baseline lactate dehydrogenase (LDH) showed that only lower LDH was associated with improved PFS (p=0.008) and OS (p=0.008). Over 2 decades, treatment practices had dramatically shifted from the frequent use of high-dose/intrathecal methotrexate and whole brain radiation in 75% of patients in Cohort 1, to use of first-line rituximab in 83% of patients in Cohort 2. Despite these key changes, there was no significant difference in OS or PFS between decades. Our findings support the use of less toxic therapies such as rituximab for PCNS-PTLD and encourage further investigation to optimize therapeutic approaches.
Keywords
Post-transplant lymphoproliferative disorder, Immune suppression, Solid organ transplant, Lymphoma, Rituximab, Methotrexate, Brain radiotherapy
Abbreviations:
ABTCR: Antibody-like T-cell Receptor; BBB: Blood-Brain Barrier; CI: Confidence Interval; CSF: Cerebrospinal Fluid; EBER: EBV-Encoded RNA; EBV: Epstein-Barr Virus; ECOG: Eastern Cooperative Oncology Group; HD-MTX: High-Dose Methotrexate; IT-MTX: Intrathecal Methotrexate; LDH: Lactate Dehydrogenase; OS: Overall Survival; PCNSL: Primary Central Nervous System Lymphoma; PCNS-PTLD: Primary Central Nervous System Post-Transplant Lymphoproliferative Disorder; PFS: Progression-Free Survival; PTLD: Post-Transplant Lymphoproliferative Disorder; ROC: Receiver Operator Characteristic; TCR: T-cell Receptor; UW: University of Wisconsin; WBRT: Whole-Brain Radiotherapy.
Introduction
Post-transplant lymphoproliferative disorders (PTLD) include a wide spectrum of diseases involving lymphoid proliferations in the setting of a solid organ or allogeneic hematopoietic stem cell transplant [1–4]. While extra-nodal presentation of PTLD is common, primary central nervous system PTLD (PCNS-PTLD) is a distinct entity involving the abnormal expansion of a lymphoproliferative population within the CNS without evidence of systemic PTLD [1,5–9]. Because PCNS-PTLD is a relatively rare complication of transplantation, its understanding is limited to case reports and retrospective case series without any unifying treatment guidelines [5–9].
The development of PTLD is predominantly associated with immunosuppressive therapy and Epstein-Barr Virus (EBV)-driven lymphoproliferation [10,11]. In EBV-associated PTLD, it is believed the impaired T-cell surveillance from immunosuppressive therapies enables EBV-transformed B-cells to undergo proliferation and expansion [12]. In contrast, the pathogenesis of EBV-negative PTLD is less clearly defined. Nevertheless, genomic profiling has identified key differences between EBV-positive and EBV-negative PTLD, with EBV-negative disease sharing more common clinical and biological characteristic to de novo lymphomas of immunocompetent patients [13].
As PTLD is likely driven by immunosuppression, treatment options generally include reduction of immunosuppression in addition to cytotoxic chemotherapy, monoclonal antibody therapy, and/or radiation therapy. Given the limited therapies that adequately penetrate the blood-brain barrier (BBB) with activity in lymphoproliferative disease, the treatment approaches for PCNS-PTLD have historically been similar to that of primary CNS lymphoma [6, 8,14–20]. Overall, treatment responses in PCNS-PTLD have been reported to be largely variable, with mortality rates of approximately 60% [6,8,20].
In 2013, our group published a case series of 10 patients with PCNS-PTLD [9]. Over the last 10 years, we hypothesized that additional cases of PCNS-PTLD diagnosed and treated within the University of Wisconsin (UW) system could provide additional insight into optimal treatments and modern outcomes of this disease. Our primary aim was to provide descriptive information on patient characteristics and therapies administered, with additional comparisons made between patient cohorts diagnosed and treated from 2000–2010 and 2011–2022. The time-derived cohort comparison was primarily based on the initial decade of observational data from this institution’s previously published experience in PCNS-PTLD [9], and not based on a defined practice or guideline shift from 2010 to 2011.
Methods
Data collection and inclusion criteria
Cases of PCNS-PTLD were identified at UW from 1/1/2000 through 1/1/2022 utilizing three separate clinical, transplant, and pathology databases. Institutional Review Board approval was obtained for this investigation. Patients aged ≥18 years who had received a solid organ transplant and subsequent diagnosis of PCNS-PTLD were eligible for inclusion. Patients were excluded if any additional systemic sites of lymphoma were identified, if treatment was not administered at UW, or if ≥2 years of follow-up data was not available. Once patients were identified, patient and disease characteristics were collected by manual chart review.
Statistical analyses
To determine statistical significance, due to the small sample size, Fisher's Exact test was used for all categorical outcomes and Student's t-test was used for all continuous outcomes. To evaluate survival outcomes, we conducted a Kaplan-Meier survival analysis to estimate overall survival (OS) and/or progression-free survival (PFS) rates. The Kaplan-Meier survival curves were generated to visualize survival distributions. Median survival times and survival probabilities at specific time points were calculated along with their 95% confidence intervals (CIs). Differences between survival curves were assessed using the log-rank test, a statistical test to compare the equality of survival distributions across groups. Multivariable survival analysis was performed using proportional hazard regression to analyze the effect of Eastern Cooperative Oncology Group (ECOG) performance status, lactate dehydrogenase (LDH), and age at diagnosis with time from diagnosis to death or last visit as a response variable.
Results
Patient characteristics
Twenty cases of PCNS-PTLD were identified with key characteristics presented in Table 1. The median age at transplant was 47 years (range, 23–66 years). Twelve patients were women and 8 were men. Twelve patients were Caucasian, 3 Black or African American, 2 Hispanic, 2 Native American/Alaskan, and 1 Asian. The majority had undergone solitary kidney transplant (12), with 7 having kidney/pancreas and 1 having liver transplant. The most common indications for transplant were type I/II diabetes (9), focal segmental glomerulosclerosis (5), hypertensive nephropathy (3) and IgA nephropathy (3).
|
|
All Patients (n=20) |
Cohort 1 (1/1/2000–1/1/2011) (n=8) |
Cohort 2 (1/2/2011–1/1/2022) (n=12) |
P-value |
|
Sex (n) |
|
|
|
0.373 |
|
Male |
12 |
6 (75%) |
6 (50%) |
|
|
Female |
8 |
2 (25%) |
6 (50%) |
|
|
Median Age at Transplant (years) |
47 (range 23-66) |
42 (range 23-56) |
48 (range 25-66) |
0.695 |
|
Race (n) |
|
|
|
0.620 |
|
Caucasian |
12 |
6 (75%) |
6 (50%) |
|
|
Black |
3 |
1 (13%) |
2 (17%) |
|
|
Hispanic |
2 |
1 (13%) |
1 (8%) |
|
|
Native American/Alaskan |
2 |
0 (0%) |
2 (17%) |
|
|
Asian |
1 |
0 (0%) |
1 (8%) |
|
|
Median ECOG Performance Status |
3 |
2 |
3 |
0.107 |
|
Type of Transplant (n) |
|
|
|
0.461 |
|
Kidney |
12 |
6 (75%) |
6 (50%) |
|
|
Kidney/pancreas |
7 |
2 (25%) |
5 (42%) |
|
|
Liver |
1 |
0 (0%) |
1 (8%) |
|
|
Indication for Transplant (n) |
|
|
|
0.647 |
|
Type I/II Diabetes Mellitus |
9 |
3 (38%) |
6 (50%) |
|
|
Focal Segmental Glomerulosclerosis |
5 |
3 (38%) |
2 (17%) |
|
|
Hypertensive Nephropathy |
3 |
2 (25%) |
1 (8%) |
|
|
IgA Nephropathy |
3 |
1 (13%) |
2 (17%) |
|
|
NASH Cirrhosis |
1 |
0 (0%) |
1 (8%) |
|
|
Median Age at Diagnosis (years) |
57 (range 27–69) |
46 (range 42–67) |
62 (range 27–69) |
0.179 |
|
Median Time from Transplant to Diagnosis (months) |
74 (range 9–364)
|
53 (range 21–244)
|
114 (range 9–364)
|
0.261 |
|
Disease Characteristics at Diagnosis (n) |
|
|
|
|
|
Leptomeningeal involvement |
4 |
3 (38%) |
1 (8%) |
0.255 |
|
CSF-positive Cytology |
1 |
1 (20%) |
0 (0%) |
0.400 |
|
EBV Viremia |
8 |
4 (57%) |
4 (40%) |
0.161 |
|
LDH 200 U/L |
8 |
2 (25%) |
6 (55%) |
0.373 |
|
Abbreviations: NASH: Non-Alcoholic Steatohepatitis; CSF: Cerebrospinal Fluid; EBV: Epstein-Barr Virus; LDH: Lactate Dehydrogenase. |
||||
Disease features at diagnosis
The median age at diagnosis of PCNS-PTLD was 57 years (range, 27–69 years) with a median time from transplant to diagnosis of 74 months (range, 9–364 months). At diagnosis, all patients had parenchymal brain lesions and 4 had leptomeningeal involvement. All diagnostic brain biopsies were positive for EBV-encoded RNA (EBER) via immunostaining. Of the 11 patients who had cytology performed on cerebrospinal fluid (CSF) at the time of PTLD diagnosis, only one sample was positive by flow cytometry. EBV viremia was detectable in 47% of patients (8 of 17 patients) at diagnosis. LDH was elevated (>200 U/L) in 42% of patients (8 of 19 patients) at the time of PTLD diagnosis. ECOG performance status was available on all patients at diagnosis.
Treatment modalities
Subtotal resection of brain lesions was performed in 5 patients. All patients underwent reduction of immune suppression at the time of PTLD diagnosis. Eleven patients received ≥2 lines of therapy; 5 received ≥3 lines and 7 received ≥4 lines. All patients had received rituximab as ≥1 line of systemic therapy, with 11 patients receiving rituximab as their only line of therapy, and 4 patients receiving ≥2 courses of rituximab. High-dose methotrexate (HD-MTX) was administered to 4 patients and whole brain radiation therapy (WBRT) to 8 patients. Other therapies included intrathecal methotrexate (IT-MTX) (4), intravenous HD-MTX (4), temozolomide (2), procarbazine chemotherapy (1), and ibrutinib (1).
Survival outcomes for all patients
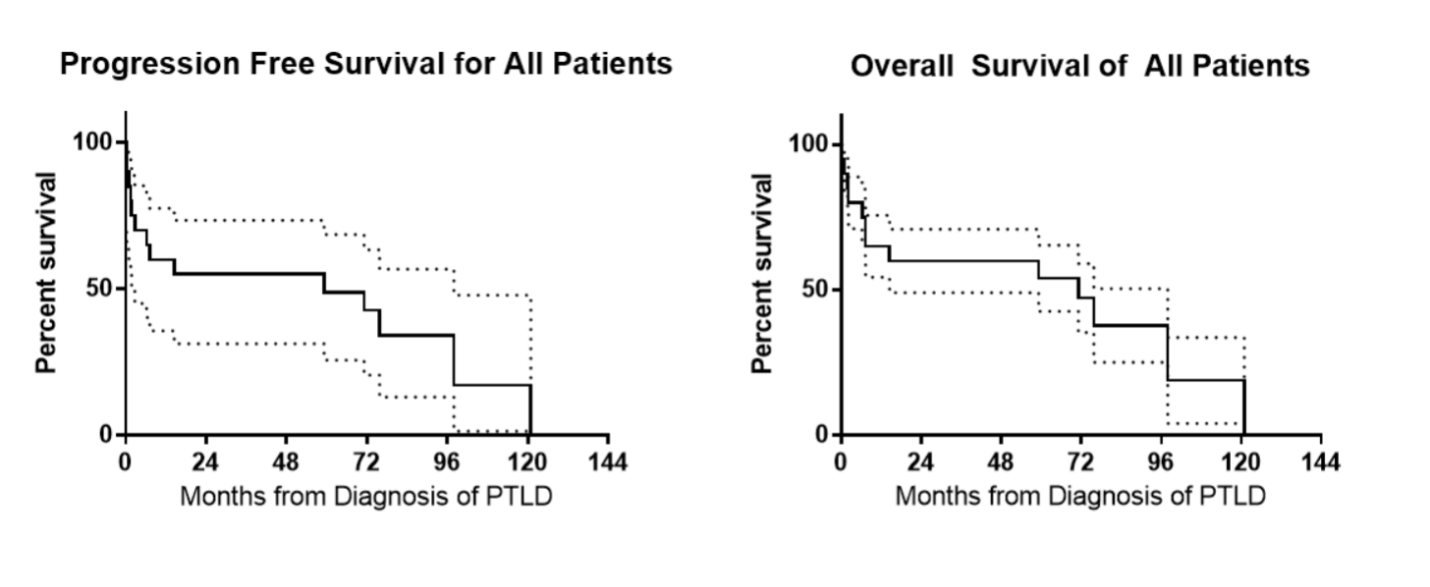
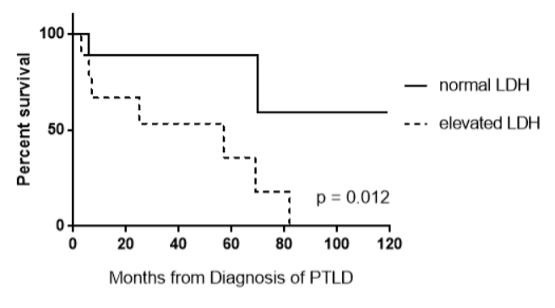
The median OS was 71.2 months (95% CI, 47.6–91.6 months). OS was analyzed with respect to the categorical variables of the type of transplant, the indication for transplant, race, date of treatment (before versus after 1/1/2011), leptomeningeal involvement, resection at diagnosis, and treatment with HD-MTX, IT-MTX, or WBRT (Table 2). The median PFS was 59.2 months (95% CI, 36.0–78.9 months). No categorical variables were predictive of worsened PFS by multivariable analysis. Evaluation of continuous variables of age at diagnosis, age at transplant, months from transplant to diagnosis, time to treatment from diagnosis, number of lines of therapy, and baseline LDH showed that only lower baseline LDH (median 206 U/L) was predictive of improved PFS between progressors versus those in continuous remission (PFS of 97.9 months [95% CI, 57.4–140.6] vs. 7.2 months [95% CI, -22.0–32.2]; p=0.008). This finding was preserved with OS as well (OS of 97.9 months [95% CI, 57.4–140.6] vs. 7.3 months [95% CI, -21.9–30.9]; p=0.008) (Figure 2). Receiver operator characteristic (ROC) analysis of LDH, identified the optimal LDH cutoff value as 238 U/L to predict death, with sensitivity of 83% and specificity of 71.5%. Thirteen deaths occurred including 3 from PTLD, 3 from infections, 1 from methotrexate toxicity, and 6 from other causes.
|
Variable |
Sub-type |
n |
Percentage |
P-value |
Hazard Ratio |
95% CI |
|
Gender |
Male |
8 |
40% |
0.129 |
2.455 |
0.771–7.824 |
|
|
Female |
12 |
60% |
|
0.407 |
0.164–2.076 |
|
Race |
White |
11 |
55% |
0.585 |
1.372 |
0.442–4.257 |
|
|
Black or African American |
3 |
15% |
0.372 |
1.555 |
0.263–9.210 |
|
|
Hispanic |
3 |
15% |
0.923 |
1.113 |
0.128–9.647 |
|
|
Asian |
1 |
5% |
0.364 |
0.336 |
0.032–3.551 |
|
|
American Indian or Native Alaskan |
2 |
10% |
0.447 |
0.579 |
0.116–2.878 |
|
Type of Transplant |
Kidney |
12 |
60% |
0.284 |
0.524 |
0.161–1.707 |
|
|
Pancreas/Kidney |
7 |
35% |
0.616 |
1.353 |
0.415–4.417 |
|
|
Liver |
1 |
5% |
0.505 |
2.899 |
0.127–66.26 |
|
Indication for Transplant |
T1DM/T2DM |
10 |
50% |
0.906 |
1.069 |
0.353–3.244 |
|
|
FSGS |
4 |
25% |
0.319 |
2.033 |
0.504–8.206 |
|
|
Hypertensive Nephropathy |
3 |
15% |
0.646 |
1.508 |
0.262–8.691 |
|
|
IgA Nephropathy |
2 |
10% |
0.093 |
0.264 |
0.056–1.249 |
|
|
NASH Cirrhosis |
1 |
5% |
0.232 |
0.307 |
0.044–2.127 |
|
Age at Diagnosis of PTLD |
65 |
16 |
80% |
0.178 |
0.359 |
0.081–1.596 |
|
|
65 |
4 |
20% |
|
2.788 |
0.626–12.41 |
|
ECOG at Diagnosis of PTLD |
0-2 |
9 |
45% |
0.070 |
0.359 |
0.118–1.089 |
|
|
2-4 |
11 |
55% |
|
2.785 |
0.918–8.446 |
|
Leptomeningeal Disease |
|
4 |
20% |
0.602 |
1.494 |
0.330–6.768 |
|
Elevated LDH |
|
8 |
42% |
0.008 |
8.980 |
2.431–33.17 |
|
EBV Viremia |
|
8 |
47% |
0.963 |
1.029 |
0.303–3.498 |
|
Decade of Diagnosis of PTLD |
2000-2010 |
8 |
40% |
0.171 |
2.567 |
0.667–9.902 |
|
|
2011-2020 |
12 |
60% |
|
0.390 |
0.1.01–1.502 |
|
Treatment Modality |
Surgical Resection |
5 |
25% |
0.709 |
1.272 |
0.360–4.493 |
|
|
IT Methotrexate |
4 |
20% |
0.651 |
1.026 |
0.329–5.929 |
|
|
HD Methotrexate |
4 |
20% |
0.598 |
0.692 |
0.176–2.723 |
|
|
WBRT |
8 |
40% |
0.808 |
1.147 |
0.378–3.487 |
|
|
First-line Rituximab |
14 |
70% |
0.518 |
0.676 |
0.206–2.217 |
|
Abbreviations: T1DM: Type-1 Diabetes Mellitus; T2DM: Type-2 Diabetes Mellitus; FSGS: Focal Segmental Glomerulosclerosis; NASH: Non-Alcoholic Steatohepatitis; ECOG: Eastern Cooperative Oncology Group; LDH: Lactate Dehydrogenase; EBV: Epstein-Barr Virus. |
||||||
Figure 1. Progression-free and overall survival of all patients.
Figure 2. Overall survival of patients with normal versus elevated LDH at the time of diagnosis.
Comparing treatment trends across decades
Outcomes were evaluated for the overall population and by decade of treatment, with Cohort 1 (n=8) including patients diagnosed from 1/1/2000–1/1/2011, and Cohort 2 including patients diagnosed from 1/2/2011–1/1/2022 (n=13) (Table 3). In comparing non-normally distributed data, distribution is reported by median (first quartile-third quartile) rather than 95% CI. Median (first-quartile-third quartile) age at diagnosis was younger in Cohort 1 (46 years, 42.5–56) versus Cohort 2 (62 years, 52.5–66), p=0.179. The median time from transplantation to the diagnosis of PCNS-PTLD (first quartile-third quartile) was 53 months (27–105) versus 114 months (49–191) for Cohort 1 and Cohort 2, respectively (p=0.261). Other characteristics at diagnosis were similar between the groups.
|
Treatment Modality |
Cohort 1 (1/1/2000–1/1/2011) (n) (N=8) |
Cohort 2 (1/2/2011–1/1/2022) (n) (N=12) |
P-value |
|
First-line Rituximab |
4 (50%) |
10 (83%) |
0.161 |
|
HD Methotrexate |
3 (38%) |
1 (8%) |
0.255 |
|
IT Methotrexate |
3 (38%) |
1 (8%) |
0.255 |
|
WBRT |
6 (75%) |
2 (17%) |
0.054 |
|
Surgical Resection |
1 (13%) |
4 (33%) |
0.603 |
|
Abbreviations: HD: High-Dose; IT: Intrathecal; WBRT: Whole Brain Radiation Therapy |
|||
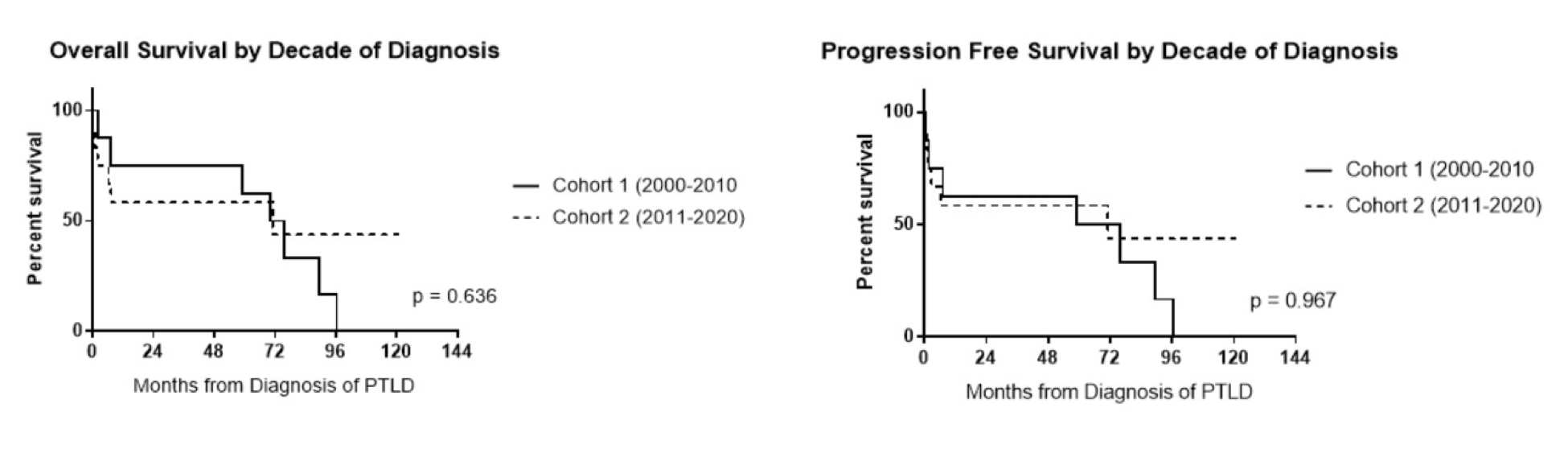
There were notable treatment differences between Cohort 1 and 2 (Table 1). First-line rituximab was given to 4/8 (50%) of patients in Cohort 1 and 10/12 (83%) of patients in Cohort 2. HD-MTX was given to 3 patients (38%) in Cohort 1 versus 1 patient (8%) in Cohort 2. Similarly, 38% of patients received IT-MTX in Cohort 1 compared to 9% in Cohort 2. WBRT was utilized more often in Cohort 1 (75%) compared with Cohort 2 (17%). PFS and OS outcomes were similar between the cohorts regardless of treatment modality (Table 1). Median PFS was 59.2 months (95% CI, 24.4–86.7) for Cohort 1 and 71.2 months (95% CI, 40.6–99.2) for Cohort 2 (p=0.967) (Figure 3). The median OS was 70.1 months (95% CI, 35.3–97.6) for Cohort 1 and 71.2 months (95% CI, 40.6–98.8) for Cohort 2 (p=0.636).
Figure 3. Overall survival and progression-free survival compared between decades.
Discussion
Central nervous system involvement of PTLD has been reported in up to 13% of all PTLD cases and is associated with inferior survival compared with systemic PTLD involvement [21]. Long-term survival in PCNS-PTLD has been reported in 43% of patients [8] as compared with an OS of >70% in all patients with PTLD, including those with systemic disease [22]. Given the relative rarity of PCNS-PTLD, prospective data are lacking regarding optimal therapy and have largely been extrapolated from treatment approaches in systemic PTLD, primary CNS lymphoma in immunocompetent patients, and therapeutic outcomes for PCNS-PTLD reported in case series.
Although there is a lack of clear consensus on management of PCNS-PTLD, there has been careful reconsideration as to whether front-line management should so closely mimic treatment approaches for PCNS lymphoma in the immunocompetent population with therapies such as HD-MTX chemotherapy and WBRT. Additionally, a conclusion of the International Primary Central Nervous System Lymphoma Collaborative Group Report in 2010 was that “an aggressive approach to tissue confirmation of diagnosis and treatment [of PCNS-PTLD] with chemotherapy or radiotherapy should be strongly considered” [20]. However, the publication of the phase II PTLD-1 trial in systemic PTLD showed benefit with a risk-stratified approach of initial rituximab monotherapy followed by escalation to chemoimmunotherapy based on response [23]. As such, it is possible that such a risk-adapted approach could also be applied to PCNS-PTLD. Based on these evolving trends in systemic PTLD, our institutional practice over the last decade has been close evaluation of response to reduction of immunosuppression and rituximab monotherapy before determining if additional systemic chemotherapy or radiotherapy is indicated in patients who are clinically stable without progressive neurologic deficits. This institutional trend in management of PCNS-PTLD is the basis for comparing treatment approaches and patient outcomes over the last 20 years.
The mechanism of rituximab activity in PCNS-PTLD is somewhat unclear, as the large molecular size of rituximab limits penetration of the BBB, and measurable CSF levels of rituximab are typically less than 1 to 2% of serum levels [24]. Some data have demonstrated that CSF penetration may increase to 3–4% of serum levels with leptomeningeal involvement by lymphoma [25]. In the setting of active CNS lymphoma/PTLD, it may be that disruption of the BBB at presentation may allow increased rituximab perfusion into CSF, which historically has been the rationale for early intensified dosing of rituximab in induction therapy for primary CNS lymphoma (PCNSL) before hypothesized reconstitution of the BBB [26]. However, in the PCNSL literature, there is debate about the additive benefit of rituximab, with data both supporting and negating the benefit of rituximab [26]. There may be properties with PCNS-PTLD that are unique to this disease entity compared with PCNSL in an immunocompetent population, including that immune reconstitution with reduction in immune suppression may be contributing to the observed benefit post-rituximab. This combination of increased rituximab penetration past a disrupted BBB and simultaneous immune reconstitution with reduction of immunosuppression may have concurrent contributing roles in the mechanism of rituximab activity in PCNS-PTLD.
The median time from transplantation to diagnosis of PCNS-PTLD tends to be a more prolonged interval than the that of systemic PTLD [8,9]. The highest incidence of systemic PTLD is reported in the first year following transplantation as opposed to the longer average reported intervals of transplant to PCNS-PTLD diagnosis of 43–54 months [27,28]. Interestingly, the reported rates of time to PCNS-PTLD more closely parallels the observed interval in our earlier cohort compared with our more modern cohort. It is possible that other factors including changes in immunosuppressive conditioning regimens pre-transplant could be contributing to this observation.
While groups investigating systemic PTLD have identified key variables such as sex, age at diagnosis, PTLD sub-type, extra-nodal site, and allograft type as significant prognostic factors [29–32], these have not been consistently reported in outcomes for PCNS-PTLD. In a multicenter retrospective analysis of 84 cases of PCNS-PTLD published in 2013, Evens et al. reported increased LDH as a predictor of inferior OS, and poor performance status as a predictor of inferior PFS [8]. In concordance with other reports of systemic PTLD [32,33], we also observed that an elevated LDH at the time of diagnosis was predictive of a shorter OS. We also observed a trend to significance of ECOG scores of 0–2 versus 3–4 for inferior PFS (p=0.068) and OS (p=0.070), but these results were not statistically significant. Multivariable survival analysis using proportional hazard regression model was also performed with time from diagnosis to death or last visit as a response variable and grouped ECOG status and other possible confounders as predictors, with similar results obtained for ECOG performance status as above.
These data demonstrate that survival outcomes for PCNS-PTLD in the last 10-15 years have not worsened at our institution despite changes in therapeutic management. We observed a significant increase in the initial use of rituximab monotherapy with more selective application of HD-MTX and WBRT, which carry increased risks for short- and long-term morbidities. This observation was maintained even with a trend towards advanced age at the time of PTLD diagnosis in Cohort 2 patients. Several phase 2 studies have shown responses to rituximab monotherapy in systemic PTLD, and current practice is to limit the application of chemoimmunotherapy to a more risk-adapted approach in patients who do not achieve sufficient response with single-agent rituximab [23,34,35]. However, rituximab has been reported to have limited CNS penetrance, which has raised questions regarding its efficacy as potential monotherapy in PCNS-PTLD [25,36,37]. While these data presented from our longitudinal single-institution analysis suggests that rituximab monotherapy may be non-inferior to HD-MTX and WBRT in regards to OS and PFS in the setting of PCNS-PTLD after solid organ transplantation, additional studies are needed to determine the optimal first-line therapy for PCNS-PTLD.
Limitations of this analysis include the small number of patients and the low incidence of PCNS-PTLD. Given the length of this study and its retrospective nature, patients did not receive a uniform evaluation at the time of PCNS-PTLD diagnosis, which may underestimate the impact of certain disease characteristics on survival outcomes that were not initially reported. Given our geography and high-volume of kidney transplantations, our patients have limited representation regarding self-identified race and transplant type, both of which may limit this study’s generalizability.
Next steps in PCNS-PTLD management should include incorporation of novel and targeted agents to improve outcomes beyond what has been realized with risk-adaptive therapy following front-line rituximab. Additional approaches such as application of cell-free DNA and circulating tumor DNA testing techniques on the CSF, and EBV titer monitoring in both the CSF and serum, may also identify patients at risk for developing PCNS-PTLD and/or relapse post-rituximab therapy. Novel therapies for PCNS-PTLD have been described in case reports including intra-thecal rituximab and chimeric antigen receptor T-cell (CAR-T) therapy that may serve as additional options in patients who fail first-line therapy [38,39]. The EBV-specific allogenic T-cell-directed agent tabelecleucel has demonstrated clinical benefit in patients with relapsed or refractory EBV-positive PTLD in a phase 3 multicenter, open-label trial or patients with systemic PTLD [40]. Based on the activity of CAR T-cell therapy in secondary CNS lymphoma observed in B-cell non-Hodgkin lymphomas in immunocompetent patients [41], it is possible tabelecleucal may ultimately prove to have activity in PCNS-PTLD. Given that the overwhelming majority of PCNS-PTLD cases are EBV+ [8,9,42], newer therapies directed at EBV, such as T-cell receptor (TCR)-like or antibody-like TCR T-cells (abTCR) targeting intracellular viral antigens like LMP2, may provide a novel means of targeted therapy in PTLD [43]. Recent studies in animal models with AbTCR T-cells targeting HLA-A*02:01-restricted LMP2 peptides (a typical EBV latency protein) found that compared with classic CAR T-cells targeting the same epitope, abTCR T-cells were observed to have increased efficiency and enhanced cytotoxicity [43].
In conclusion, PCNS-PTLD is a rare yet devastating consequence of transplantation that differs from other types of PTLD in terms of the potential for morbidity and effect on a patient's functional and cognitive status. By contrasting different decades of treatment practices, first-line rituximab monotherapy may be non-inferior to other treatment modalities while avoiding more toxic side-effects from chemotherapy. Further work is needed to characterize patients at heightened risk of developing PCNS-PTLD, to identify patients at high risk for relapse after front-line therapy, and to develop optimal treatment strategies incorporating newer novel therapies.
Acknowledgements
This study was supported by the National Cancer Institute Cancer Center Support Grant P30 CA014520.
Disclosures
Dr. Chang has received research funding from Genentech.
None of the authors have any relevant conflicts of interest to report.
Ethics Approval Statement
This study was conducted in accordance with the ethical standards of the University of Wisconsin–Madison Protocol Review and Monitoring System (PRMS). Approval was granted by the University of Wisconsin Health Sciences PRMS (Protocol No. UW23128) on November 30, 2023.
Patient Consent Statement
This case series involved a retrospective analysis of anonymized patient data. Therefore, individual patient consent was not required in accordance with institutional guidelines.
Permission to Reproduce Material from Other Sources
All content presented in this article is original and has not been reproduced from other sources.
Clinical Trial Registration
Not applicable.
Data Availability Statement
Raw data and statistical analyses are available on request.
Author Contributions
Bradley Uyemura: Data collection, wrote and edited manuscript; Zhanhai Li: Biostatical analysis, edited manuscript; David Yang: Identified cases through data base search, edited manuscript; Michael J. Fallon: Identified cases through data base search, edited manuscript; Jon S. Odorico: Identified cases through data base search, edited manuscript; Kristin Bradley: Edited manuscript; Julie E. Chang: Study concept and design, data collection, wrote and edited manuscript.
References
2. Randhawa PS, Jaffe R, Demetris AJ, Nalesnik M, Starzl TE, Chen YY, et al. Expression of Epstein-Barr virus-encoded small RNA (by the EBER-1 gene) in liver specimens from transplant recipients with post-transplantation lymphoproliferative disease. N Engl J Med. 1992 Dec 10;327(24):1710–4.
3. Patton DF, Wilkowski CW, Hanson CA, Shapiro R, Gajl-Peczalska KJ, Filipovich AH, et al. Epstein-Barr virus--determined clonality in posttransplant lymphoproliferative disease. Transplantation. 1990 Jun;49(6):1080–4.
4. Hanto DW, Frizzera G, Gajl-Peczalska KJ, Simmons RL. Epstein-Barr virus, immunodeficiency, and B cell lymphoproliferation. Transplantation. 1985 May;39(5):461–72.
5. Crane GM, Powell H, Kostadinov R, Rocafort PT, Rifkin DE, Burger PC, et al. Primary CNS lymphoproliferative disease, mycophenolate and calcineurin inhibitor usage. Oncotarget. 2015 Oct 20;6(32):33849–66.
6. Castellano-Sanchez AA, Li S, Qian J, Lagoo A, Weir E, Brat DJ. Primary central nervous system posttransplant lymphoproliferative disorders. Am J Clin Pathol. 2004 Feb;121(2):246–53.
7. Schneck SA, Penn I. Cerebral neoplasms associated with renal transplantation. Arch Neurol. 1970 Mar;22(3):226–33.
8. Evens AM, Choquet S, Kroll-Desrosiers AR, Jagadeesh D, Smith SM, Morschhauser F, et al. Primary CNS posttransplant lymphoproliferative disease (PTLD): an international report of 84 cases in the modern era. Am J Transplant. 2013 Jun;13(6):1512–22.
9. Lake W, Chang JE, Kennedy T, Morgan A, Salamat S, Başkaya MK. A case series of primary central nervous system post transplantation lymphoproliferative disorder: imaging and clinical characteristics. Neurosurgery. 2013 Jun;72(6):960–70.
10. Sampaio MS, Cho YW, Shah T, Bunnapradist S, Hutchinson IV. Impact of Epstein-Barr virus donor and recipient serostatus on the incidence of post-transplant lymphoproliferative disorder in kidney transplant recipients. Nephrol Dial Transplant. 2012 Jul;27(7):2971–9.
11. Green M, Michaels MG. Epstein-Barr virus infection and posttransplant lymphoproliferative disorder. Am J Transplant. 2013 Feb;13 Suppl 3:41–54.
12. Young L, Alfieri C, Hennessy K, Evans H, O'Hara C, Anderson KC, et al. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989 Oct 19;321(16):1080–5.
13. Ferreiro JF, Morscio J, Dierickx D, Vandenberghe P, Gheysens O, Verhoef G, et al. EBV-Positive and EBV-Negative Posttransplant Diffuse Large B Cell Lymphomas Have Distinct Genomic and Transcriptomic Features. Am J Transplant. 2016 Feb;16(2):414–25.
14. Twombley K, Pokala H, Ardura MI, Harker-Murray P, Johnson-Welch SF, Weinberg A, et al. Intraventricular rituximab and systemic chemotherapy for treatment of central nervous system post-transplant lymphoproliferative disorder after kidney transplantation. Pediatr Transplant. 2012 Sep;16(6): E201–9.
15. Nabors LB, Palmer CA, Julian BA, Przekwas AM, Kew CE. Isolated central nervous system posttransplant lymphoproliferative disorder treated with high-dose intravenous methotrexate. Am J Transplant. 2009 May;9(5):1243–8.
16. Patrick A, Wee A, Hedderman A, Wilson D, Weiss J, Govani M. High-dose intravenous rituximab for multifocal, monomorphic primary central nervous system posttransplant lymphoproliferative disorder. J Neurooncol. 2011 Jul;103(3):739–43.
17. Bonney DK, Htwe EE, Turner A, Kelsey A, Shabani A, Hughes S, et al. Sustained response to intrathecal rituximab in EBV associated post-transplant lymphoproliferative disease confined to the central nervous system following haematopoietic stem cell transplant. Pediatr Blood Cancer. 2012 Mar;58(3):459–61.
18. Lieberman F, Yazbeck V, Raptis A, Felgar R, Boyiadzis M. Primary central nervous system post-transplant lymphoproliferative disorders following allogeneic hematopoietic stem cell transplantation. J Neurooncol. 2012 Apr;107(2):225–32.
19. Izadi M, Fazel M, Saadat SH, Taheri S. Radiotherapy is the best treatment method in post transplant lymphoproliferative disorders localizing in brain: a review of the literature. Ann Transplant. 2011 Oct-Dec;16(4):126–33.
20. Cavaliere R, Petroni G, Lopes MB, Schiff D; International Primary Central Nervous System Lymphoma Collaborative Group. Primary central nervous system post-transplantation lymphoproliferative disorder: an International Primary Central Nervous System Lymphoma Collaborative Group Report. Cancer. 2010 Feb 15;116(4):863–70.
21. Maecker B, Jack T, Zimmermann M, Abdul-Khaliq H, Burdelski M, Fuchs A, et al. CNS or bone marrow involvement as risk factors for poor survival in post-transplantation lymphoproliferative disorders in children after solid organ transplantation. J Clin Oncol. 2007 Nov 1;25(31):4902–8.
22. Jagadeesh D, Tsai DE, Wei W, Alvarez Bustamante J, Wagner-Johnston ND, Berg S, et al. Post-transplant lymphoproliferative disorder (PTLD) after solid organ transplant (SOT): A multicenter real world analysis (RWA) of 877 patients (pts) treated in the modern era. Journal of Clinical Oncology. 2020;38(15_suppl):e20026-e.
23. Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012 Feb;13(2):196–206.
24. Petereit HF, Rubbert-Roth A. Rituximab levels in cerebrospinal fluid of patients with neurological autoimmune disorders. Mult Scler. 2009 Feb;15(2):189–92.
25. Rubenstein JL, Combs D, Rosenberg J, Levy A, McDermott M, Damon L, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003 Jan 15;101(2):466–8.
26. Bromberg JEC, van der Meulen M, Doorduijn JK. The Role of Rituximab in Primary Central Nervous System Lymphoma. Curr Oncol Rep. 2020 Jun 29;22(8):78.
27. Caillard S, Lelong C, Pessione F, Moulin B; French PTLD Working Group. Post-transplant lymphoproliferative disorders occurring after renal transplantation in adults: report of 230 cases from the French Registry. Am J Transplant. 2006 Nov;6(11):2735–42.
28. Trofe J, Buell JF, Beebe TM, Hanaway MJ, First MR, Alloway RR, et al. Analysis of factors that influence survival with post-transplant lymphoproliferative disorder in renal transplant recipients: the Israel Penn International Transplant Tumor Registry experience. Am J Transplant. 2005 Apr;5(4 Pt 1):775–80.
29. Ullah A, Lee KT, Malham K, Yasinzai AQK, Khan I, Asif B, et al. Post-transplant Lymphoproliferative Disorder (PTLD) in the US Population: Demographics, Treatment Characteristics, and Survival Analysis. Cureus. 2023 May 31;15(5):e39777.
30. Tajima T, Hata K, Haga H, Nishikori M, Umeda K, Kusakabe J, et al. Post-transplant Lymphoproliferative Disorders After Liver Transplantation: A Retrospective Cohort Study Including 1954 Transplants. Liver Transpl. 2021 Aug;27(8):1165–80.
31. Bishnoi R, Bajwa R, Franke AJ, Skelton WP 4th, Wang Y, Patel NM, et al. Post-transplant lymphoproliferative disorder (PTLD): single institutional experience of 141 patients. Exp Hematol Oncol. 2017 Sep 29;6:26.
32. Pearse WB, Vakkalagadda CV, Helenowski I, Winter JN, Gordon LI, Karmali R, et al. Prognosis and outcomes of patients with post-transplant lymphoproliferative disorder: a single center retrospective review. Blood. 2020 Nov 5;136:9–10.
33. Dierickx D, Tousseyn T, Sagaert X, Fieuws S, Wlodarska I, Morscio J, et al. Single-center analysis of biopsy-confirmed posttransplant lymphoproliferative disorder: incidence, clinicopathological characteristics and prognostic factors. Leuk Lymphoma. 2013 Nov;54(11):2433–40.
34. Choquet S, Leblond V, Herbrecht R, Socié G, Stoppa AM, Vandenberghe P, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood. 2006 Apr 15;107(8):3053–7.
35. González-Barca E, Domingo-Domenech E, Capote FJ, Gómez-Codina J, Salar A, Bailen A, et al. Prospective phase II trial of extended treatment with rituximab in patients with B-cell post-transplant lymphoproliferative disease. Haematologica. 2007 Nov;92(11):1489–94.
36. Larouche JF, Bergeron M, Hampson G, Illidge T, Delage R. Rituximab cerebrospinal fluid levels in patients with primary central nervous system lymphoma treated with intravenous high dose rituximab. Blood. 2011;118(21):1644.
37. Petereit HF, Rubbert-Roth A. Rituximab levels in cerebrospinal fluid of patients with neurological autoimmune disorders. Mult Scler. 2009 Feb;15(2):189–92.
38. Rösler W, Bink A, Bissig M, Imbach L, Marques Maggio E, Manz MG, et al. CAR T-cell Infusion Following Checkpoint Inhibition Can Induce Remission in Chemorefractory Post-transplant Lymphoproliferative Disorder of the CNS. Hemasphere. 2022 Jun 17;6(7):e733.
39. Aslan F, Güler S, Sezgin Evim M, Aslier M, Yazici Z, Öztürk Nazlioğlu H, et al. Successful Treatment of Central Nervous System Involvement in Posttransplant EBV-related Lymphoproliferative Disease With Intrathecal Rituximab Therapy. J Pediatr Hematol Oncol. 2023 Jul 1;45(5):e628–30.
40. Mahadeo KM, Baiocchi R, Beitinjaneh A, Chaganti S, Choquet S, Dierickx D, et al. Tabelecleucel for allogeneic haematopoietic stem-cell or solid organ transplant recipients with Epstein-Barr virus-positive post-transplant lymphoproliferative disease after failure of rituximab or rituximab and chemotherapy (ALLELE): a phase 3, multicentre, open-label trial. Lancet Oncol. 2024 Mar;25(3):376–87.
41. Cook MR, Dorris CS, Makambi KH, Luo Y, Munshi PN, Donato M, et al. Toxicity and efficacy of CAR T-cell therapy in primary and secondary CNS lymphoma: a meta-analysis of 128 patients. Blood Adv. 2023 Jan 10;7(1):32–9.
42. Velvet AJJ, Bhutani S, Papachristos S, Dwivedi R, Picton M, Augustine T, et al. A single-center experience of post-transplant lymphomas involving the central nervous system with a review of current literature. Oncotarget. 2019 Jan 11;10(4):437–48.
43. Cheng J, Hu X, Dai Z, Zeng Y, Jin J, Mu W, et al. Targeting intracellular LMP2 with costimulatory signal-armed antibody-like TCR T cells. JCI Insight. 2025 May 22;10(10):e178572.