Editorial
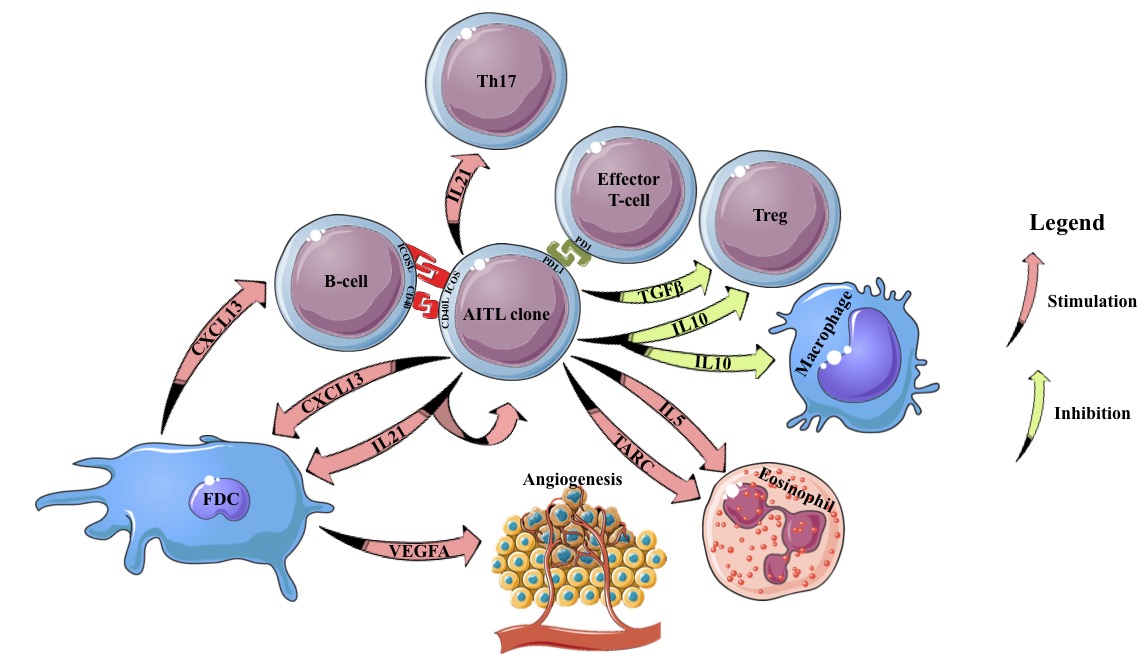
Angioimmunoblastic T cell lymphoma (AITL) is one of the most common T-cell lymphomas, second only to peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) [1,2]. Initially AITL was considered a non-malignant lymphadenopathy with immune hyperactivation [3], nowadays being classified as a PTCL [4]. The former classification was implied because of the autoimmune events that present alongside this entity with a myriad of autoantibodies being elevated [5]. These autoimmune events are being caused through the hyperstimulation of B-cells [5] (Figure 1). Today, the entity is considered to be a clonal proliferation of T follicular helper (TFH) cells, fact that could be inferred from the clinical presentation of AITL and from the expression signature of AITL clone similar to TFH cells [6,7].
Figure 1. Interactions of the AITL clone with the microenvironment. FDC: Follicular Dendritic Cell; IL: Interleukin; Treg: Regulatory T-cell; Th: T helper cell.
Regarding the mutational patterns that occur in AITL, one of the most frequently mutated genes in AITL is represented by TET2. This has been shown to be associated with T-cell lymphomas presenting a TFH phenotype, showing a possible causal link between TET2 inactivation and the acquirement of this phenotype [8,9]. Aside the role in the initiation of AITL, it has also been shown that TET2 mutations lead to a reduction in T regulatory cells (Tregs) and increased number of TFH cells [10]. Additionally, TET2 mutations were shown to destabilize FOXP3, inhibiting Treg formation, and to increase the number of T helper 17 (Th17) cells, frequently associated with autoimmune disease [11].
Two of the most important signaling molecules that lead to this immune hyperactivity are represented by CXCL13 and IL21 [12]. Both of those not only lead to B-cell stimulation, but also to Th17, in the case of IL21, and autocrine stimulation of the TFH cells [12,13]. In turn, the stimulation of Th17 cells lead to an increased probability of developing autoimmune events [14]. Moreover, the AITL clone has also been shown not only to inhibit the Treg activity through TGFβ and IL10 [12], but also to deplete these cells [15], leading to an imbalance between the Th17 and Treg cells and an increase in autoimmune events [14]. Aside these paracrine and autocrine interactions, the normal TFH cells, and probably the AITL clone by extension, interact with B-cells through direct contact. Some of the important molecules in this case are represented by CD40L [16] and ICOS [17], leading to further co-stimulation of the B-cells and exacerbation of the autoimmune events. From a pathology standpoint, these TFH cells were shown to be rosetting around B-cells, confirming their direct interaction [12].
Another important cell in the AITL microenvironment is represented by the follicular dendritic cell (FDC), which has been shown to frequently express CXCL13 and to be located in proximity to the AITL clone [18,19]. These results not only show that some of the architecture of the normal germinal center is maintained, with CXCL13 leading to a stimulation of the TFH cells, but also the possibility that FDC also lead to a supplementary activation of the B-cells through the secretion of CXCL13 [18].
Regarding the resident macrophages it has been shown that IL10 elevation and an increase in M2 polarized macrophages are associated with a worse prognosis, showing a possible immune evasion and an increase in AITL activity as IL10 secretion represents one of the functions of the normal TFH cell [20,21]. Considering the immune evasion that AITL can present, it has also been shown that PDL1 expression is frequently positive on T-cell lymphoma with a TFH phenotype, showing a potential “shield” that the AITL clone presents against the immune hyperactivation it induced [22,23].
One pathology aspect of AITL that can also be mentioned is the high degree of neo-angiogenesis present, this being caused by the AITL clone and the microenvironment it orchestrates [12]. It has been shown that VEGFA is positive both on the AITL clone and on the endothelial cells of this microenvironment, which, alongside the typical highly vascularized pathology of this entity, shows it’s importance in orchestrating the neo-angiogenesis in this case [24,25]. Furthermore, the BCL2 mRNA levels in neo-angiogenic cells was shown to correlate with VEGFA levels and with clinical prognosis, showing that in AITL the high vascular density is not only caused by the higher stimulation of the neo-angiogenic cells, but also by the decreased apoptosis in those [26].
As eosinophils were recurrently shown to be involved in variety of autoimmune diseases AITL is no exception, with high eosinophil infiltrates being present in the microenvironment. This increase in the eosinophils was explained through the increased secretion of IL5 and TARC by the AITL clone, which were also shown to correlate with the prognosis of these patients [27].
Thus, the AITL microenvironment shows the effects of the AITL clone on several immune and non-immune cells, with potential implications in a better understanding of the fundamental biology of TFH cells.
Conflict of Interest
The authors declare no conflict of interest.
Author Contribution Statement
All authors contributed to the writing and revision of this article.
References
2. Rüdiger T, Weisenburger DD, Anderson JR, Armitage JO, Diebold J, MacLennan KA, Nathwani BN, Ullrich F, Müller-Hermelink HK. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin’s Lymphoma Classification Project. Annals of Oncology. 2002 Jan 19;13(1):140-9.
3. Lukes RJ, Tindle BH. Immunoblastic Lymphadenopathy a Hyperimmune Entity Resembling Hodgkin’s Disease. New England Journal of Medicine. 1975 Jan 2;292(1):1-8.
4. Jiang M, Bennani NN, Feldman AL. Lymphoma classification update: T-cell lymphomas, Hodgkin lymphomas, and histiocytic/dendritic cell neoplasms. Expert Review of Hematology. 2017 Mar 4;10(3):239-49.
5. Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood, The Journal of the American Society of Hematology. 2017 Mar 2;129(9):1095-102.
6. Witalis M, Chang J, Zhong MC, Bouklouch Y, Panneton V, Li J, Buch T, Kim SJ, Kim WS, Ko YH, Veillette A. Progression of AITL-like tumors in mice is driven by Tfh signature proteins and TB cross talk. Blood Advances. 2020 Mar 10;4(5):868-79.
7. Fujisawa M, Chiba S, Sakata-Yanagimoto M. Recent progress in the understanding of angioimmunoblastic T-cell lymphoma. Journal of Clinical and Experimental Hematopathology. 2017;57(3):109-19.
8. Sakata-Yanagimoto M, Enami T, Yoshida K, Shiraishi Y, Ishii R, Miyake Y, Muto H, Tsuyama N, Sato-Otsubo A, Okuno Y, Sakata S. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nature Genetics. 2014 Feb;46(2):171.
9. Lemonnier F, Couronné L, Parrens M, Jaïs JP, Travert M, Lamant L, Tournillac O, Rousset T, Fabiani B, Cairns RA, Mak T. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood, The Journal of the American Society of Hematology. 2012 Aug 16;120(7):1466-9.
10. Nakatsukasa H, Oda M, Yin J, Chikuma S, Ito M, Koga-Iizuka M, Someya K, Kitagawa Y, Ohkura N, Sakaguchi S, Koya I. Loss of TET proteins in regulatory T cells promotes abnormal proliferation, Foxp3 destabilization and IL-17 expression. International Immunology. 2019 May;31(5):335-47.
11. Yue X, Lio CW, Samaniego-Castruita D, Li X, Rao A. Loss of TET2 and TET3 in regulatory T cells unleashes effector function. Nature Communications. 2019 May 1;10(1):1-4.
12. Gaulard P, de Leval L. The microenvironment in T-cell lymphomas: emerging themes. InSeminars in cancer biology 2014 Feb 1 (Vol. 24, pp. 49-60). Academic Press.
13. Kazanietz MG, Durando M, Cooke M. CXCL13 and its receptor CXCR5 in cancer: inflammation, immune response, and beyond. Frontiers in Endocrinology. 2019;10.
14. Lee GR. The balance of Th17 versus Treg cells in autoimmunity. International Journal of Molecular Sciences. 2018 Mar;19(3):730.
15. Bruneau J, Canioni D, Renand A, Marafioti T, Paterson JC, Martin-Garcia N, Gaulard P, Delfau MH, Hermine O, Macintyre E, Brousse N. Regulatory T-cell depletion in angioimmunoblastic T-cell lymphoma. The American Journal of Pathology. 2010 Aug 1;177(2):570-4.
16. Hale JS, Ahmed R. Memory T follicular helper CD4 T cells. Frontiers in Immunology. 2015 Feb 2;6:16.
17. Wan Z, Lin Y, Zhao Y, Qi H. TFH cells in bystander and cognate interactions with B cells. Immunological Reviews. 2019 Mar;288(1):28-36.
18. Ohtani H, Komeno T, Agatsuma Y, Kobayashi M, Noguchi M, Nakamura N. Follicular Dendritic Cell Meshwork in Angioimmunoblastic T-Cell Lymphoma Is Characterized by Accumulation of CXCL13+ Cells. Journal of Clinical and Experimental Hematopathology. 2015;55(2):61-9.
19. de Leval L, Rickman DS, Thielen C, Reynies AD, Huang YL, Delsol G, Lamant L, Leroy K, Briere J, Molina T, Berger F. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood, The Journal of the American Society of Hematology. 2007 Jun 1;109(11):4952-63.
20. Niino D, Komohara Y, Murayama T, Aoki R, Kimura Y, Hashikawa K, Kiyasu J, Takeuchi M, Suefuji N, Sugita Y, Takeya M. Ratio of M2 macrophage expression is closely associated with poor prognosis for Angioimmunoblastic T-cell lymphoma (AITL). Pathology international. 2010 Apr;60(4):278-83.
21. Ham JS, Park HY, Ryu KJ, Ko YH, Kim WS, Kim SJ. Elevated serum interleukin-10 level and M2 macrophage infiltration are associated with poor survival in angioimmunoblastic T-cell lymphoma. Oncotarget. 2017 Sep 29;8(44):76231.
22. Buchwalow I, Tiemann M, Samoilova V, Atiakshin D. Immunophenotyping of the PD-L1-positive cells in angioimmunoblastic T cell lymphoma and Hodgkin disease. BMC Research Notes 2020;13:139.
23. Kim S, Kwon D, Koh J, Nam SJ, Kim YA, Kim TM, Kim CW, Jeon YK. Clinicopathological features of programmed cell death-1 and programmed cell deathligand-1 expression in the tumor cells and tumor microenvironment of angioimmunoblastic T cell lymphoma and peripheral T cell lymphoma not otherwise specified. Virchows Archives. 2020 Mar 13:1-2.
24. Zhao, W.L., Mourah, S., Mounier, N., Leboeuf, C., Daneshpouy, M.E., Legrès, L., Meignin, V., Oksenhendler, E., Le Maignin, C., Calvo, F. and Brière, J., 2004. Vascular endothelial growth factor-A is expressed both on lymphoma cells and endothelial cells in angioimmunoblastic T-cell lymphoma and related to lymphoma progression. Laboratory Investigation, 84(11), pp.1512-1519.
25. Wang F, Zhang GH, Ding KY, Liu L, Weng HY. Expression and clinical pathological significance of EBER, PTEN and VEGF in angioimmunoblastic T-cell lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015 Jun;23(3):663-8.
26. Ratajczak P, Leboeuf C, Wang L, Brière J, LoiselFerreira I, Thiéblemont C, Zhao WL, Janin A. BCL2 expression in CD105 positive neoangiogenic cells and tumor progression in angioimmunoblastic T-cell lymphoma. Modern Pathology. 2012 Jun;25(6):805-14.
27. Thielen C, Radermacher V, Trimeche M, Roufosse F, Goldman M, Boniver J, de Leval L. TARC and IL-5 expression correlates with tissue eosinophilia in peripheral T-cell lymphomas. Leukemia Research. 2008 Sep 1;32(9):1431-8.