Abstract
Full-thickness skin graft (FTSG) donor sites are often closed primarily, particularly when small and located in areas with adequate tissue laxity. Despite primary closure, these sites may still experience delayed healing, discomfort, or hypertrophic scarring. Placental extract gel, derived from human placenta, is rich in growth factors, cytokines, and bioactive peptides known to facilitate wound healing and modulate scar formation through enhanced angiogenesis, anti-inflammatory action, and microbial resistance. Its biocompatibility and regenerative properties make it a promising adjunct in wound care. We report the case of a 12-year-old child with electrical burn raw areas over bilateral feet, for which reconstruction was done using an FTSG harvested from the left groin. The donor site was closed primarily, and placental extract gel was applied topically along the suture line. The wound healed without complications, with suture removal on day 7, and the resultant scar was cosmetically satisfactory. This case highlights the potential role of placental extract gel in enhancing healing and improving early scar quality, even in surgically closed wounds. Further studies may help establish its routine use as a cost-effective and easily applicable adjunct in post-operative wound care.
Keywords
Placental extract gel, Full-thickness skin graft, Wound healing, Scar modulation
Introduction
Optimal healing of a full thickness skin graft (FTSG) donor site is crucial not only for minimizing patient discomfort, but also for preventing complications such as infection, delayed epithelialization, and unsightly scarring [1,2]. Over the past few decades, researchers have explored various topical agents to enhance wound healing outcomes. Placental extract gel (PEG) has emerged as a promising candidate in wound management due to the presence of various biologically active compounds. Placental tissues are known to contain a broad spectrum of growth factors, cytokines, and extracellular matrix components that play vital roles in tissue repair and regeneration [3]. These substances contribute not only to cell proliferation and migration but also to angiogenesis and modulation of the inflammatory response, all of which are essential processes in wound healing [4]. Moreover, the anti-microbial and anti-inflammatory properties of placental extracts may provide a dual advantage in minimizing infection risks as well. Several studies have demonstrated the beneficial effects of placental extracts in various tissue models [5]. Given the unique healing requirements and potential for morbidity at donor sites, there is a compelling need to explore innovative treatment modalities that could optimize healing outcomes while preserving cosmetic integrity. Here we report the role of placental extract gel as a topical agent for enhancing the healing of FTSG donor sites.
Case Presentation
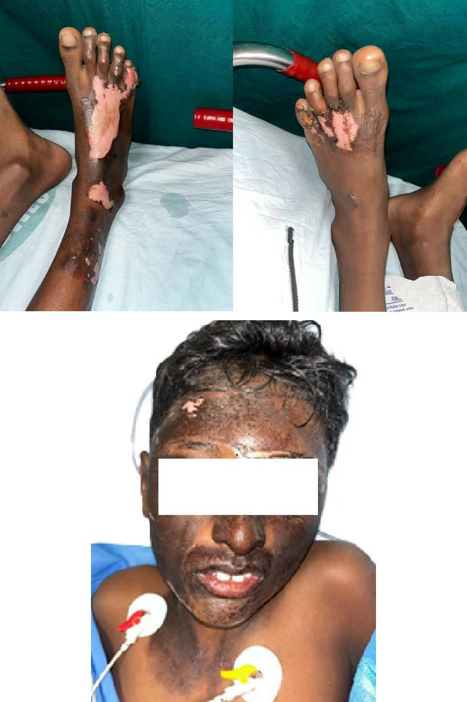
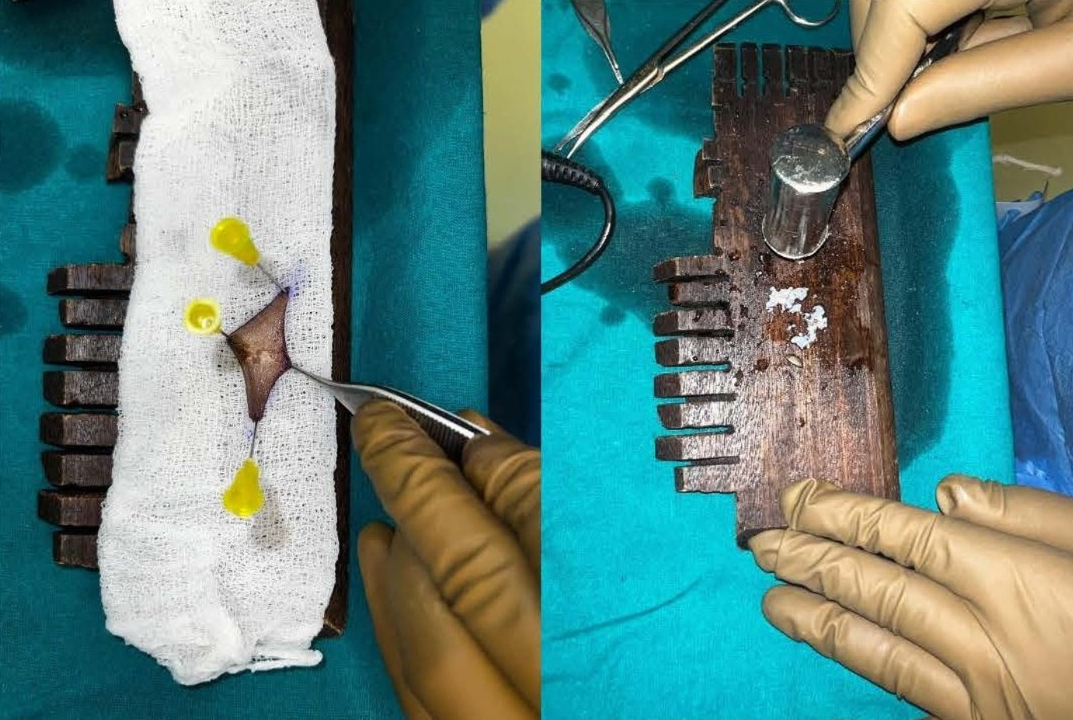
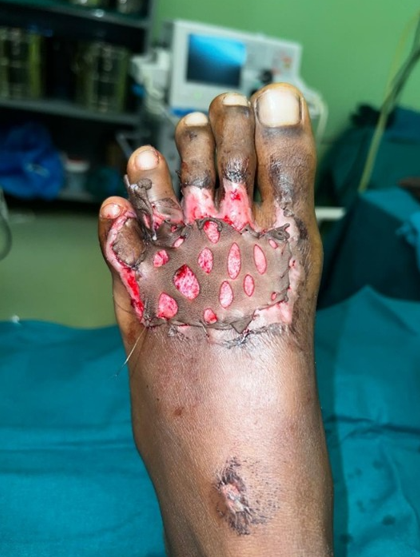
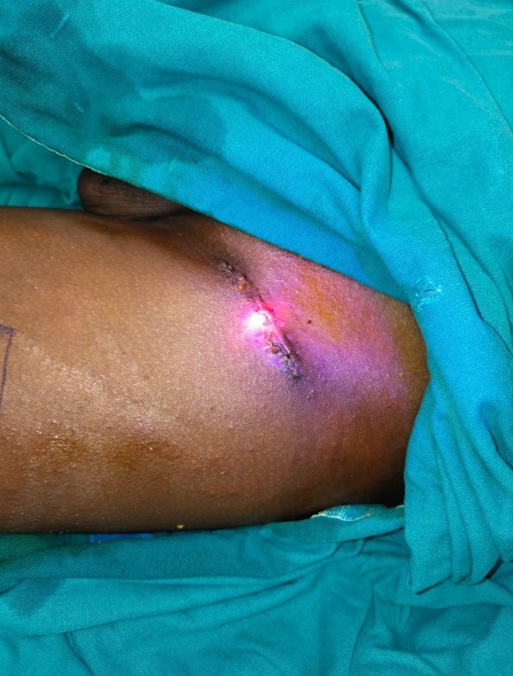
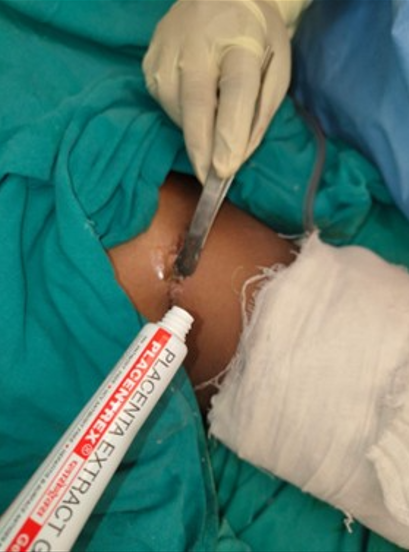
This study was conducted in a Tertiary Care Centre in the Department of Plastic Surgery after getting the departmental ethical committee approval. Informed consent was obtained from the patient's legal guardian as the patient was a minor. The patient was a 12-year-old male child who had sustained high voltage electrical burns of mixed second degree to his face, neck, and both lower limbs involving 15% total body surface area (Figure 1). He was admitted to our Tertiary Burns Centre, and initial fluid resuscitation was given as per standard guidelines. He underwent dermabrader-assisted tangential excision of deep burns over bilateral lower limbs under general anesthesia using a handheld high-speed rotating head dermabrader. A full-thickness skin graft approximately 5 cm by 2 cm was harvested from his left groin, and a dermal extract was prepared (Figure 2). A dermal extract-collagen scaffold and the harvested graft were used to cover post-burn raw areas (Figure 3). The FTSG donor site was closed primarily (Figure 4). Five grams of Human Placenta purified extract gel (Placentrex 0.1/1g, w/w) was applied to the donor site (Figure 5), and CRO-NPWT was applied. The dressing was changed every 3 days with reapplication of placental extract gel during each dressing change. Suture removal was done on 7th post operative day and placental extract gel application was continued on alternate days for another week on the maturing scar.
Figure 1. Mixed second degree patient’s face, neck, and both lower l imbs, involving 15% total body surface area.
Figure 2. FTSG harvested from left groin and used to obtain dermal extract.
Figure 3. FTSG used to cover foot raw area.
Figure 4. FTSG donor site.
Figure 5. Topical application of Placenta extract gel at FTSG donor site.
Results
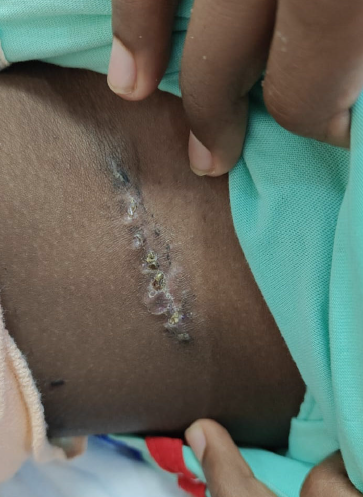
There was minimal erythema with no wound edge maceration noted during dressing changes. By post-operative day 7, the wound was well approximated with no signs of dehiscence or discharge, and hence suture removal was done (Figure 6). The surgical scar was supple with no induration at 2 weeks. The patient reported no discomfort and no adverse effects were noted during the treatment period.
Figure 6. FTSG donor site on POD-7.
Discussion
The human placenta is a rich source of various growth factors which are essential for fetal development, such as insulin-like growth factors (IGFs), epidermal growth factor (EGF), transforming growth factor-beta (TGF-β), and vascular endothelial growth factor (VEGF). These growth factors are also involved in tissue repair and regeneration in adults and play a vital role in wound healing. Placental-derived biomaterials are known for their biocompatibility, biodegradability, and low immunogenicity making them ideal for medical use [6]. The first description of preparation of placental extract was given by Prof Filatov. Various extracts of placenta have been described; however, only an aqueous extract of fresh full-term human placenta has been shown to have therapeutic potential [7]. Filatov’s procedure comprises single hot and cold aqueous extractions prepared after incubating dissected and minced placenta at 900°C and 60°C respectively. The extract is then sterilized under saturated steam (pressure 15-lbs/sq inch at 1200°C for 40 min). After filtration and addition of 1.5% (v/v) benzyl alcohol as preservative, ampoules are filled and sterilized once again under the said condition for 20 min.
Human placenta extract prevents exudate formation, aids in the removal of unhealthy tissue, and decreases bacterial load, which are required for good wound bed preparation [8]. Topical application supplements growth factors and small peptides that help in matrix formation and cell adhesion, thereby promoting fibrogenesis, neo-angiogenesis, and epithelialization. In addition, it also has anti-inflammatory and antiplatelet aggregation activity by directly modulating prostaglandin (PG) production by suppression of cyclooxygenase (COX) [9]. Current indications of the hydroalcoholic extract of human placenta include chronic nonhealing wounds, burn wounds, post-surgical wounds, and bedsores. Additionally, placental extract is useful in the management of vitiligo, alopecia, and wrinkle management [10].
Conclusion
It is anticipated that PEG could serve as a valuable adjunct in the post-operative management of patients undergoing skin graft procedures, ultimately leading to improved patient satisfaction and reduced postoperative complications. However, additional clinical research is required to establish its use in routine clinical practice with further emphasis of different extraction techniques, application forms and clinical indications.
Conflicts of Interest
None.
References
2. Miller A, Roberts D, Harrison P. Challenges in full-thickness skin grafting: Optimizing outcomes through innovative therapies. Arch Plast Surg. 2017; 44(3):189–95.
3. Wang L, Zhang X, Li M. Bioactive compounds in placental extracts and their roles in tissue repair and regeneration. Int J Mol Med. 2016; 38(2):441–8.
4. Singh R, Gupta V, Sharma P. Growth factor-mediated healing: Potential roles of placental extracts in regenerative medicine. Regen Med Res. 2019;5(1):11–9.
5. Patel R, Kumar S. Anti-inflammatory effects of placental extracts: Implications for wound healing. J Clin Invest. 2015; 125(9):3503–11.
6. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008 Apr 29; 15:88–99.
7. Wu CH, Chang GY, Chang WC, Hsu CT, Chen RS. Wound healing effects of porcine placental extracts on rats with thermal injury. Br J Dermatol. 2003 Feb; 148(2):236–45.
8. Hong JW, Lee WJ, Hahn SB, Kim BJ, Lew DH. The effect of human placenta extract in a wound healing model. Ann. Plast. Surg. 2010; 65: 96–100.
9. Shukla VK, Rasheed MA, Kumar M, Gupta SK, Pandey SS. A trial to determine the role of placental extract in the treatment of chronic non-healing wounds. J Wound Care. 2004 May; 13(5):177–9.
10. Lee S, Park J, Kim H. Placental extract in wound care: Experimental evidence and clinical applications. Wound Repair Regen. 2018; 26(2):118–25.