Abstract
Coenurosis is a socioeconomically significant zoonotic disease. The neuroimmunopathology of this disease is complex, involving a dynamic interplay between the parasite and immune system dynamics of several hosts. Although current diagnostic and treatment approaches remain inadequate, there is some solace in the recent investigations leading to proper prevention and control mechanisms of coenurosis with the One Health philosophy, integrating veterinary medicine, parasitology, immunology, community medicine, and public health—while also addressing other neglected trematode pathogens. Comprehensive research into the molecular mechanisms of coenurosis pathogenesis is still required to develop accurate diagnostic tools and effective therapeutic approaches, ultimately enabling adequate public health intervention strategies to limit the effect of this disease.
Keywords
Coenurosis, Zoonotic disease, Immune system dynamics, One Health approach, Pathogenesis
Introduction
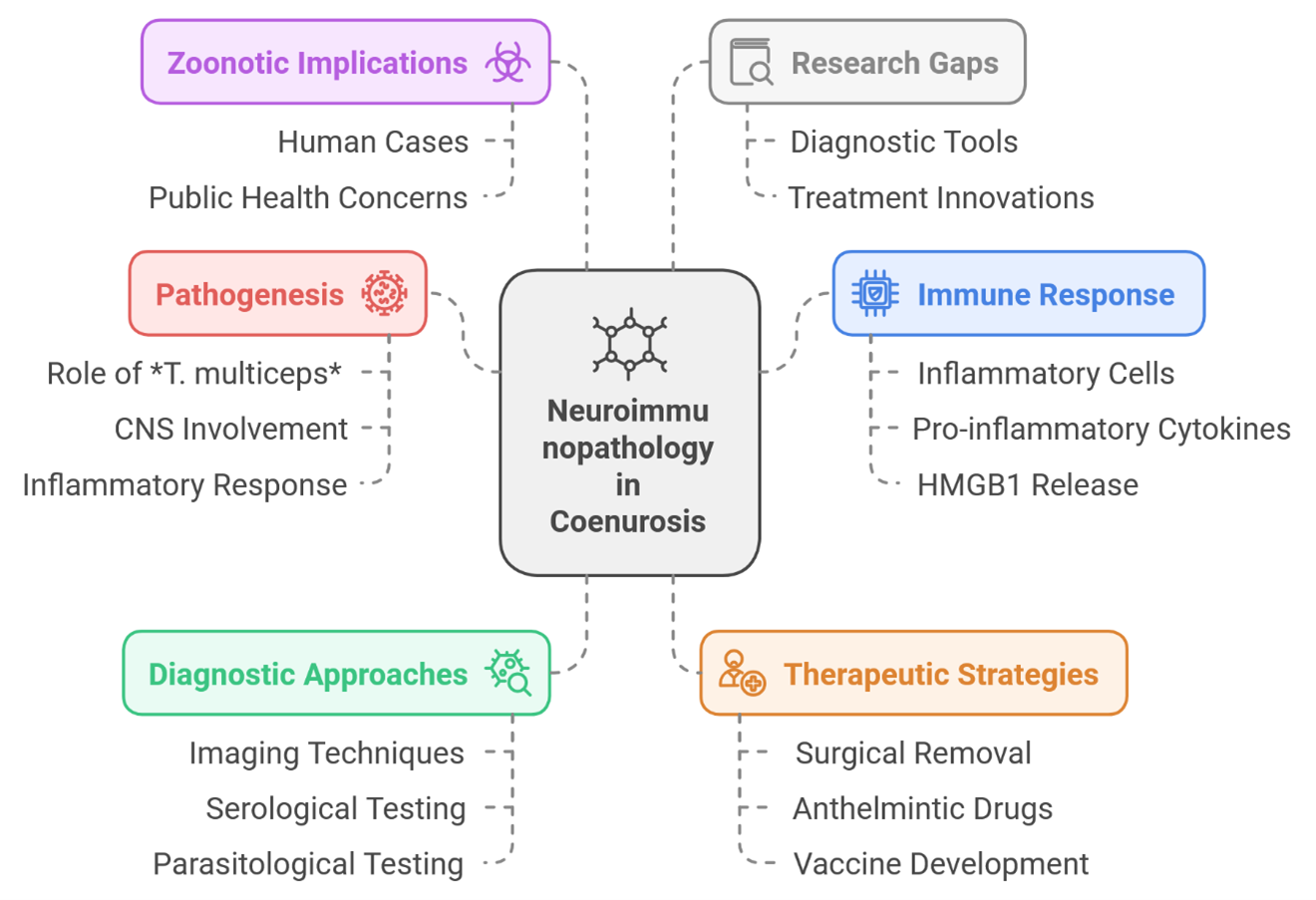
Coenurosis is the cerebrospinal cestode parasite of sheep and goat larvae of Taenia multiceps in cestode Coenura cerebralis [1,2]. This review was done to present the neuroimmune pathogenesis of coenurosis induced by its parasite and host immune response, causing severe central nervous system (CNS) damage [1]. Although primarily of veterinary importance, coenurosis is also a zoonotic disease that can affect humans [3,4]. A full description of the neuroimmunological process is necessary to shift diagnostics and therapeutics from principles to practice (Figure 1).
The Pathogenesis of Coenurosis: A Complex Interaction
Definitive hosts (canids) and intermediate hosts (sheep, goats and sometimes humans) [1,5] are necessary for the T. multiceps life cycle [1]. Intermediate hosts ingest T. multiceps eggs, and upon release from the eggs, oncospheres are released into circulation and appear in the CNS [1]. Inside this region, cystic structures (coenuri) arise, which contain a large number of protoscolices that can be enormous enough to push the surrounding brain parenchyma [2,6]. Neurological signs are a consequence of mechanical pressure. Nevertheless, the immunopathogenesis in coenurosis entirely depends on the host's inflammatory response. The initial immune access to the parasite is by recruitment of inflammatory cells, mainly lymphocytes and macrophages [6,7]. These cells try to contain the parasite, eventually leading to pro-inflammatory cytokines and HMGB1 (high mobility group box 1) release [7]. Interestingly, the upregulation of HMGB1 in neurons, endothelial cells, and glial cells disrupts the integrity of the brain parenchyma [7]. However, the host immune response is not entirely detrimental; for instance, the upregulation of ADAMTS-13 may contribute to maintaining blood-brain barrier integrity and, ultimately, promote neuroprotection by interacting with other protective systems [7]. This balance of either pro- or anti-inflammatory regulators seemingly determines how aggressive the inflammatory response and subsequent neurological damage is. Additional studies are needed to better understand these intricate interactions.
Immune Response and Neurological Damage
Inflammatory response from coenurosis cysts is due to the lysis of the cyst wall by their immediate parasiticidal activity. For years, several studies have linked the infection capable of generating a systemic inflammatory response to neurological symptoms. This widespread inflammation may affect multiple brain structures, which likely explains the extensive range of neurological symptoms—such as ataxia, seizures, and paralysis [1]. Direct neurological damage correlates with the dissemination pattern of inflammatory foci in CNS [6]. Lastly, serious qualitative features of neuroimmunopathology in coenurosis are related to an increase in oxidative stress [7]. This leads to the generation of reactive oxygen species, which, despite their origin, become harmful by damaging cellular components and contributing to neurodegeneration [7]. In coenurosis-infected mice, the levels of 8-OHdG are significantly high which is a well-established marker of oxidative DNA damage [7]. Oxidative stress might play a role in the pathogenesis of coenurosis, which needs to be scientifically verified before pursuing targeted strategies to mitigate oxidative damage.
Diagnostic Approaches and Therapeutic Strategies
Currently, coenurosis is clinically diagnosed using imaging approaches (i.e. computed tomography (CT) and magnetic resonance imaging (MRI), along with parasitological testing [1,2,8]. CT is useful for imaging cysts in the CNS [2], whereas MRI provides more detailed characterization of the cyst features and associated edema [3]. Nevertheless, these approaches might only sometimes be feasible or cheap, particularly in resource-poor countries where coenurosis is predominant [2]. In addition, early diagnosis is challenging as the clinical signs become apparent only when lesions have reached a considerable size [17]. Many studies are exploring the potential of serological testing as a tool for early detection [10–12]. These assays aim to detect early-phase infections by identifying antibodies against various parasite antigens present in the blood [10]. The antigen GP50/TmGP50 is a very exciting one with high sensitivity and specificity to detect Taenia multiceps infection in goats [10]. TmP2, an acidic ribosomal protein P2 (rP2) has also shown potential as a diagnostic marker. However, further research is warranted to optimize and assess these assays in various populations and epidemiologic settings. An emerging approach for the prediagnostic detection of preclinical cases involves measuring kynurenic acid (KYNA) levels in blood plasma using fluorescence spectroscopy [2]. Therapies available for coenurosis are scarce; surgical cyst removal remains the best treatment for cerebral coenurosis [1,13]. Surgery is always not feasible due to economic constraints and the technical challenges associated with the procedure [1]. Anthelmintic drugs such as praziquantel can eliminate the parasite, however, their efficacy is limited, and they may induce neurological symptoms and inflammatory responses [3,10]. Treatment is necessary not only to reduce burden of coenurosis but also to enable a vaccine-mediated immune response against specific parasite antigens [1].
Zoonotic Implications and Public Health
Despite the rare nature of human cases of coenurosis described in the literature [3], they serve as an interesting example to highlight the zoonotic potential of T. multiceps. While this infection primarily affects the CNS [1], it can also cause damage to extracerebral tissues throughout the body. The clinical course of coenurosis in humans is neurological, with symptom severity varying according to the size and location of the cysts which is similar to its presentation in animals. To ensure effective management, surveillance systems and public health measures must be strengthened [6]. Risk reduction can be achieved through educational and preventive programs targeting at-risk populations to lower transmission rates. Additionally, implementing control measures to reduce the prevalence of T. multiceps in dog populations is essential for breaking the cycle of transmission [14].
Research Gaps and Future Directions
Despite tremendous advances in coenurosis research, several gaps still exist. Further studies are needed to fully elucidate the complex interplay between the parasite and the host immune system, with particular emphasis on the roles of specific cytokines and chemokines in the inflammatory pathogenesis of neurological damage.
Expertise must also be developed in establishing sensitive and specific diagnostic tools for early detection of infection. At the same time, innovative treatment strategies—including vaccine development and pharmacologically effective treatments—must be designed to improve outcomes in coenurosis. Additionally, more research is needed to better understand the epidemiology of human coenurosis and to identify effective public health interventions for its prevention and control.
Conclusion
Coenurosis is a zoonotic disease of considerable socioeconomic importance. Its neuroimmunopathology is highly complex, characterized by a dynamic interaction between the parasite and the immune responses of various hosts. To develop precise diagnostic tools and effective therapeutic strategies, further in-depth research into the molecular mechanisms driving its pathogenesis is essential. Such advancements will be crucial for implementing effective public health interventions to reduce the disease's impact.
References
2. Olar LE, Tomoiagă VD, Mârza SM, Papuc I, Beteg IF, Peștean PC, et al. Computed Tomography and Fluorescence Spectroscopy Blood Plasma Analysis Study for Kynurenic Acid as a Diagnostic Approach to Chronic Coenurosis in Sheep. Life (Basel). 2024 Sep 5;14(9):1121.
3. Ing MB, Schantz PM, Turner JA. Human coenurosis in North America: case reports and review. Clin Infect Dis. 1998 Sep;27(3):519–23.
4. Otranto D, Eberhard ML. Zoonotic helminths affecting the human eye. Parasit Vectors. 2011 Mar 23;4:41.
5. Varcasia A, Tamponi C, Tosciri G, Pipia AP, Dore F, Schuster RK, et al. Is the red fox (Vulpes vulpes) a competent definitive host for Taenia multiceps? Parasit Vectors. 2015 Sep 25;8:491.
6. El-Neweshy MS, Khalafalla RE, Ahmed MMS, Al Mawly JH, El-Manakhly EM. First report of an outbreak of cerebral coenurosis in Dhofari goats in Oman. Rev Bras Parasitol Vet. 2019 Aug 1;28(3):479–88.
7. Dincel GC, Yavuz O, Yildirim S, Al-Olayan EM, El-Ashram S. ADAMTS-13 and HMGB1-induced oxidative stress in Taenia multiceps-infected animals. Sci Rep. 2023 Oct 20;13(1):17929.
8. El Akkad DM, Ramadan RM, Auda HM, Abd El-Hafez YN, El-Bahy MM, Abdel-Radi S. Improved Dot-ELISA assay using purified Sheep Coenurus cerebralis antigenic fractions for the diagnosis of Zoonotic Coenurosis. World's Veterinary Journal. 2022(3):237–49.
9. Ahn S, Oh H, Choi SY, Kim JT, Kim HC. Cerebral Coenurosis of a Long-Tailed Goral, Naemorhedus caudatus, in Korea. Korean J Parasitol. 2021 Feb;59(1):55–9.
10. Huang X, Xu J, Wang Y, Guo C, Chen L, Gu X, et al. GP50 as a promising early diagnostic antigen for Taenia multiceps infection in goats by indirect ELISA. Parasit Vectors. 2016 Dec 1;9(1):618.
11. Liu Y, Guo C, Dong X, Gu X, Xie Y, Lai W, et al. Molecular characterisation and expression analysis of two heat-shock proteins in Taenia multiceps. Parasit Vectors. 2019 Mar 12;12(1):93.
12. Huang X, Chen L, Yang Y, Gu X, Wang Y, Lai W, et al. Expression, tissue localization and serodiagnostic potential of Taenia multiceps acidic ribosomal protein P2. Parasit Vectors. 2015 Dec 1;8:613.
13. Vieitez V, Álvarez Gómez de Segura I, López Rámis V, Santella M, Ezquerra LJ. Total intravenous anaesthesia in a goat undergoing craniectomy. BMC Vet Res. 2017 Sep 15;13(1):287.
14. Cleaveland S, Thumbi SM, Sambo M, Lugelo A, Lushasi K, Hampson K, et al. Proof of concept of mass dog vaccination for the control and elimination of canine rabies. Rev Sci Tech. 2018 Aug;37(2):559–68.