Abstract
Collagen is a biopolymer with regenerative and tissue reconstruction properties used in various treatments in medicine, but few research studies were dedicated to the electrospinning of collagen derivatives loaded with essential oils as efficient antimicrobial biomaterial.
This research aimed to obtain antibacterial nanofibers based on concentrated collagen hydrolysate loaded with cloves and cinnamon essential oils as natural alternatives to synthesis products. The essential oils were successfully incorporated into collagen using electrospinning process, resulting in nanofibers with diameter from 404.8 nm to 717.6 nm and porous structure. The presence of essential oils in collagen nanofiber mats was confirmed by Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR), Ultraviolet–visible spectroscopy (UV–VIS) and antibacterial activity assays. Scanning Electron Microscopy with Energy Dispersive Spectroscopy analyses allowed evaluating the morphology and constituent elements of the nanofiber networks. Microbiological tests performed against Escherichia coli, Staphylococcus aureus and Candida albicans showed that the presence of essential oils supplemented the new collagen nanofibers with antibacterial properties. Optimization of electrospinning parameters has led to the obtaining of new collagen electrospun nanofiber mats loaded with essential oils with potential use for wound dressings, tissue engineering or protective clothing.
Keywords
Antibacterial nanofibers, Collagen hydrolysate, Clove and cinnamon essential oils, Bioactive nanofibers
Introduction
The manufacture of scaffolds for wound healing has become important, especially the formation of tissuespecific scaffolding [1,2]. In order to create scaffolds, it is essential to mimic the chemical composition, physical morphology and biological functions of the human body [3]. Scaffolds can be created by using synthetic polymers (e.g. polycaprolactone) or natural polymers (e.g. chitosan) or a combination of both. The addition of natural polymers can be very advantageous as they can avoid chronic inflammation, immune reactions and toxicity [4]. Collagen is another example of natural polymer, that is the most abundant protein in the human body and the key element of the extracellular matrix. It imprints structural integrity and tensile strength of tissues [5]. The use of collagen in scaffolds for tissue engineering and skin reconstruction provides a high stability in vivo and high biomechanical power over time [6,7].
One method used for the manufacturing of scaffolds for tissue engineering is the electrospinning process [8-10]. This technique uses electrostatic forces to stretch a dilute polymer solution as it solidifies [11]. It is a manufacturing technique of micro- and nanofibers for tissue engineering, because the nanofibers from scaffold closely mimic the size and structure of the native extracellular matrix [12]. The use of solvent and water-based systems are useful for the dissolving of collagen with important regenerative properties [13-15]. Various practices of collagen processing by electrospinning, including collagen-elastin mixtures [16], collagen-polycaprolactone scaffolds for vascular tissue engineering [17] and fibrinogen fibers allow native collagen deposits during cell growth [18]. Despite these examples of electrospinning involving collagen, there is an apparent gap in research on the integration of drugs from these electrospun scaffolds. The integration of drugs, such as antibiotics, with polymer-collagen mixtures may be critical in the future success of human tissue to accept scaffolding; there are many clinical applications for scaffolding that require controlling the bacterial growth [1]. For example, one of the requirements for the control of intra-abdominal infection is to maintain the concentration of antimicrobial drug during drug administration [19]. In this regard, sustained release of drugs is important for the success of treatment. Continuous drug release from electrospun materials has been studied, including polylactic acid matrix with diclofenac sodium (antiinflammatory) [20], polycaprolactone nanofibers loaded with metronidazole [21] and polyvinylidene fluoride with enrofloxacin (antibiotic) [22]. Given the range of success that has existed with drug-loaded electrospinning polymer and polymer-collagen blends, the next area of research could be the possibility of modifying the characteristics of scaffolds with the inclusion of collagen, whether it be mechanical, chemical or drug release characteristics [1].
The understanding of electrospinning parameters in correlation to structural characteristics of electrospun biomaterial will allow us to manipulate processes in order to optimize their suitable characteristics for different applications [23-26].
The purpose of this study was to obtain electrospun nanofibers with antioxidant and antimicrobial properties by using of collagen hydrolysate loaded with essential oils. The essential oils of clove, Eugenia caryophyllata, riched in eugenol (96.9%) and cinnamon, Cinnamomum verum, riched in cinnamic aldehyde (84.1%) [27] were used. The antimicrobial activity of cinnamon and clove essential oils is well known; however, their application in biopolymeric materials is limited [27-29]. Electrospun of collagen nanofibres loaded with clove and cinnamon essential oils were characterized by different methods: scanning electron microscopy (SEM) for the morphology, Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR / FTIR) for composition and antimicrobial activity against Escherichia coli, Staphylococcus aureus and Candida albicans.
Materials and Methods
Materials
Essential oils of clove, Eugenia caryophyllata, and cinnamon, Cinnamomum verum, from Plant SRL-Radix, Darmstadt, Germany, Alcalase 2.4 L from Novozymes, calcium oxide hydrated from Cristal R Chim SRL, Bucharest, Romania and pearl sodium hydroxide from Lachner, Neratovice, Czech Republic were using in this study.
Preparation of concentrated collagen hydrolysate
Collagen was extracted from bovine leather by alkalineenzymatic hydrolysis with 10% CaO (p.a.) (w/w) at 80oC and 0.4% Alcalase 2.4L (w/w) at 60oC. Collagen hydrolysate obtained was concentrated in a Rotary Evaporator at 60oC up to ~60% (w/w) when the collagen viscosity increased until to reach the optimal viscosity for nanofibers electrospinning [30,31].
Characterization of concentrated collagen hydrolysate
Collagen was characterized based on the following physical-chemical analyses: dry matter SR EN 4684:2006), ash content (SR EN ISO 4047:2008), total nitrogen and protein (SR ISO 5397:1996), and aminic nitrogen (in house ICPI method). The viscosity was measured with a Brookfield AMETEK DV2T TC-550 Viscometer at 25oC. The size of collagen dispersion’s particles and Zeta potential were measured by Dynamic Light Scattering (DLS) technique with Zetasizer Nano-ZS device from Malvern. The analyses were expressed as the average values of three determinations and standard deviation was mentioned.
Characterization of essential oils (EO)
Essential oils (EO) are mixtures of compounds in different compositions with specific properties. To determine the compounds from essential oils, a Thermo Scientific gas chromatograph coupled with mass spectrometer, DSQ II MS, equipped with TR-5 MS non-polar capillary column (60 m × 0.25 μm × 0.25 μm) was used. The temperature was set in the range between room temperature to 350°C, the heating rate has been scheduled between 0.1 and 120°C/min, the temperature of split/spitless injector was between 50°C and 375°C, and the pneumatic control system of pressure/carrying gas flow was of 5%.
The total content of polyphenols (TCP) was determined using the Folin-Ciocalteu reagent (p.a.), by measuring the absorbance at 740 nm (Helios Alpha UV/VIS Spectrometer, Thermospectronic). The concentration of samples in ethanol was 2620 μg/mL for clove EO and 2085 μg/mL for cinnamon EO. The samples were prepared by dissolving 0.2 g from each essential oil into 10 mL of methanol and completed with distilled water to 100 mL. For assay, a volume of 240 μL Folin-Ciocalteu’s phenol reagent and 0.68 mL Na2CO3 solution of 7.5% w/v were added to 3.6 mL distilled water and 50 μL sample. A calibration graph with gallic acid covered the range 0-1500 mg/L (y = 0.000109x, R2 = 0.996) was used. The total phenol content was calculated as a gallic acid equivalent (GAE) in mg per g of dry mass.
The radical scavenging activity (RSA) assay of essential oils was evaluated by their capacity to reduce the 2,2-diphenyl- 1-picrylhydrazyl radical (DPPH) and ABTS+ radical cation. For antioxidant assay, various concentrations from 10-500 μg/mL from clove EO and 20-2085 μg/mL from cinnamon EO were prepared from the stock sample solutions; 0.5 mL from each diluted solution was directly put into 2.5 mL DPPH solution in ethanol solution (150 μM) and gently mixed and left to stand 30 min in darkness at room temperature. For ABTS free radical scavenging assay, 140 μL of each diluted sample were mixed with 4 mL of ABTS solution and incubated for 6 min in the dark. Then the absorbance was recorded at 750 nm using UVspectroscopy. The ascorbic acid (vitamin C) (2-150 μg/ mL), as highly antioxidant agent, was used for comparison. Then the absorbance was read at 517 nm, against blank, by using a UV/VIS spectrometer (Helios Alpha UV/VIS, Thermospectronic).
Electrospinning of collagen nanofibers loaded with essential oils
Collagen hydrolysate was mixed with 10% clove or cinnamon essential oil by mechanical mixing for 30 minutes and was electrospun using a Fluidnatek equipment manufactured by Bioinicia, Spain. The electrospinning setup consisted of a high voltage power supply, a syringe pump with a needle and a plate collector. The prepared solutions were loaded in syringe and injected through the needle at a constant flow rate of 0.9 mL/h. The distance between the needle and the collector was fixed at 13 cm, and the applied voltage was set to 22 kV. Both collagen nanofibers and collagen nanofibers loaded with essential oils were electrospun on waxed paper, cotton textile and leather supports.
Attenuated total reflectance fourier-transform infrared (ATR-FTIR) analysis
ATR-FTIR analyses were performed with a Jasco 4200 FTIR Spectrometer operating in the range of 4000 to 650 cm-1, with a spectral resolution of 0.5 cm-1.
Scanning electron microscopy (SEM) analysis
The microscopic images were obtained using a FEI Quanta 200 Scanning Electron Microscope with high vacuum, at 15 kV and GSED detector at 8000x magnification.
Microbiological analyses
The antibacterial and antifungal activity of collagen nanofibers and collagen nanofibers loaded with essential oils were analyzed against Escherichia coli ATCC 11229 (gram negative) and Staphylococcus aureus ATCC 6538 (gram positive) bacteria and Candida albicans ATCC 10231 fungus, respectively. The microbiological analyses were performed by absorption method, adapted according to ISO 20743:2013 “Textiles - Determination of antibacterial activity of textile products”.
Statistical analysis
All values were expressed as a mean value ± standard deviation (SD) of three independent samples (n=3). Statistical analysis of the antioxidant scavenging assay was performed using the analysis of variance (ANOVA) (95% significant level) on each pair of interest and differences at p lower than 0.05, mean there is a statistically significant difference.
Results and Discussions
Physical-chemical characterization of concentrated collagen hydrolysate: Collagen physical-chemically analyses show the following values: dry matter of 60.4%, ash content, total nitrogen and protein reported on dry matter basis of 6.2%, 14.6% and 82.4%, respectively, pH of 8.5 and aminic nitrogen reported on protein basis of 1.4%. The viscosity was of 1623 cP.
The viscosity of collagen hydrolysate at 60.4% concentration is very high with a value of 1623 cP which makes the collagen with low molecular weight (3000 Da) to be a spinnable material as compared to low concentrated collagen hydrolysate with viscosity of 1.5 cP and without spinnable properties [30,31]. The low concentration in water, the high viscosity and known tensioactive properties of collagen hydrolysate allow a good mixture of essential oils without the need of synthesis ten sides which is an important advantage for non-toxicity potential of new collagen nanofibers.
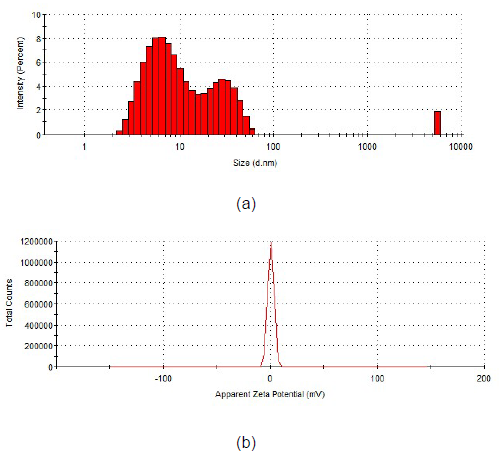
Particle size measurement for concentrated collagen hydrolysate shows the average size of 794.5 nm, with three major populations at 7.3 nm (66.8%), 29 nm (31.4%) and 5560 nm (1.8%), with polydispersity of 0.718 and Zeta potential of 0.51mV (Figure 1). The results show a low stability of concentrated collagen hydrolysate, with high polydispersity and high concentration of low particle sizes which confirm the associative properties of collagen hydrolysate.
Figure 1. Histogram of particle size distribution (a) and zeta potential (b) in concentrated collagen hydrolysate.
Characterization of essential oils
GS-MS analysis: The compounds from essential oils were quantified by gas chromatography coupled with mass spectroscopy-GC-MS. The retention times of the compounds identified in the composition of essential oils, the percentage of area in which they were found, are given in Table 1 and Table 2.
| No. | Retention time, min. | Compound name | Compound formula | Area percentage, % |
|---|---|---|---|---|
| 1 | 24.708 | Eugenol | C10H12O2 | 96.999 |
| 2 | 26.224 | a-Caryophyllene | C15H24 | 1.971 |
| 3 | 29.198 | Acetyl eugenol | C12H14O3 | 0.728 |
| 4 | 30.453 | Caryophyllene oxide | C15H24O | 0.122 |
| No. | Retention time, min. |
Compound name | Compound formula |
Area percentage, % |
|---|---|---|---|---|
| 1 | 5.707 | Cardene | C8H8 | 0.067 |
| 2 | 6.775 | Tricyclene | C10H16 | 0.039 |
| 3 | 7.256 | Bicyclo[3.1.1]hept-2-ene, 2,6,6-trimethyl- | C10H16 | 3.314 |
| 4 | 7.793 | Camphene | C10H16 | 1.874 |
| 5 | 8.338 | Benzaldehyde | C7H6O | 0.809 |
| 6 | 8.974 | L-ß-pinene | C10H16 | 0.323 |
| 7 | 10.856 | 1,5,5-Trimethyl-6-methylene-cyclohexene | C10H16 | 0.031 |
| 8 | 11.225 | o-Cymol | C10H14 | 0.144 |
| 9 | 11.391 | ß-Terpinyl acetate | C H O 12 20 2 |
0.796 |
| 10 | 11.447 | Eucalyptol | C10H18 | 2.237 |
| 11 | 12.856 | 4-Carene | C10H16 | 0.051 |
| 12 | 13.152 | Acetophenone | C8H8O | 0.034 |
| 13 | 14.216 | Terpinolen | C10H16 | 0.132 |
| 14 | 14.950 | Nerolido | C15H26O | 0.114 |
| 15 | 15.341 | Fenchol, exo- | C10H18 | 0.077 |
| 16 | 16.656 | Cinnamaldehyde | C9H8O | 0.049 |
| 17 | 16.853 | Bicyclo [4.1.0] heptane, 7-(1-methylethylidene)- | C10H16 | 0.116 |
| 18 | 17.299 | Isobornylthiocyanoacetate | C13H19NO2 S | 0.054 |
| 19 | 17.704 | 5-Methylene-1,3a,4,5,6,6a-hexahydropentalen-1-ol | C9H12O | 1.160 |
| 20 | 17.873 | 1,2-Epoxy-5,9-cyclododecadiene | C12H18O | 0.026 |
| 21 | 17.986 | 2-Methylbenzofuran | C9H8O | 0.097 |
| 22 | 18.238 | 5-Isopropyl-2-methylbicyclo [3.1.0] hexan-2-ol | C10H18O | 0.404 |
| 23 | 18.826 | a-Terpineol | C10H18O | 1.367 |
| 24 | 21.862 | Cinnamaldehyde | C9H8 O | 84.125 |
| 25 | 22.285 | Bornyl acetate | C12H20O2 | 1.746 |
| 26 | 24.969 | Copaene | C15H24 | 0.128 |
| 27 | 26.171 | Aromadendrene | C15H24 | 0.108 |
| 28 | 26.968 | Cinnamyl acetate | C H O 11 12 2 |
0.236 |
| 29 | 28.989 | d-Cadinene | C15H24 | 0.034 |
| 30 | 31.523 | ß-Guaiene | C15H24 | 0.020 |
Eugenol is the major and characteristic compound in clove essential oil with an area percentage of 96.999% of the composition, while the other compounds have an area percentage of less than 2%.
A large amount of clove essential oil is used in dental therapy. Also, it is used in pharmaceuticals with carminative and antiemetic action.
The major compound found in cinnamon essential oil is cinnamaldehyde with an area percentage of 84.125%, while the other compounds have an area percentage less than 3%. Cinnamon essential oil is used in pharmaceuticals as digestive and carminative.
TCP Assay: The total phenol content was determined for clove and cinnamon essential oils. Clove EO shows 578.24 ± 48 mg GAE/g dry substance, while the cinnamon EO contains 24.58 ± 0.58 mg GAE/g dry substance.
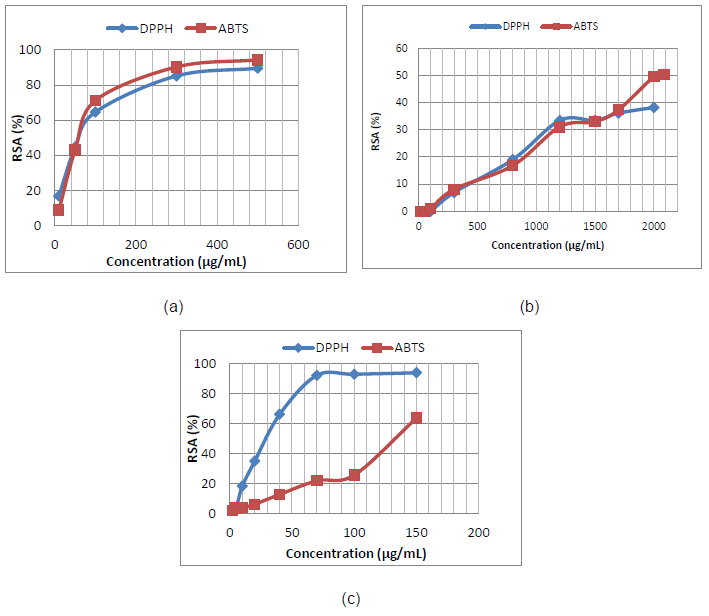
Antioxidant activities: The DPPH and ABTS free radical scavenging assay of clove EO at different concentrations such as 50-500 μg/mL (p = 0.002 and and p = 0.005) and cinnamon EO in concentrations of 20-2085 μg/mL (p = 0.007 and and p = 0.002) was investigated and shown in Figures 2a and 2b.
Figure 2. DPPH and ABTS free radical scavenging activity for clove EO (a); cinnamon EO (b) in comparison with ascorbic acid (c).
Overall, the clove EO shows the higher antioxidant activity. The EC50 values of ~60 μg/mL were found for DPPH and ABTS antioxidant assays of clove EO (for DPPH, p = 0.002, and for ABTS, p = 0.005), while cinnamon EO shows EC50 value of 2000 μg/mL for ABTS. The EC50 in the case of DPPH assay was not detected for cinnamon EO at 30 min, highlighting the lowest antioxidant potential of cinnamon EO. The antioxidant activities of ascorbic acid (control) were also shown in Figure 2(c). The EC50 values for ascorbic acid were 39 μg/mL for DPPH and 131 μg/mL for ABTS assay (p = 0.003 for both assays) [32,33].
ATR-FTIR analysis
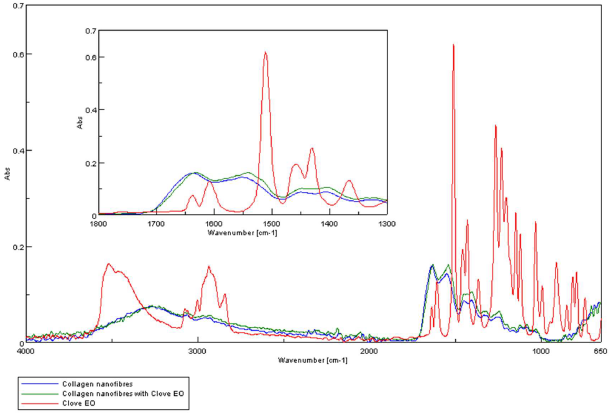
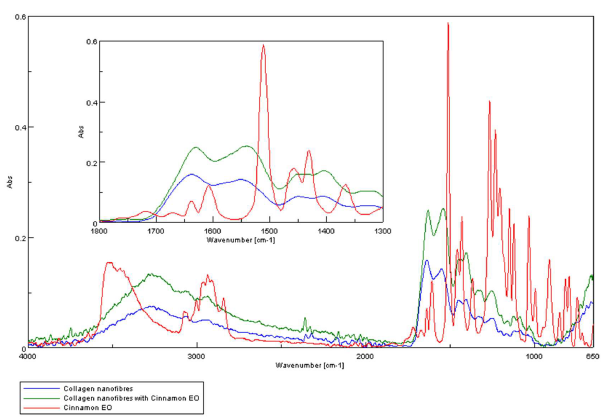
The FTIR analysis aimed at identification of clove and cinnamon essential oils in collagen nanofibers loaded with essential oils. ATR-FTIR spectra for clove essential oil, collagen nanofibers and collagen nanofibers loaded with clove essential oil are presented in Figure 3. The ATR-FTIR spectra for cinnamon essential oil, collagen nanofibers and collagen nanofibers loaded with cinnamon essential are presented in Figure 4.
Figure 3. ATR-FTIR spectra for: clove essential oil, collagen hydrolysate nanofibers loaded with clove essential oil and collagen hydrolysate nanofibers.
Figure 4. ATR-FTIR spectra for: cinnamon essential oil, collagen hydrolysate nanofibers loaded with cinnamon essential oil and collagen hydrolysate nanofibers.
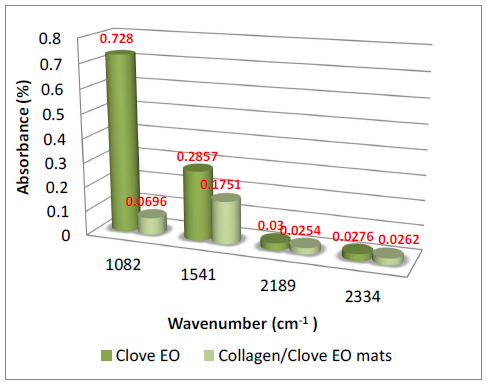
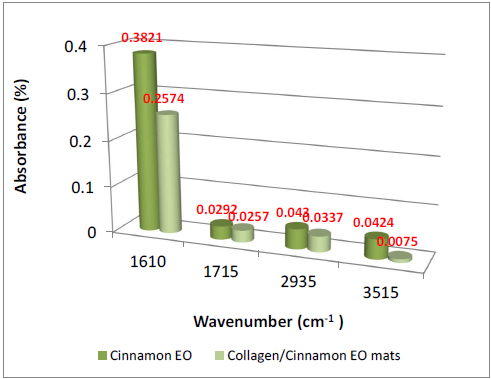
The presence of essential oils is given by the existence of maximum absorption of characteristic bands at: 3868- 3727 cm-1 (-OH groups attributed to phenols), 2944-2935 cm-1 (-CH2 group), 1637 cm-1 (-C=O), 1461-1456 cm-1(-CH2- bend), 1430 cm-1 (-C-H group), 1366 cm-1-1082 cm-1 (-C-O-C group); 1121 cm-1 (C-C group), and 502 cm-1-851 cm-1 (alkyl group) [23-25] (Figures 3 and 4).The absorption bands of clove essential oil from 1082 cm-1, 1541 cm-1, 2189 cm-1 and 2334 cm-1 were found in collagen hydrolysate nanofibers loaded with clove essential oil too proving the successfully essential oil loading. The absorption bands of cinnamon essential oil from 1610 cm-1, 1715 cm-1, 2935 cm-1 and 3515 cm-1 were found in collagen hydrolysate nanofibers loaded with cinnamon essential oil too proving the successfully essential oil loading.
Collagen nanofibers and collagen nanofibers loaded with essential oil (clove or cinnamon) show amide A band at 3283-3267cm-1, associated with the stretching vibrations of N-H groups, amide I band around 1629-1637 cm-1 (stretching vibrations of peptide C=O groups), amide II (around 1539-1549 cm-1, N-H bending vibrations coupled to C-N stretching vibrations) and amide III (around 1256- 1245 cm-1, C-N stretching and N-H bending vibrations of amide linkages) [30,31] (Figures 5,6).
Figure 5. Dependence of specific groups on clove essential oil in collagen hydrolysate nanofibers loaded with clove essential oil.
Figure 6. Dependence of specific groups on cinnamon essential oil in collagen hydrolysate nanofibers loaded with cinnamon essential oil.
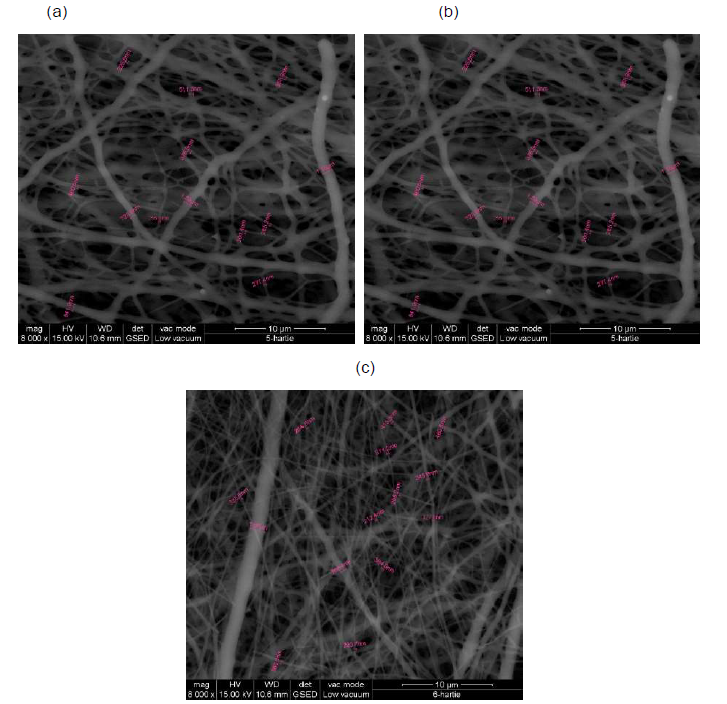
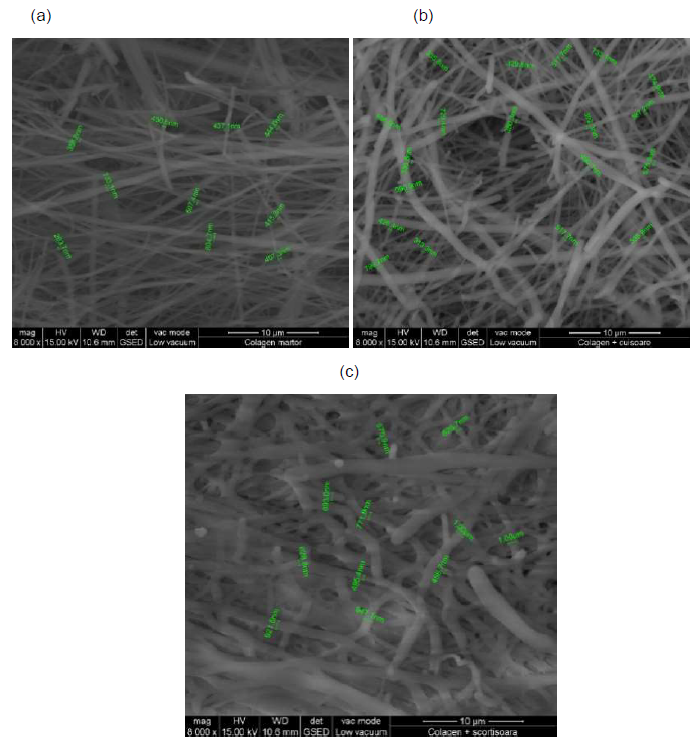
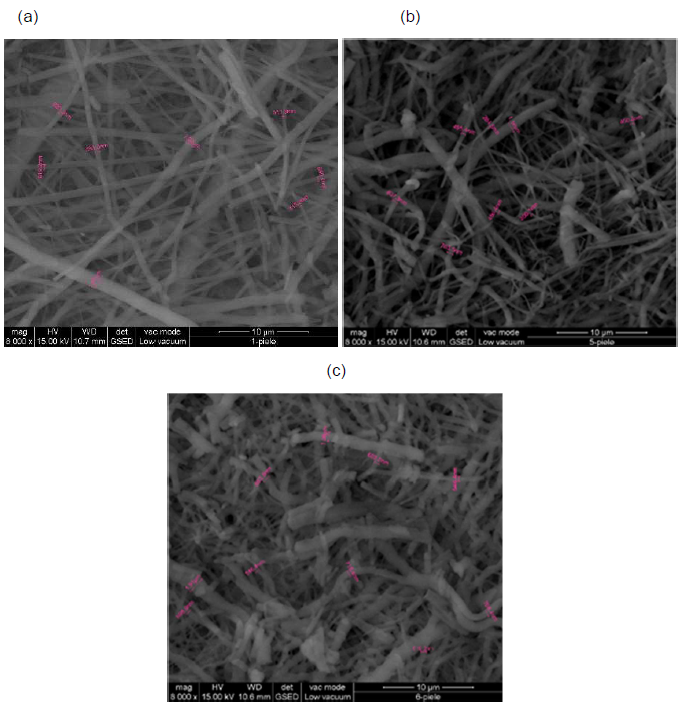
SEM analysis: SEM images for collagen hydrolysate nanofibers and collagen hydrolysate nanofibers loaded with clove and cinnamon essential oils were observed at 8000x deposited on waxed paper, cotton and leather supports are shown in Figures 7-9.
Figure 7. SEM images of collagen hydrolysate nanofibers (a), collagen hydrolysate nanofibers loaded with clove essential oil (b) and collagen hydrolysate nanofibers loaded with cinnamon essential oil (c) deposited on waxed paper support, at 8000x.
Figure 8. SEM images of collagen hydrolysate nanofibers (a), collagen hydrolysate nanofibers loaded with clove essential oil (b) and collagen hydrolysate nanofibers loaded with cinnamon essential oil (c) on cotton support, at 8000x.
Figure 9. SEM images of collagen hydrolysate nanofiber (a), collagen hydrolysate nanofibers loaded with clove essential oil (b) and collagen hydrolysate nanofibers loaded with cinnamon essential oil (c) on leather support, at 8000x.
SEM images show well-defined nanofibers with porous 3D structure with different average diameters depending on the support and the loaded essential oil in the collagen hydrolysate (Figures 7-9 and Table 3).
| Sample | The average diameter of the nanofibers, nm | ||
|---|---|---|---|
| on waxed paper | on cotton | on leather | |
| Collagen hydrolysate nanofibers | 404.8 | 485.2 | 531.2 |
| Collagen hydrolysate nanofibers loaded with clove essential oil |
429.6 | 546.4 | 568.3 |
| Collagen hydrolysate nanofibers loaded with cinnamon essential oil |
463.2 | 683.1 | 717.6 |
The average diameter of nanofibers increases from collagen nanofibers, between 404.8-531.2 nm to collagen nanofibers loaded with essential oils between 429.6-568.3 nm for clove essential oil and between 463.2 - 717.6 nm for cinnamon essential oil (Table 3).
Similar increase of nanofiber diameter was recorded when other essential oils (thyme, oregano essential oils [30,31]) loaded collagen nanofibers. The influence of different supports on nanofiber diameter wasn’t reported and represents an original contribution for future applications in the area of wound healing materials, protective cloths, etc.
Microbiological analyses
Antibacterial and antifungal activity of collagen hydrolysate nanofibers and collagen hydrolysate nanofibers loaded with clove and cinnamon essential oil against Escherichia coli, Staphylococcus aureus, and Candida albicans are presented in Tables 4-6.
| Sample | Result, CFU/mL | R, % | Log10 red. |
|---|---|---|---|
| Inoculum concentration | T0=2.4x104 | - | - |
| Collagen nanofibers | T0 =2.4x104 T24 =7.2x102 |
70 | 0.52 |
| Collagen nanofibers loaded with clove essential oil |
T0 =2.4x104 T24 =2.2x102 |
90.83 | 1.04 |
| Collagen nanofibers loaded with cinna- mon essential oil | T0 =2.4x104 T24=0 |
100 | - |
| Sample | Result, CFU/mL | R, % | Log10 red. |
|---|---|---|---|
| Inoculum concentration | T0=3.6x104 | - | - |
| Collagen nanofibers | T0 =3.6x104 T24=1.2x102 |
96.67 | 1.48 |
| Collagen nanofibers loaded with clove essential oil |
T0 =3.6x104 T24 =5x102 |
98.61 | 1.86 |
| Collagen nanofibers loaded with cinnamon essential oil |
T0=3.6x104 T24=0 |
100 | 0 |
Antibacterial activity
Antibacterial tests show reduction of colony forming unities by 90.83% for collagen nanofibers loaded with clove essential oil and 100% for collagen nanofibers loaded with cinnamon essential oil against Escherichia coli as compared to collagen nanofibers with 70% reduction properties (Table 4).
The results of microbiological analyzes show resistance against the bacterial and fungal strains used and suggest that there is a direct relationship between damage caused to the cell membrane and cell death [34]. At the bactericidal concentration used, the main mechanism of action of the major compounds in clove and cinnamon essential oil (eugenol and cinnamic aldehyde) is the disruption of the cytoplasmic membrane, which increases its nonspecific permeability [35-37].
Antifungal activity: Microbiological tests were performed for evaluation of antifungal resistance of collagen nanofibers and collagen nanofibers loaded with essential oils against Candida albicans.
Antifungal tests performed for collagen nanofibers loaded with clove and cinnamon essential oils, respectively show 100% resistance against Candida albicans while the control collagen nanofibers showed colony forming unities reduction of 76.67 % against Candida albicans (Table 6). Collagen nanofibers loaded with cinnamon essential oils show 100% resistance against Escherichia coli, Staphylococcus aureus and Candida albicans and collagen nanofibers loaded with clove essential oil show 90.83% resistance against Escherichia coli, 98.61% resistance against Staphylococcus aureus and 100% resistance against Candida albicans.
| Sample | Result, CFU/mL | R, % | Log10 red. |
|---|---|---|---|
| Inoculum concentration | T0 =2.8x104 | - | - |
| Collagen nanofibers | T0 =2.8x104 T24 =5.8x103 |
76.67 | 0.63 |
| Collagen nanofibers loaded with clove essential oil |
T0 =2.8x104 T0=0 |
100 | - |
| Collagen nanofibers loaded with cinnamon essential oil |
T0 =2.8x104 T24=0 |
100 | - |
Besides the antimicrobial activity of essential oils, other authors described the better antimicrobial activity of nanosized metals, and potent cytotoxicity effect against growth of carcinoma cells. For example, nano-sized Fe(II), Cd(II) and Zn(II) Schiff base complexes were tested on three selected bacteria (Escherichia coli, Microccus luteus and Serratia marcescence) and three fungi (Aspergillus flavus, Getrichm candidum and Fusarium oxysporum) [38]. In other paper, Abdel-Rahman et al. [39-41] investigated the cytotoxicity of Cr(III),VO(II), Ni(II), Cu (II), Pd (II), Ag (I), Pd(II), Ag(I) and Cu(II) complexes against growth of carcinoma cells. It was reported that the antimicrobial resistance of these metal oxides is better than that of their Schiff base complex and could be explained by the small particles of metal oxides which possess a higher surface / volume ratio, contributing to the high efficiency of antibacterial activities. Furthers studies regarding the biocompatibility of electrospun collagen embedded with essential oils will be necessary for validation,
Conclusions
Bioactive nanofibers were obtained based on collagen hydrolysate loaded with 10% clove or cinnamon essential oil by the electrospinning process. The collagen hydrolysate was extracted from bovine leather by alkaline-enzymatic hydrolysis having 82.4% content in protein and it was concentrated to a viscosity of 1623 cP in order to fulfill the optimal conditions for electrospinning process. The measurement of the particle size of the collagen hydrolysate revealed a major dimension of 7.3 nm in a proportion of 66.8%, followed by 29 nm (31.4%) and 5560 nm (1.8%). GC-MS analysis of essential oils shows that the eugenol was the major compound in clove essential oil, having an area percentage of 96.999%, while the major compound in cinnamon essential oil was cinnamic aldehyde with an area percentage of 84.125%.
Clove EO shows the highest antioxidant potential proved both by DPPH and ABTS assays. The morphology and diameter of nanofibers were observed by scanning electron microscopy on different support (waxed paper, cotton textiles and leather) and confirmed the increased nanofiber diameter for loaded nanofibers and for cotton and leather support. The ATR-FTIR analysis have identified the specific absorption bands of clove and cinnamon essential oils in electro spun samples loaded with essential oils.
Antibacterial tests showed 100% resistance of collagen nanofibers loaded with cinnamon essential oil against Escherichia coli, Staphylococcus aureus and Candida albicans and 90.83%, 98.61% and 100% resistance against Escherichia coli, Staphylococcus aureus Candida albicans, respectively, in the case of collagen nanofibers loaded with clove essential oil. Electro spun collagen-based nanofibers with porous 3 D structure loaded with essential oils can be used for wound dressings, tissue engineering or protective clothing.
Acknowledgements
The works were funded by the Romanian Ministry for Education and Research under research projects no PN 19170102_CREATIV-PIEL/2020 and 6PFE_4PERFORMTEX- PEL/2018.
References
2. Van Winterswijk PJ, Nout E. Tissue engineering and wound healing: an overview of the past, present, and future. Wounds: a compendium of clinical research and practice. 2007 Oct 1;19(10):277-84.
3. Jiang Q, Reddy N, Zhang S, Roscioli N, Yang Y. Waterstable electrospun collagen fibers from a non-toxic solvent and crosslinking system. Journal of Biomedical Materials Research Part A. 2013 May;101(5):1237-47.
4. Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. Journal of the Royal Society Interface. 2007 Dec 22;4(17):999-1030.
5. Sell SA, McClure MJ, Garg K, Wolfe PS, Bowlin GL. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Advanced Drug Delivery Reviews. 2009 Oct 5;61(12):1007-19.
6. Tillman BW, Yazdani SK, Lee SJ, Geary RL, Atala A, Yoo JJ. The in vivo stability of electrospun polycaprolactonecollagen scaffolds in vascular reconstruction. Biomaterials. 2009 Feb 1;30(4):583-8.
7. Agarwal S, Wendorff JH, Greiner A. Use of electrospinning technique for biomedical applications. Polymer. 2008 Dec 8;49(26):5603-21.
8. Reneker DH, Yarin AL. Electrospinning jets and polymer nanofibers. Polymer. 2008 May 13;49(10):2387- 425.
9. Baranauskaite J, Adomaviciute E, Jankauskaite V, Marksa M, Barsteigiene Z, Bernatoniene J. Formation and investigation of electrospun Eudragit E100/oregano mats. Molecules. 2019 Jan;24(3):628.
10. Unalan I, Endlein SJ, Slavik B, Buettner A, Goldmann WH, Detsch R, et al. Evaluation of electrospun poly (e-caprolactone)/gelatin nanofiber mats containing clove essential oil for antibacterial wound dressing. Pharmaceutics. 2019 Nov;11(11):570.
11. Del Valle LJ, Camps R, Díaz A, Franco L, Rodríguez- Galán A, Puiggalí J. Electrospinning of polylactide and polycaprolactone mixtures for preparation of materials with tunable drug release properties. Journal of polymer research. 2011 Nov 1;18(6):1903-17.
12. Tan AR, Ifkovits JL, Baker BM, Brey DM, Mauck RL, Burdick JA. Electrospinning of photocrosslinked and degradable fibrous scaffolds. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2008 Dec 15;87(4):1034-43.
13. Russo N, Cassinelli C, Torre E, Morra M, Iviglia G. Improvement of the physical properties of guided bone regeneration membrane from porcine pericardium by polyphenols-rich pomace extract. Materials. 2019 Jan;12(16):2564.
14. Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Advanced Drug Delivery Reviews. 2007 Dec 10;59(14):1413-33.
15. Hofman K, Tucker N, Stanger J, Staiger M, Marshall S, Hall B. Effects of the molecular format of collagen on characteristics of electrospun fibres. Journal of Materials Science. 2012 Feb 1;47(3):1148-55.
16. Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Vermes I, et al. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006 Feb 1;27(5):724-34.
17. Venugopal J, Zhang YZ, Ramakrishna S. Fabrication of modified and functionalized polycaprolactone nanofibre scaffolds for vascular tissue engineering. Nanotechnology. 2005 Aug 9;16(10):2138.
18. McManus MC, Boland ED, Simpson DG, Barnes CP, Bowlin GL. Electrospun fibrinogen: feasibility as a tissue engineering scaffold in a rat cell culture model. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2007 May;81(2):299-309.
19. Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surgical Infections. 2010 Feb 1;11(1):79-109.
20. Toncheva A, Paneva D, Manolova N, Rashkov I. Electrospun poly (L-lactide) membranes containing a single drug or multiple drug system for antimicrobial wound dressings. Macromolecular Research. 2011 Dec 1;19(12):1310-9.
21. He M, Jiang H, Wang R, Xie Y, Zhao C. Fabrication of metronidazole loaded poly (e-caprolactone)/zein core/ shell nanofiber membranes via coaxial electrospinning for guided tissue regeneration. Journal of Colloid and Interface Science. 2017 Mar 15;490:270-8.
22. He T, Wang J, Huang P, Zeng B, Li H, Cao Q, et al. Electrospinning polyvinylidene fluoride fibrous membranes containing anti-bacterial drugs used as wound dressing. Colloids and Surfaces B: Biointerfaces. 2015 Jun 1;130:278-86.
23. Heino J. The collagen family members as cell adhesion proteins. Bioessays. 2007 Oct;29(10):1001-10.
24. McCarthy JB, Vachhani B, Iida J. Cell adhesion to collagenous matrices. Peptide Science. 1996;40(4):371-81.
25. Aldana AA, Abraham GA. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. International journal of pharmaceutics. 2017 May 25;523(2):441-53.
26. Sizeland KH, Hofman KA, Hallett IC, Martin DE, Potgieter J, Kirby NM, et al. Nanostructure of electrospun collagen: Do electrospun collagen fibers form native structures?. Materialia. 2018 Nov 1;3:90-6.
27. Souza AC, Goto GE, Mainardi JA, Coelho AC, Tadini CC. Cassava starch composite films incorporated with cinnamon essential oil: Antimicrobial activity, microstructure, mechanical and barrier properties. LWTFood Science and Technology. 2013 Dec 1;54(2):346-52.
28. Wu J, Sun X, Guo X, Ge S, Zhang Q. Physicochemical properties, antimicrobial activity and oil release of fish gelatin films incorporated with cinnamon essential oil. Aquaculture and Fisheries. 2017 Jul 1;2(4):185-92.
29. WANG GS, DENG JH, Min SH, Bo LI. Mechanisms, clinically curative effects, and antifungal activities of cinnamon oil and pogostemon oil complex against three species of Candida. Journal of Traditional Chinese Medicine. 2012 Mar 1;32(1):19-24.
30. Berechet MD, Gaidau C, Miletic A, Pilic B, Râpa M, Stanca M, Ditu LM, Constantinescu R, Lazea-Stoyanova A. Bioactive Properties of Nanofibres Based on Concentrated Collagen Hydrolysate Loaded with Thyme and Oregano Essential Oils. Materials. 2020 Jan;13(7):1618.
31. Râpa M, Gaidau C, Stefan LM, Matei E, Niculescu M, Berechet MD, et al. New Nanofibers Based on Protein By- Products with Bioactive Potential for Tissue Engineering. Materials. 2020 Jan;13(14):3149.
32. Abdel-Rahman LH, Abu-Dief AM, Shehata MR, Atlam FM, Abdel-Mawgoud AA. Some new Ag (I), VO (II) and Pd (II) chelates incorporating tridentate imine ligand: Design, synthesis, structure elucidation, density functional theory calculations for DNA interaction, antimicrobial and anticancer activities and molecular docking studies. Applied Organometallic Chemistry. 2019 Apr;33(4):e4699.
33. Abu-Dief AM, Abdel-Rahman LH, Abdel-Mawgoud AA. A robust in vitro Anticancer, Antioxidant and Antimicrobial Agents Based on New Metal-Azomethine Chelates Incorporating Ag (I), Pd (II) and VO (II) Cations: Probing the Aspects of DNA Interaction. Applied Organometallic Chemistry. 2020 Feb;34(2):e5373.
34. Broxton PM, Woodcock PM, Gilbert P. A study of the antibacterial activity of some polyhexamethylene biguanides towards Escherichia coli ATCC 8739. Journal of Applied Bacteriology. 1983 Jun;54(3):345-53.
35. Gill AO, Holley RA. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. International journal of food microbiology. 2006 Apr 15;108(1):1-9.
36. Nuñez L, D’Aquino M. Microbicide activity of clove essential oil (Eugenia caryophyllata). Brazilian Journal of Microbiology. 2012 Dec;43(4):1255-60.
37. Zhang Y, Liu X, Wang Y, Jiang P, Quek S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016 Jan 1;59:282-9.
38. Abdel-Rahman LH, Abu-Dief AM, El-Khatib RM, Abdel-Fatah SM. Some new nano-sized Fe (II), Cd (II) and Zn (II) Schiff base complexes as precursor for metal oxides: Sonochemical synthesis, characterization, DNA interaction, in vitro antimicrobial and anticancer activities. Bioorganic chemistry. 2016 Dec 1;69:140-52.
39. Abdel-Rahman LH, Abu-Dief AM, Basha M, Hassan Abdel-Mawgoud AA. Three novel Ni (II), VO (II) and Cr (III) mononuclear complexes encompassing potentially tridentate imine ligand: Synthesis, structural characterization, DNA interaction, antimicrobial evaluation and anticancer activity. Applied Organometallic Chemistry. 2017 Nov;31(11):e3750.
40. Abdel-Rahman LH, Adam MS, Abu-Dief AM, Moustafa H, Basha MT, Aboraia AS, et al. Synthesis, theoretical investigations, biocidal screening, DNA binding, in vitro cytotoxicity and molecular docking of novel Cu (II), Pd (II) and Ag (I) complexes of chlorobenzylidene Schiff base: Promising antibiotic and anticancer agents. Applied Organometallic Chemistry. 2018 Dec;32(12):e4527.
41. Abdel-Rahman LH, Adam MS, Abu-Dief AM, Ahmed HE, Nafady A. Non-Linear Optical Property and Biological Assays of Therapeutic Potentials Under In Vitro Conditions of Pd (II), Ag (I) and Cu (II) Complexes of 5-Diethyl amino- 2-({2-[(2-hydroxy-Benzylidene)-amino]-phenylimino}- methyl)-phenol. Molecules. 2020 Jan;25(21):5089.