Abstract
Lenalidomide maintenance following autologous stem cell transplantation for treatment of multiple myeloma is a highly effective treatment strategy with large, randomized clinical trials and a meta-analysis demonstrating improved progression-free and overall survival. Our analysis of 1,256 patients from the Canadian Myeloma Research Group Multiple Myeloma Database (CMRG-DB) demonstrated a 24-month improvement in progression-free survival (58.2 months vs 34.6 months p <0.0001) and improved overall survival (NYR vs 98 months, p <0.0001). Although the survival benefits of lenalidomide maintenance are well established, many unanswered questions still exist regarding its use.
At maintenance doses, the efficacy of lenalidomide is thought to rely on its immunomodulatory effect and action against minimal residual disease (MRD) evidenced by improved rates of MRD negativity in patients on maintenance. However, the optimal duration, dosing schedule and possibility of discontinuation remain unclear. As these patients inevitably progress, the optimal sequence of subsequent therapy is of key importance. Historically, lenalidomide-exposed patients have been excluded from trials examining treatment-dose lenalidomide in the relapsed setting. This limits the generalizability of this important data to most patients as lenalidomide maintenance is increasingly adopted as the standard of care in first-line therapy. Large, retrospective datasets such as the CMRG-DB provide a critical tool to help evaluate the questions left unanswered by prospective clinical trials.
Here within we review current evidence surrounding lenalidomide maintenance and future areas of investigation.
Background
Low-dose, lenalidomide maintenance following autologous stem cell transplantation (ASCT) is the current standard of care in patients with newly diagnosed multiple myeloma (NDMM) based on results from large, randomized controlled trials and a meta-analysis which demonstrated improved overall (OS) and progressionfree survival (PFS) [1-5]. In our recent manuscript, published in Heamatologica we document the impact of lenalidomide maintenance in the real-world setting [6]. Our retrospective analysis of 1,256 patients with NDMM treated with bortezomib-based induction and ASCT as firstline therapy analyzed survival outcomes based on the use of lenalidomide maintenance (n=723) or no maintenance (n=533).6 We identified a 24-month advantage in PFS (58.2 months vs 34.6 months p <0.0001) and an improvement in overall survival (NYR vs 98 months, p <0.0001) in favour of those who received lenalidomide maintenance [6].The survival benefits persisted in all response groups and cytogenetic risk categories [6]. Higher rates of very good partial response (VGPR) or greater were seen in patients receiving maintenance (93.9% versus 80.7 %, p < 0.01) [6]. In a subset analysis, no appreciable differences in OS (p=0.75) or PFS (p=0.66) were seen in patients receiving a 21 of 28-day versus continuous lenalidomide maintenance dosing schedule nor were there statistically significant difference in the frequency of secondary primary malignancies or thrombosis during frontline therapy (p=0.32 and p=0.5 respectively) [6]. In the same study, only 19.6% of patient discontinued lenalidomide maintenance prior to relapse corroborating the findings of prospective studies that maintenance is well tolerated [6]. Taken together, this real-world data confirms the survival advantage conferred by maintenance lenalidomide observed in phase 3 randomized, controlled trials and reaffirms this therapy as a standard of care [6]. However, these observations raise a number of questions regarding the mechanism of action of lenalidomide at maintenance doses, the potential role of minimal residual disease (MRD) testing, optimal dose and duration of maintenance, and whether progression on lenalidomide represents resistance to the drug at treatment doses. Furthermore, it presents an opportunity to discuss the role of large databases in the research setting.
Lenalidomide: Mechanism of Action
Lenalidomide is an oral immunomodulator (IMID) with multiple mechanisms of action that works primarily through the protein cereblon (CRBN) [7]. CRBN forms a portion of the protein complex cullin-4 RING E3 ligase complex (CRL4) [7,8]. Immunomodulators have been shown to stabilize CRBN and in turn increase CRL4- meditated ubiquitination then degradation of protein targets [7-9]. Down regulation and genetic variants in CRBN have been shown to induce resistance to IMIDs such as lenalidomide and pomalidomide [7-9]. The zinc finger proteins Aiolos (IKZF3) and Ikaros (IKZF1), which are integral to lymphocyte and plasma cells development, are targets of lenalidomide enhanced CRL4 mediated degradation [9,10]. Reduced levels of IKZF1 and IKZF3 lead to reduced expression of interferon regulation factor-4 (IRF4) and regulatory factor c-Myc on which myeloma cells are dependent for survival and gene dysregulation [11-15].
Although these mechanisms are critical for action of lenalidomide at full treatment doses, the effect of lenalidomide at maintenance lower doses is thought to primarily be related to its immunomodulatory effects on the bone marrow microenvironment. IMIDs have also been shown to provide immunoregulatory function through T cell mediated mechanisms. In response to pneumococcal 7-valent conjugate vaccine, patients on IMIDs have demonstrated increased IFN-γ–producing T cells and decreased T helper 17 (Th-17) cells possibly related to reduced IRF-4 levels [16,17]. The same changes have been observed in the bone marrow microenvironment of patients treated with IMIDs with increasing Th-17 subsets correlating with relapsed disease [13]. Reduced expression of programmed death-1 (PD-1) protein in patients with complete remission on maintenance was observed in all CD4+ and CD8+ expressing T cells and an increase in number of peripheral natural killer (NK) cells [18,19]. However, the impact of lenalidomide on the regulatory T cell compartment is not entirely clear. Fostier et al. demonstrated that lenalidomide maintenance increased the number of regulatory T cells with a suppressive phenotype (namely CD45RA-) in the bone marrow microenvironment [19]. Additionally, they demonstrated increased naïve CD8+ T cells and memory CD4+ and CD8+ T cells in bone marrow samples of patients receiving lenalidomide maintenance which play a critical role in sustained response to lenalidomide [19].
CD8+ T cells have increased inflammatory cytokine producing potential and an increased CD4+/CD8+ T cell ratio was observed, the opposite of which is seen at disease progression [19]. Their analysis also showed that high levels of T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif protein (TIGIT) was expressed in T cells from multiple myeloma patients post ASCT. Patients with residual myeloma post ASCT who received lenalidomide maintenance had decreased numbers of TIGIT+ CD8+ T cells [19]. Taken together this data suggests that the improved PFS and OS observed with lenalidomide maintenance may relate to increased immune-stimulation against residual disease in patient with myeloma post-ASCT [19].
Minimal Residual Disease: Where Does Maintenance Fit in?
The advent of minimal residual disease (MRD) monitoring in multiple myeloma has revolutionized response assessment and treatment goals for the disease [20,21]. The correlation of MRD negativity with improved overall and progression free survival and has led to adoption of this endpoint in the clinical trial and realworld setting [20-24]. In vivo, longitudinal MRD data has allowed investigators to test the hypothesis that maintenance lenalidomide enhances immune-stimulation against residual disease.
Previous data from Hahn et al. demonstrated improved PFS and OS in patients with MRD-negative status treated with ASCT (single or tandem) and 1 year of finite-duration lenalidomide maintenance [25]. Perrot et al. demonstrated that rates of MRD negativity by next generation sequencing increased from 30% to 38% during treatment with lenalidomide maintenance following ASCT [24]. Alonso et al. verified these results in the real-world setting demonstrating 34.3% of MRD-positive patients post-ASCT subsequently became MRD-negative while on lenalidomide maintenance with a mean time to MRD negativity of 18.5 months. Importantly, there were no differences in survival outcomes between these patients and those who achieved MRD negativity immediately following ASCT [26]. These results serve to validate the hypothesis that lenalidomide maintenance improves survival, at least in part, through increased rates of MRD negativity. Given that the majority of patients remain MRD-positive following ASCT, lenalidomide maintenance serves to benefit the majority of transplanted patients with multiple myeloma.
This raises several questions particularly with regards to continuation of maintenance lenalidomide in patients who achieve MRD-negative status [6]. Though lenalidomide is generally well tolerated with only 19.6% of patients discontinuing therapy prior to progression in our analysis, it does carry notable adverse effects such as cytopenias, risk of infection, rash, GI upset, teratogenicity and financial burden [6]. Gu et al. in their evaluation of 104 patients treated with bortezomib-based induction, ASCT and maintenance therapy (which comprised of lenalidomide or thalidomide with or without interferon-α) noted that subgroup of patients who achieved an MRDnegative status pre-transplant and sustained it for 24 months continued to remain MRD-negative during their extended follow-up (median follow-up of 31 months) [27]. Though future studies are needed, this suggests that patients achieving MRD-negative status for 24 months post-ASCT may be able to discontinue maintenance therapy [27]. In their study, all progressions on protocol were heralded by MRD- positive status further cementing the importance of MRD status monitoring [27]. However, this leaves several unanswered questions. Is it truly safe to discontinue maintenance in patients with prolonged MRD-negative status? If patients develop MRD positivity while off maintenance chemotherapy, then is re-initiation of maintenance doses adequate therapy? Should patients with persistent MRD-positive status on maintenance receive further treatment doses of chemotherapy to attempt to achieve MRD negativity? Clearly further research is required in this area.
Second-Line Therapy: Life after Maintenance
Numerous phase 3 trials have been performed which included patients progressing after 1 prior line of therapy (Table 1) [28-38]. These studies have compared a new regimen, most often a triplet to a doublet of dexamethasone combined with either lenalidomide or bortezomib or carfilzomib as the control arm. Due to the era in which they were performed, or due to the trial design, the majority have a limited number of lenalidomide-exposed patients (Table 2). This finding poses difficulty in selecting the optimal regimen for our patients who are now frequently experiencing their first relapse on lenalidomide, particularly at the low doses used for maintenance. Another complicating factor is that the definition of lenalidomideexposed or -refractory is not consistent between trials as essentially all studies have grouped patients exposed to treatment and maintenance doses of lenalidomide together [32,33,35,38]. Since the new triplet regimens containing lenalidomide use full treatment doses of this drug, it is not known if such regimens might still have utility for patients progressing on lenalidomide maintenance. Further study elucidating mechanisms of relapse on lenalidomide maintenance, as well as prospective trials to address the inclusion of this agent in second-line therapy, would be helpful in this regard.
| Trial | N | Median prior lines Rx | Len- exposed (%) | Len- refractory (%) | PFS (months) ITT group | PFS (months) in 2nd line Rx | PFS (months) Len- exposed | PFS (months) Len- refractory |
|---|---|---|---|---|---|---|---|---|
| ASPIRE (KRd) [38,47] | 792 | 2 | 19.9 | 7.2 | 26.3 | 29.6 | 19.4 | 11.3 |
| TOURMALINE- MM1 (IxaRd) [35] | 722 | 1 | 12 | NE | 20.6 | 20.6 | NA | -- |
| ELOQUENT-2 (ELO-Rd) [33] | 646 | 2 | 6 | NE | 19.4 | NA | NA | -- |
| POLLUX (DRd) [28,32] | 569 | 1 | 21 | NE | 44.5 | 53.6 | 38.8 | -- |
| ENDEAVOR (Kd) [31,48] | 929 | 2 | 38 | 25 | 18.7 | 22.2 | 12.9 | 8.6 |
| CASTOR (DVd) [36,49] | 498 | 2 | 35 | 18 | 16.5 | 27 | 9.4 | 7.8 |
| OPTIMMISM (PVd) [37] | 599 | 2 | 100 | 71 | 11.2 | 20.73 | -- | 9.3 ( 2nd line-7.8 ) |
| APOLLO (DPd) [30] | 304 | 2 | 100 | 80 | 12.4 | NA | -- | -- |
| CANDOR (KDd) [29] | 466 | 2 | 42 | 27 | 28.6 | NA | NA | NA |
| IKEMA (IsaKd) [50] | 302 | 2 | 90 | NA | NYR (median F/U 20.7 mo) | NA | NA | NA |
| Abbreviations: d: low-dose dexamethasone; D: Daratumumab; Elo: Elotuzumab, IIT: Intention-to-treat; Isa: Isatuximab; Ixa: Ixazomib; K: Carfilzomib; Len: Lenalidomide; NA: Not Available; PFS: Progression-Free Survival; R: Lenalidomide; Rx: Therapy; V: Bortezomib | ||||||||
| Trial | Lenalidomide (R)-related exclusion criteria excluding intolerance | Distinction between treatment vs maintenance doses of lenalidomide |
|---|---|---|
| ASPIRE KRd vs Kd [38] |
|
no |
| POLLUXDRd vs Rd [35] |
|
no |
| ELOQUENT-2 EloRd vs Rd [33] |
|
no |
| TOURMALINEMM1 IxaRd vs Rd [35] |
|
no |
| OPTIMISIMM PomVd vs Vd [37] |
|
no |
| Abbreviations: d: low-dose dexamethasone; Elo: Elotuzumab; Ixa: Ixazomib; K: Carfilzomib; R: Lenalidomide; V: Bortezomib | ||
Another consideration relates to the unprecedented efficacy reported with the combination of daratumumab, lenalidomide and dexamethasone (DRd) when administered as second-line therapy. The PFS in patients progressing after 1 prior line of therapy was 44 months, and, although patients progressing on any dose of lenalidomide were excluded, those with prior exposure to this IMiD experienced a PFS of 38.8 months [28]. This has led to some hematologists questioning if lenalidomide maintenance should be withheld, or truncated, to allow the possibility of using DRd at first progression. There is no prospective data to support this approach, and there is considerable uncertainty involved in recommending a strategy in which the PFS with first-line therapy is compromised in the hopes that a potent second-line regimen may possibly lead to the longest second PFS and PFS2 Fortunately, this dilemma is expected to be relatively short-lived as daratumumab and similar immunotherapies are integrated into the initial regimen.
Using the CMRG national Canadian myeloma database, we have previously reported the outcomes of first- and second-line therapy sequencing in patients treated with and without lenalidomide maintenance after ASCT [6]. Patients had all received bortezomib-based induction and ASCT and were analyzed by whether or not they received lenalidomide maintenance and whether or not they received lenalidomide-containing therapy in the second-line setting. Patients treated with lenalidomide as maintenance and as part of second-line therapy had the longer median overall survival (106.9 months) compared to in those who receive lenalidomide only in second-line therapy (93.9 months). Our real-world data reinforces the importance of using lenalidomide maintenance in the front-line setting and suggests that saving lenalidomide until second-line therapy may compromise overall survival [6]. Moreover, it suggests that some patients benefit from continuing lenalidomide after progression on maintenance. More work is needed to identify the appropriate patient for this strategy compared to introduction of different agents at disease progression.
Optimal Dosing Schedules: How Much for How Long?
Multiple dosing schedules of lenalidomide maintenance have been evaluated in clinical trials though none have been prospectively compared head to head. In the pivotal trials evaluating lenalidomide maintenance multiple regimens were used. Attal et al. examined lenalidomide 10 mg daily increased to 15 mg daily as tolerated until progression [1]. However, based on review of safety data and increased incidence of secondary primary malignancies the study was amended and the duration of lenalidomide maintenance was limited to 2 years [1]. McCarthy et al. examined lenalidomide maintenance at 10 mg daily whereas Palumbo et al. utilized 10 mg given for 21 of 28-days; both trials continued maintenance until progression [4,5]. Finally, Jackson et al. utilized 25 mg for 21 of 28 days which was amended to 10 mg for 21 of 28 days in a protocol change [2].
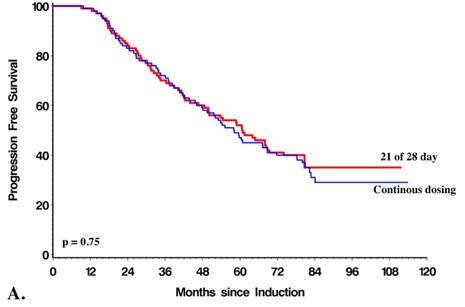
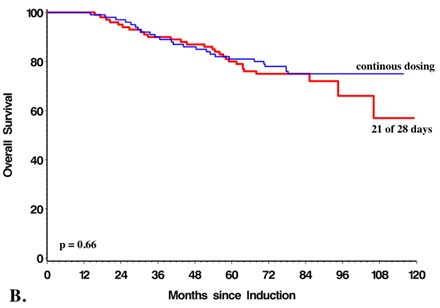
All of these trials demonstrated similar improvement in PFS with lenalidomide maintenance with McCarthy et al. also demonstrating improved 3-year overall survival (Table 3) [1,2,4,5]. In our analysis there was no difference in PFS or OS in patients who received a 21 of 28-day cycle or continuous dosing schedule (Figures 1a and 1b). A 21 of 28-day dosing schedule has the advantage of reduced cost and potentially reduced adverse effects.
Figure 1. Progression free (A) and overall (B) survival were no different in patients receiving 21 of 28 days or continuous doing lenalidomide maintenance.
| Trial | LM* schedule | PFS | OS | Additional survival outcomes |
|---|---|---|---|---|
| Attal et al. [1] | 10 mg daily increased to 15 mg daily as tolerated. Limited duration to 2 years in protocol amendment. | LM 41months No-LM 23 months p <0.001 | NYR | 4-year OS 73% vs 75% |
| McCarthy et al. [4] | 10 mg daily until progression | LM 46months No-LM 27 months p <0.001 | NYR | 3-year OS 88% vs 80% p= 0.008 |
| Palumbo et al. [5] *ASCT subgroup | 10 mg for 21 of 28 days until progression | LM 54.7 months No-LM 37.4 months p <0.001 | NYR | 5-year OS 78.4% vs 66.6% |
| Jackson et al. [2] | 25 mg for 21of 28 days which was amended to 10 mg for 12 of 28 days in a protocol change | LM 39 months No-LM 20 months p <0.001 | NYR | 5-year OS 60% vs 50% p = 0.15 |
| Abbreviations: *LM: Lenalidomide Maintenance; NYR: Not Yet Reached; PFS: Progression-Free Survival, OS: Overall Survival | ||||
Another smaller Canadian real-world analysis of patients treated with post-ASCT maintenance at Princess Margaret Cancer Centre also reported reassuring results when doses of lenalidomide less than 10 mg per day were used. Dose reductions from the target of 10 mg per day, either at the start or during maintenance, were documented in the majority of patients (70%) but the median PFS of ~42 months after starting maintenance (rather than the start of induction therapy or from the date of ASCT) was similar to that reported in prospective trials [39]. There was no difference in outcome between patients who did and did not need reductions, and the routine symptom assessment tool used in the clinic demonstrated a wellpreserved quality of life [39]. Based on this analysis, and the experience described above, we would advocate the use of a 21 of a 28-day lenalidomide maintenance dosing schedule in most patients.
Furthermore, recent studies have examined the utility of a finite period of maintenance [40]. In the landmark IFM2009 trial examining the timing and role of transplantation in the context of RVD induction/consolidation, Attal et al. utilized lenalidomide maintenance at 10 mg daily increased to 15 mg daily as tolerated for 1 year [1]. A treatmentfree interval would benefit patients from a side-effect standpoint with added financial benefit given the high cost of lenalidomide. However, the median PFS in this study is ~50 months, 8 months shorter than data presented in our real-world study [39].The STAMMiNA trial also utilized finite duration of lenalidomide maintenance therapy of 10 mg daily starting 3 months after transplant increased to 15 mg daily as tolerated for 3 years in all treatment arms [41].A recent presentation of their data with 6-years of follow-up indicated that discontinuation of lenalidomide even at 38 months was associated with shorter PFS [42]. The upcoming Dana Farber partner analysis of the IFM2009 study (NCT01191060) in which maintenance lenalidomide was continued until progression has yet to read out, which potentially implies a superior PFS with this non-finite approach. Therefore, a definitive answer to the question of whether lenalidomide maintenance should always be continued until disease progression remains unanswered for now, particularly in the era of MRD testing, but our current policy continues to advocate continuous administration until myeloma relapse [6,43].
Ongoing trials are attempting to elucidate the role of maintenance based on MRD status. The ongoing DRAMMATIC trial (NCT04071457) investigating the use of lenalidomide maintenance 10 mg daily with or without daratumumab maintenance involves evaluation of MRD status during maintenance and plans for discontinuation of maintenance in certain MRD negative arms. They define MRD negative as <1 × 106 using next generation sequencing. The AURIGA trial (NCT03901963) also investigates lenalidomide maintenance with or without daratumumab maintenance with the same dosing strategy but a finite duration of 36 months.
Novel, combination maintenance strategies are also actively being investigated. Recent updates from the FORTE trial specifically examining patients with respect to maintenance strategy demonstrated an improved 3-year PFS with carfilzomib and lenalidomide maintenance rather than lenalidomide alone (75% vs 66%, HR 0.63; p=0.026) [44] Patients in the carfilzomib and lenalidomide maintenance group had higher rates of transition from MRD-positive to MRD-negative during maintenance (46% vs 32%, p=0.04) [44]. The superiority of “double” maintenance in high-risk patients is of particular interest since many Canadian myeloma experts are already recommending a combination of lenalidomide plus a proteasome inhibitor (bortezomib or ixazomib) as maintenance in such patients. Future analysis of this strategy in the real-world setting using the CMRG database is planned after the data matures. With respect to other new maintenance approaches, extension of the CASSIOPEIA trial is ongoing and examining the use of single-agent daratumumab maintenance continued for 2 years’ duration [45]. The GRIFFIN trial has also examined the role of lenalidomide maintenance with or without daratumumab for 26 cycles [46]. Results with respect to the impact of this novel maintenance strategy is eagerly awaited. These trials represent a new era of combination maintenance strategies involving multiple novel agents which may be “practice changing” in the treatment of myeloma.
Big Data: The Benefits of Real-World Datasets
Large, real-world datasets such as the CMRG-DB provide a unique opportunity to analyze critical research questions in the real-world setting. Though clinical trials often provide an inaugural answer to important research questions, they contain a fundamental lack of generalizability given strict inclusion and exclusion criteria. Real-world data is critical to validate these results in a broader sense. The CMRG-DB includes data from 14 academic institutions across Canada and over 7000 patients. This provides an enormous resource for evaluating clinical questions much more quickly than in the prospective setting. For example, the impact of treatment holidays from lenalidomide maintenance on MRD status could be much more rapidly addressed by retrospective evaluation and MRD testing of patient samples contained within the database rather than constructing a costly and time-consuming clinical trial which may only duplicate data already available through datasets such as the CMRG-DB. In addition, database analyses can identify subgroups with suboptimal outcomes to help guide the design of future prospective clinical trials.
The most common argument against retrospective datasets is that they are inherently biased based on the lack of standardized treatment that is encompassed in a clinical trial setting. Though this may be true, the patients we see every day in clinic receive treatment based on their unique patient and disease related factors. Decisions regarding the treatment of myeloma patients are not made in a randomized, controlled vacuum in which they are uniformly treated with a common protocol. Care providers consider numerous facets of a patient’s disease and circumstances under the guidance of results identified in the randomized controlled trials. The results of these decisions, and therefore the applicability of prospective trial to the real-world setting, can only be truly elucidated in retrospective datasets.
Further arguments in retrospective dataset analysis include the use of historical controls as was the case in our article. This practice has been criticized on the basis that patients from the “pre-lenalidomide maintenance era” may not have had the same access to more effective disease monitoring or second line therapy. Though this may be true, our historical control group has very similar progression-free and overall survival to that documented in phase 3 trials evaluating lenalidomide maintenance in a randomized, controlled setting [1,2,4,5]. Furthermore, the main outcome of PFS is only influenced by the treatment given within the frontline of therapy; therapy consistently given as per the accepted standard of bortezomib-based induction followed by an autologous stem cell transplant. Appropriate cohort matching and evaluation of data against prospective trials can potentially overcome this obstacle.
Conclusions
Although the positive impact of lenalidomide maintenance on PFS and OS are undisputed and have led to its adoption as a standard of care after ASCT, several questions regarding the optimal use of lenalidomide maintenance remain unanswered. In vitro studies suggest lenalidomide maintenance works by immunoregulation against minimal residual disease. However, mechanisms of resistance to lenalidomide doses utilized for maintenance versus to full treatment doses are not clear and whether further lenalidomide can be leveraged for relapse on maintenance is uncertain. The role of lenalidomide in patients who obtain a prolonged MRD-negative status also remains unstudied. Given the critical importance of selecting the optimal sequence of available regimens for the repeated recurrences seen in myeloma patients, large retrospective datasets can provide critical insight into clinical questions through the lens of real-world data. These datasets, which must incorporate effective monitoring and quality policies to ensure accuracy and integrity, can be of great utility in answering questions not readily addressed in the clinical trial setting and provide research with a breadth of readily accessible and useful data.
References
2. Jackson GH, Davies FE, Pawlyn C, Cairns DA, Striha A, Collett C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. The Lancet Oncology. 2019 Jan 1;20(1):57-73.
3. McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. Journal of Clinical Oncology. 2017 Oct 10;35(29):3279.
4. McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stemcell transplantation for multiple myeloma. New England Journal of Medicine. 2012 May 10;366(19):1770-81.
5. Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. New England Journal of Medicine. 2014 Sep 4;371(10):895- 905.
6. Cherniawsky HM, Kukreti V, Reece D, Masih-Khan E, McCurdy A, Jimenez-Zepeda VH, et al. The survival impact of maintenance lenalidomide: an analysis of realworld data from the Canadian Myeloma Research Group national database. Haematologica. 2020 Oct 13.
7. Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood, The Journal of the American Society of Hematology. 2011 Nov 3; 118(18):4771-9.
8. Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010 Mar 12; 327(5971):1345-50.
9. Zhu YX, Braggio E, Shi CX, Kortuem KM, Bruins LA, Schmidt JE, et al. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood. 2014 Jul 24;124(4):536-45.
10. Krönke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014 Jan 17;343(6168):301-5.
11. Agnarelli A, Chevassut T, Mancini EJ. IRF4 in multiple myeloma—biology, disease and therapeutic target. Leukemia Research. 2018 Sep 1;72:52-8.
12. Bjorklund CC, Lu L, Kang J, Hagner PR, Havens CG, Amatangelo M, et al. Rate of CRL4 CRBN substrate Ikaros and Aiolos degradation underlies differential activity of lenalidomide and pomalidomide in multiple myeloma cells by regulation of c-Myc and IRF4. Blood Cancer Journal. 2015 Oct; 5(10):e354-.
13. Di Lullo G, Marcatti M, Heltai S, Tresoldi C, Paganoni AM, Bordignon C, et al. Immunomodulatory drugs in the context of autologous hematopoietic stem cell transplantation associate with reduced pro-tumor T cell subsets in multiple myeloma. Frontiers in Immunology. 2019 Jan 21; 9:3171.
14. Holien T, Våtsveen TK, Hella H, Waage A, Sundan A. Addiction to c-MYC in multiple myeloma. Blood, The Journal of the American Society of Hematology. 2012 Sep 20;120(12):2450-3.
15. Shaffer AL, Emre NT, Lamy L, Ngo VN, Wright G, Xiao W, et al. IRF4 addiction in multiple myeloma. Nature. 2008 Jul;454(7201):226-31.
16. Brüstle A, Heink S, Huber M, Rosenplänter C, Stadelmann C, Yu P, et al. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nature Immunology. 2007 Sep; 8(9):958-66.
17. Noonan K, Rudraraju L, Ferguson A, Emerling A, Pasetti MF, Huff CA, et al. Lenalidomide-induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clinical Cancer Research. 2012 Mar 1;18(5):1426-34.
18. Danhof S, Schreder M, Knop S, Rasche L, Strifler S, Löffler C, Gogishvili T, Einsele H, Hudecek M. Expression of programmed death-1 on lymphocytes in myeloma patients is lowered during lenalidomide maintenance. Haematologica. 2018 Mar;103(3):e126.
19. Fostier K, Caers J, Meuleman N, Broos K, Corthals J, Thielemans K, et al. Impact of lenalidomide maintenance on the immune environment of multiple myeloma patients with low tumor burden after autologous stem cell transplantation. Oncotarget. 2018 Apr 17; 9(29):20476.
20. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. The Lancet Oncology. 2016 Aug 1;17(8):e328-46.
21. Munshi NC, Avet-Loiseau H, Anderson KC, Neri P, Paiva B, Samur M, et al. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Advances. 2020 Dec 8;4(23):5988-99.
22. Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncology. 2017 Jan 1;3(1):28-35.
23. Paiva B, Vidriales MB, Cerveró J, Mateo G, Pérez JJ, Montalbán MA, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood, The Journal of the American Society of Hematology. 2008 Nov 15;112(10):4017-23.
24. Perrot A, Lauwers-Cances V, Corre J, Robillard N, Hulin C, Chretien ML, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018 Dec 6;132(23):2456-64.
25. Hahn TE, Wallace PK, Fraser R, Fei M, Tario JD, Howard A, et al. Minimal residual disease (MRD) assessment before and after autologous hematopoietic cell transplantation (AutoHCT) and maintenance for multiple myeloma (MM): results of the Prognostic Immunophenotyping for Myeloma Response (PRIMeR) study. Biology of Blood and Marrow Transplantation. 2019 Mar 1;25(3):S4-6.
26. Bahlis NJ, Dimopoulos MA, White DJ, Benboubker L, Cook G, Leiba M,. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia. 2020 Jul; 34(7):1875-84.
27. Gu J, Liu J, Chen M, Huang B, Li J. Longitudinal flow cytometry identified “minimal residual disease”(MRD) evolution patterns for predicting the prognosis of patients with transplant-eligible multiple myeloma. Biology of Blood and Marrow Transplantation. 2018 Dec 1; 24(12):2568-74.
28. Bahlis NJ, Dimopoulos MA, White DJ, Benboubker L, Cook G, Leiba M,. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia. 2020 Jul; 34(7):1875-84.
29. Dimopoulos M, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. The Lancet. 2020 Jul 18;396(10245):186-97.
30. Dimopoulos MA. APOLLO: Phase 3 Randomized Study of Subcutaneous Daratumumab Plus Pomalidomide and Dexamethasone (D-Pd) Versus Pomalidomide and Dexamethasone (Pd) Alone in Patients (Pts) with Relapsed/ Refractory Multiple Myeloma (RRMM). Abstract.
31. Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. The Lancet Oncology. 2016 Jan 1;17(1):27-38.
32. Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. New England Journal of Medicine. 2016 Oct 6;375(14):1319-31.
33. Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. New England Journal of Medicine. 2015 Aug 13;373(7):621-31.
34. Moreau P, Dimopoulos M, Mikhael J, Yong K, Capra M, Facon T, et al. Isatuximab plus carfilzomib and dexamethasone vs carfilzomib and dexamethasone in relapsed/refractory multiple myeloma (IKEMA): interim analysis of a phase 3 randomized, open-label study. EHA25 Open Access Library. 2020;Oral Abstract Presentation
35. Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. New England Journal of Medicine. 2016 Apr 28; 374(17):1621-34.
36. Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. New England Journal of Medicine. 2016 Aug 25; 375(8):754-66.
37. Richardson PG, Oriol A, Beksac M, Liberati AM, Galli M, Schjesvold F, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. The Lancet Oncology. 2019 Jun 1; 20(6):781-94.
38. Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špicka I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. New England Journal of Medicine. 2015 Jan 8; 372(2):142-52.
39. Yang C, Jiang H, Masih-Khan E, Chen CI, Prica A, Reece D, et al. Tolerability and efficacy of post transplant lenalidomide maintenance therapy in multiple myeloma: a real world single centre experience. Blood. 2017 Dec 7; 130(Supplement 1):3462-.
40. Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. New England Journal of Medicine. 2017 Apr 6; 376(14):1311- 20.
41. Stadtmauer EA, Pasquini MC, Blackwell B, Hari P, Bashey A, Devine S, et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: results of the BMT CTN 0702 trial. Journal of Clinical Oncology. 2019 Mar 1; 37(7):589.
42. Hari P, Pasquini MC, Stadtmauer EA, Fraser R, Fei M, Devine SM, et al. Long-term follow-up of BMT CTN 0702 (STaMINA) of postautologous hematopoietic cell transplantation (autoHCT) strategies in the upfront treatment of multiple myeloma (MM). Journal of Clinical Oncology. 2020; 38(15_suppl):8506-8506.
43. Ludwig H, Zojer N. Fixed duration vs continuous therapy in multiple myeloma. Hematology 2014, the American Society of Hematology Education Program Book. 2017 Dec 8;2017(1):212-22.
44. Gay F, Musto P, Scalabrini DR, Galli M, Belotti A, Zamagni E, et al. Survival Analysis of Newly Diagnosed Transplant-Eligible Multiple Myeloma Patients in the Randomized Forte Trial. InBlood 2020 Nov 5 (Vol. 136). 2021 L ST NW, SUITE 900, WASHINGTON, DC 20036 USA: AMER SOC HEMATOLOGY.
45. Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. The Lancet. 2019 Jul 6;394(10192):29-38.
46. Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood, The Journal of the American Society of Hematology. 2020 Aug 20;136(8):936-45.
47. Dimopoulos MA, Stewart AK, Masszi T, Špicka I, Oriol A, Hajek R, et al. Carfilzomib–lenalidomide– dexamethasone vs lenalidomide–dexamethasone in relapsed multiple myeloma by previous treatment. Blood Cancer Journal. 2017 Apr;7(4):e554.
48. Moreau P, Joshua D, Chng WJ, Palumbo A, Goldschmidt H, Hájek R, et al. Impact of prior treatment on patients with relapsed multiple myeloma treated with carfilzomib and dexamethasone vs bortezomib and dexamethasone in the phase 3 ENDEAVOR study. Leukemia. 2017 Jan;31(1):115-22.
49. Spencer A, Lentzsch S, Weisel K, Avet-Loiseau H, Mark TM, Spicka I, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018 Dec;103(12):2079.
50. Moreau P, Dimopoulos MA, Yong K, Mikhael J, Risse ML, Asset G, et al. Isatuximab plus carfilzomib/ dexamethasone versus carfilzomib/dexamethasone in patients with relapsed/refractory multiple myeloma: IKEMA Phase III study design. Future Oncology. 2020 Jan;16(02):4347-58.