Abstract
Introduction: Multiple myeloma is a malignant clonal proliferation of immunoglobulin secreting plasma cells in bone marrow. This immunoglobulin produced by neoplastic plasma cells is mostly in the form of a single prominent monoclonal protein. Having two such distinct bands is rarely seen. We present an interesting case with two monoclonal bands on serum protein electrophoresis. Case Report: A middle aged woman presented with skull, vertebral, bilateral femoral lytic lesions. Laboratory investigations revealed elevated serum immunoglobulin G that was both Kappa and lambda light chain restricted on immunofixation electrophoresis (bi-clonal). The presentation was with aggressive disease, the patient did not respond well with conventional chemotherapy regime, requiring upgrading of chemotherapeutic regime. Conclusion: Bi-clonal gammopathy in plasma cell dyscrasia can be a product of clonal evolution, thus can be a marker of poor prognosis.
Keywords
Biclonal, Gammopathy, Multiple myeloma, Aggressive, Disease
Abbreviations
MM: Multiple Myeloma; IMWG: International Myeloma Working Group; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; FBP: Full Blood Picture; IgG: Immuno-globulin G; IgA: Immuno-globulin A; IgM: Immuno-globulin M; IgD: Immuno-globulin D; IgE: Immuno-globlulin E; IgH: Immunoglobulin Heavy chain; IgL: Immunoglobulin Light chain
Introduction
Multiple myeloma (MM) manifests as neoplastic proliferation of plasma cells in the bone marrow associated with monoclonal paraprotein in the blood and/or urine, and evidence of end organ damage that can be attributed to the underlying plasma cell proliferative disorder [1]. The median age at diagnosis of multiple myeloma is 70 years, and the incidence increases with age [2].
Monoclonal paraprotein also known as M-protein is one of the sensitive and specific tumour markers for monoclonal gammopathies including multiple myeloma, because it reflects the clonal nature of the plasma cell proliferation [3]. M protein presents on the serum and urine protein electrophoresis as a homogeneous spike-like peak in a focal region of the gamma-globulin zone and indicates a monoclonal gammopathy. The quantity of M protein, the results of bone marrow biopsy, and other characteristics can help differentiate multiple myeloma from the other causes of monoclonal gammopathy [4].
The best method for detecting monoclonal (M) protein is high resolution agarose gel electrophoresis. M-protein is generally observed as a localized and distinct band which is frequently seen on gamma or beta globulin region. It may also be seen on a 2 globulin region but this situation is very rare [3]. Immunofixation electrophoresis is used to identify the clonality of M-proteins observed on agarose gel electrophoresis. In plasma cell neoplasms, there is production of a single type of immunoglobulin [5]. The class switching between different immunoglobulin heavy chains have been demonstrated to occur in a single clone of plasma cell neoplasm during proliferation, but class switching between kappa and lambda light chain does not occur in a single clone of plasma cells, unless there is clonal evolution. Therefore, presence of two or more M proteins with different light chain restriction would suggest presence of more than one clones of neoplastic plasma cells [6]. In our case report we present a patient with IgG kappa and IgG lambda type of biclonal gammopathy.
Case Report
A 65-years-old lady presented to orthopaedics clinic with history of pain at right hip joint at the greater trochanter. His past medical and family history was unremarkable. As an initial workup X-ray of the right hip joint showed multiple lytic lesions in greater trochanter of the right-side femur. Computed tomography CT scan of thorax, abdomen and pelvis showed Lytic lesions in the left humeral head and greater trochanter of bilateral femurs which were suggestive of multiple myeloma. Magnetic resonance imaging MRI of the spine noted compression fracture at 1st Lumbar vertebra. X-ray skull showed multiple lytic lesions in frontal bone of the skull. In the biochemical panel serum creatinine was 205 micro-moles/liter and serum calcium was 2.92 mmol/L. Full blood picture (FBP) showed haemoglobin of 80 g/ L, platelet of 87,000/mL, total white cell count of 6,000/microliter with hypochromic microcytic red blood cells. No rouleau formation was noted and there was no leucoerythroblastic reaction. Serum protein electrophoresis showed presence of a monoclonal band in the gamma region, as shown in (Figure 1). Paraprotein quantitation was 3.5 g/L. Immunofixation electrophoresis showed that the paraprotein was of two clones of immunoglobulin G (IgG), one having Lambda and the other having Kappa light chain restriction. The urine protein electrophoresis showed presence of urine paraprotein with quantitation of 1287.0 mg/L as shown in (Figure 2) whereas the immunofixation electrophoresis showed that it was Kappa light chain. Bone marrow aspirate showed plasmacytosis constituting 26% of the cells. Bone marrow trephine biopsy was hypercellular with approximately 60% cellularity having interstitial infiltration by plasma cells that were positive for CD138 with immunohistochemical staining. In-situ hybridization for kappa and lambda light chains shows kappa light chain restriction. All 3 cell lines were present but with decrease in number. Conventional karyotyping revealed presence of normal female karyotype. Initially, the patient was started on regime containing Cyclophosphamide, Borteozomab and Dexamethasone but because of poor response during the first month of therapy the patient was upgraded to regime containing Cyclophosphamide, Lenalidomide and Dexamethasone.
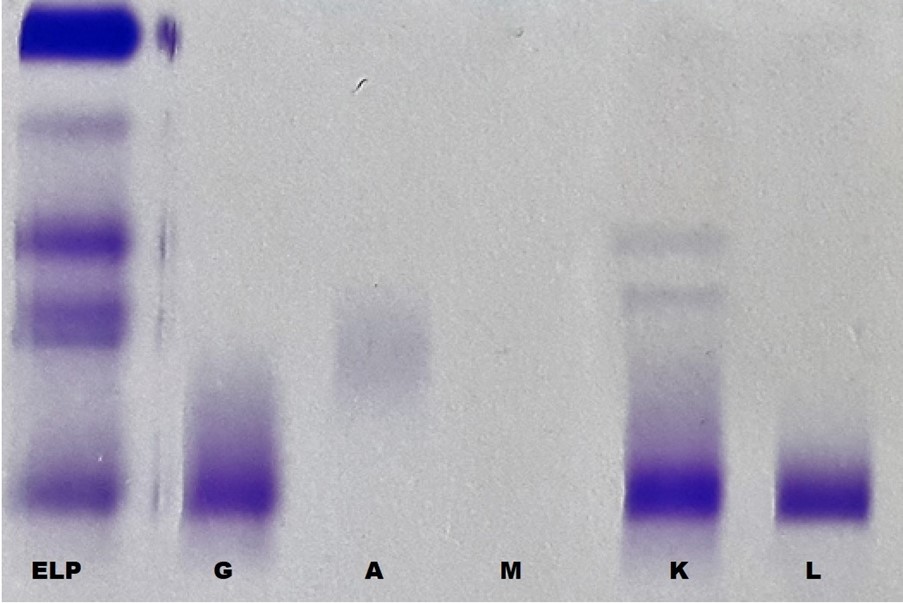
Figure 1. Serum protein electrophoresis (labeled as ELP) showed a prominent band in the gamma region. On immunofixation electrophoresis it was shown to be immunoglobulin G heavy chain (labeled as G) with both Kappa and lambda light chain restriction (Biclonal) (labeled as K and L respectively).
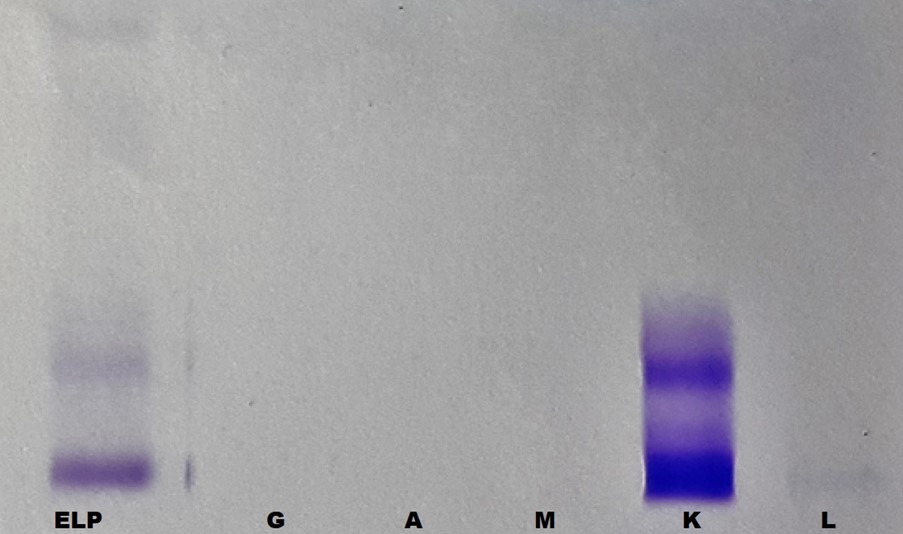
Figure 2. Urine Protein electrophoresis (labeled as ELP) showed urine paraportein which was shown to be Kappa light chain on immunofixation electrophoresis (labeled as K).
Discussion
MM is an uncommon malignancy [7]. It is a B cell neoplasm of the bone marrow with a complex array of clinical manifestations [7]. It accounts for 10%-15% of all hematologic malignancies, and 20% of deaths related to haematological malignancies [8]. The diagnosis of MM is based on the presence of neoplastic plasma cells in the bone marrow or other extramedullary sites, along with evidence of disease-related organ dysfunction [7]. According to the International Myeloma Working Group IMWG consensus updates of 2014 the disease definition of multiple myeloma to include validated biomarkers in addition to existing requirements of attributable CRAB features that includes hypercalcaemia, renal failure, anaemia, and bone lesions [1]. These changes were based on the identification of biomarkers associated with near inevitable development of CRAB features in patients who would otherwise be regarded as having smouldering MM [1]. The newly added biomarkers include presence of equal to or more than 60% plasma cells in the bone marrow, one or more lytic lesions on magnetic resonance imaging MRI, and serum free light chain ratio with involved and uninvolved ratio >100 [1].
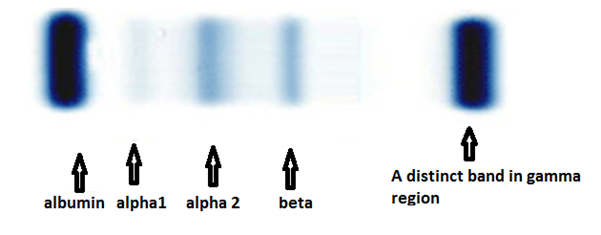
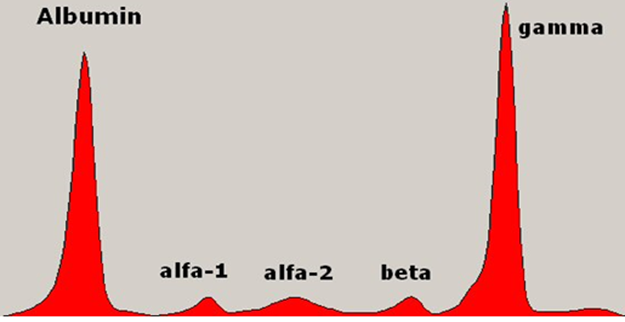
The circulating M-protein may consist of an intact immunoglobulin, only light chain, or in rare instances, only heavy chain. The heavy chain is from one of the five immunoglobulin classes G, A, M, D or E, while the light chain is either Kappa or Lambda type [8]. Serum protein electrophoresis is the commonly used technique for detection and characterisation of M protein [8]. The most important use of serum protein electrophoresis is for the detection of monoclonal paraproteins [8]. In serum of normal individual immunoglobulins are extremely heterogeneous and spread out as an ill-defined smear throughout the gamma and beta region as in (Figure 3a and 3b) [8]. In contrast, the immunoglobulin proteins produced by an abnormal monoclonal proliferation of plasma cells are homogeneous in their physico-chemical & electrophoretic properties, therefore will form sharp, tall & narrow-based peaks on densitometric tracing as in (Figure 4a and 4b) [8].
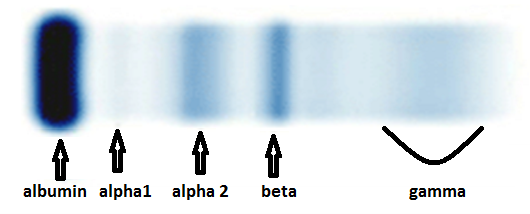
Figure 3. a. Serum protein electrophoresis in a normal individual [10]. b. Densitometric tracing of various components in normal serum separated by serum protein electrophoresis (Figure 1) [8].
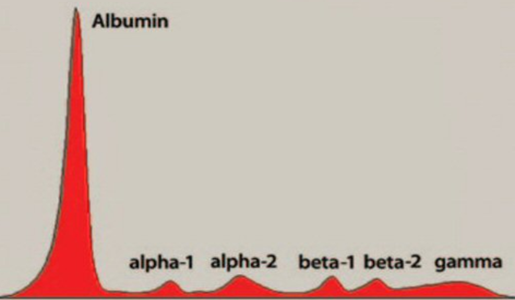
Figure 4. a. Serum protein electrophoresis in a case of multiple myeloma showing a prominent band at the gamma region [8]. b. Densitometric tracing of the serum electrophoresis in a case of multiple myeloma showing a prominent spike at the gamma region [8].
Very rarely two or three clonal bands are seen in MM and such cases constitute approximately 1% of all monoclonal gammopathies [8,9,4]. Biclonal gammopathies are characterised by simultaneous appearance of two different M protein bands on serum protein electrophoresis. The existence of true biclonal myeloma based on the presence of more than 1 immunoglobulin heavy IgH chain subtype must be carefully reviewed because both serum M components may share the same clonal origin with an identical variable region rather than being 2 independent clones [4]. In contrast presence of M protein with more than one immunoglobulin light chain restriction can be considered as truly biclonal, because, there is no molecular mechanism for changing light chain expression within the same myeloma clone [6]. There are two possible explanations for the production of two types of M protein in a plasma cell neoplasm. One possibility is that they may be due to proliferation of two clones of plasma cells, each producing an unrelated monoclonal immunoglobulin. Second possibility is that there has been clonal evolution in neoplastic plasma cells [2,6].
During the normal development and differentiation of B lymphocytes, the surface immunoglobulins change from only IgM to both IgM and IgD, and the secreted antibody switches heavy-chain class (from IgM to IgG, IgA or IgE). In-spite of the switching of heavy-chain class, the antibody molecules retain the same antigenic specificity [6]. It has been postulated that some biclonal pairs may result from a transformation event in a cell undergoing a variable-region switch from one heavy-chain class or subclass to another [6]. Biclonal myeloma cases expressing both kappa and lambda are not exceptional, although its frequency has been estimated to be only around 1% to 2% of all cases with biclonal bands. This low frequency has led to difficulty in interpreting the clinical characteristics of these patients; some authors having found no difference in the clinical characteristics and prognosis, while others suggest a poor response to therapy [6,10].
Conclusion
Biclonal gammopathy is a very rare presentation of disease in patients with multiple myeloma. Biclonality, whether determined by molecular-genetic methods or by presence of para-proteins is considered to represent clonal evolution and may therefore, be a determinant of disease prognosis.
Authors’ Contributions
AMH, SRN, SHN conceived the idea. AMH and SRN were the major contributor to the writing of the manuscript. MA collected the laboratory data via integrated laboratory management system (ILMS). AMH and SRN diagnosed the case. SHN and ASZ provided the clinical information of the patient. SRN, AMH, ASI, NJN and HAM were the major contributors for critically revising the manuscript for important intellectual content. NJN, SRN and AMH have given expert opinion and final approval of the version to be published. All authors read and approved the final manuscript.
Availability of Data and Materials
All generated data is included in this article.
Ethical Approval and Consent to Participate
Not applicable, as case acquired during routine diagnostics.
Consent for Publication
Written informed consent was obtained from patient for publication of this case report and the accompanying Figure. A copy of the written consent shall be availed to the Editor-in-Chief of this journal upon reasonable request.
Competing Interests
The authors declare to have no competing interests.
References
2. Mullikin TC, Rajkumar SV, Dispenzieri A, Buadi FK, Lacy MQ, Lin Y, et al. Clinical characteristics and outcomes in biclonal gammopathies. American Journal of Hematology. 2016 May;91(5):473-5.
3. Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. Harrison's principles of internal medicine, 19e. New York, NY, USA: Mcgraw-hill; 2015: 2958.
4. O'Connell T, Horita TJ, Kasravi B. Understanding and interpreting the serum protein electrophoresis. American Family Physician. 2005 Jan 1;71(1):105-12.
5. González D, van der Burg M, García-Sanz R, Fenton JA, Langerak AW, González M, et al. Immunoglobulin gene rearrangements and the pathogenesis of multiple myeloma. Blood, The Journal of the American Society of Hematology. 2007 Nov 1;110(9):3112-21.
6. Mahto M, Balakrishnan P, Koner BC, Lali P, Mishra TK, Saxena A. Rare case of biclonal gammopathy. International Journal of Case Reports and Images (IJCRI). 2011 Feb 1;2(2):11-4.
7. Alexander DD, Mink PJ, Adami HO, Cole P, Mandel JS, Oken MM, et al. Multiple myeloma: a review of the epidemiologic literature. International Journal of Cancer. 2007;120(S12):40-61.
8. Seifter EJ. Cancer: Principles and Practice of Oncology, Vincent T. DeVita, Jr., Samuel Hellman, Steven A. Rosenberg, JNCI Journal of the National Cancer Institute. 1997;89(6):353-353.
9. Harding SJ, Mead GP, Bradwell AR, Berard AM. Serum free light chain immunoassay as an adjunct to serum protein electrophoresis and immunofixation electrophoresis in the detection of multiple myeloma and other B-cell malignancies. Clinical Chemistry and Laboratory Medicine. 2009 Mar 1;47(3):302-4.
10. Jurczyszyn A, Gozzetti A, Gdula-Argasińska J, Czepiel J, Vij R, Fiala M, et al. Similar survival outcomes in patients with biclonal versus monoclonal myeloma: a multi-institutional matched case-control study. Annals of Hematology. 2017 Oct;96(10):1693-8.