Commentary
Allogeneic stem cell transplantation remains the only curative option for various benign and malignant hematological disorders [1-3]. The procedure of allogeneic stem cell transplantation entails the administration of adequate doses of hematopoietic progenitor cells, aiming for complete and sustained hematopoietic reconstitution. Hematopoietic progenitors are obtained either through a bone marrow harvest or from GCSF-mobilized peripheral blood of the identified donor. Over the years various modalities have been utilized to establish the adequacy of harvested stem cell product e.g. Colony-forming unit– granulocyte monocyte (CFU-GM) assay, total mononuclear cell count (MNC), and CD34+ cell count.
Previously, CFU-GM was considered the gold standard for establishing the quality and adequacy of harvested stem cell product [4-7]. However, estimation of CD34+ cell count by flowcytometry has effectively replaced this function of CFU-GM. Now, an adequately harvested stem cell product, that ensures successful and sustained engraftment and repopulation of hematopoietic tissue, is defined as one with the CD34+ cells count of ≥ 2 million cells/kg of recipient’s body weight [8-11]. In lower-middle income countries, estimation of CD34+ cell count by flowcytometry is extremely challenging in terms of its financial implications as well as the technical expertise that it requires. Furthermore, it is a time consuming procedure with a turnaround time of several hours [12-14].
Another modality with a potential to serve as a predictor of adequately harvested stem cell product is Hematopoi Progenitor Cell count (XN-HPC), estimated and reported by Sysmex XN-20 hematology analyzers (Sysmex Corporation, Cobe, Japan). Various studies have evaluated the predictive potential of peripheral blood XN-HPC for CD34+ cell count in autologous stem cell transplant setting, and encouraging results have been obtained. Easy accessibility, operator independence, cost effectiveness, and faster turnaround time (less than 2 minutes) are the major advantages of XN-HPC over CD34+ cell enumeration by flowcytometry.
Sysmex hematology analyzers are equipped with a peculiar White Precursor Cell (WPC) channel that treats the cells with a solvent-detergent system resulting in lysis of mature myeloid precursors. Immature myeloid progenitor cells are inherently resistant to the solventdetergent action due to their low cell membrane cholesterol content [15,16]. These cells are then analyzed through optical detection and fluorescent flowcytometry; computing forward scatter (FSC), side scatter (SSC) and side fluorescence scatter of the immature myeloid precursors [17,18]. FSC and SSC provide information about the cell size and internal complexity respectively. Fluorescence scatter informs about the maturity status as well as benign/malignant origin of the cell population. Hematopoietic progenitors (XP-HPC), due to their lower WPC channel reagent permeability, are then identified through their medium FSC, low SSC and relatively low side fluorescence. XN-HPC count is then reported by the analyzer as number of cells x 103/μl.
CD34 antigen positive hematopoietic stem cells obtained by flowcytometry and XN-HPC count obtained by sysmex hematology analyzers are two separate entities; the former represents an early population of stem cells without expression of lineage specific antigens while the later identifies lineage antigen negative stem cells plus hematopoietic progenitor cells with lineage commitment.
The role of pre-harvest peripheral blood XN-HPC has already been established in confidently determining the day of stem cell harvest for an effective autologous collection [19]. Similarly, the prognosticating potential of pre-harvest peripheral blood XN-HPC for the post-harvest product CD34+ cell count has also been substantiated in both autologous and allogenic stem cell transplant setting [8].
So far, there has been no published data on the enumeration of XN-HPC count and its correlation with CD34 positive cells in harvested allogeneic peripheral blood stem cell products. Both the entities are considered to represent two different cell populations with some overlap. In our recent study published in Translational Oncology [20], we have elucidated the role of XN-HPC in predicting the CD34+ cell count of harvested peripheral blood stem cell product. The objective of this study was to establish the definitive XN-HPC cut off, for the effective CD34+ cell count of ≥ 2 million cells/kg of recipient’s body weight, in harvested peripheral blood stem cell product.
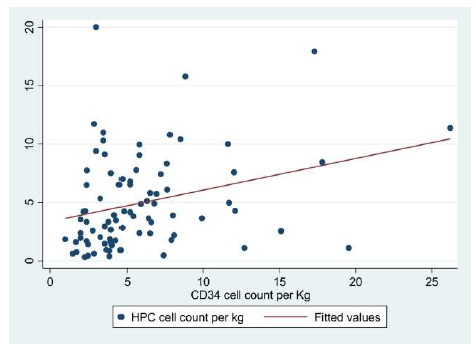
The study was carried out in 2 phases. In phase I, XN-HPC and CD34+ cell count were recorded for 101 harvested peripheral blood stem cell products by utilizing Sysmex XN-1000 and BD FASC Calibur (using ISHAGE gating strategy [21]), respectively. This data was analyzed to establish the degree of correlation between the two variables i.e. XN-HPC and CD34+ cell count by applying spearman correlation. The analysis revealed a moderately positive and statistically significant correlation (P value =0.003).
| Coordinates of the CurveMechanism/FunctionReferences | ||
|---|---|---|
| Test Result Variable(s):HPC (106 cell/kg) | ||
| Positive if Greater Than or Equal Toa | Sensitivity (%) | Specificity (%) |
| 0.358000 | 98.70 | .00 |
| 0.685000 | 93.60 | 25.00 |
| 0.805000 | 93.60 | 50.00 |
| 1.660000 | 82.10 | 75.00 |
| 1.715000 | 80.80 | 75.00 |
| 1.745000 | 79.50 | 75.00 |
| 1.795000 | 78.20 | 75.00 |
| =1.845000 | =78.20 | 100.00 |
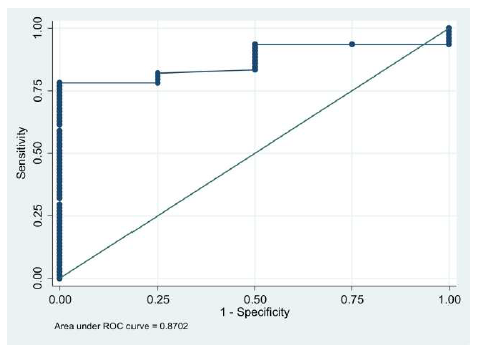
Target value of CD34+ cell count of ≥ 2 million cells/ kg of recipient’s body weight was then focused for the corresponding XN-HPC value. ROC analysis revealed area under the curve as 87.0% (P value = .013).
Multiple XN-HPC values were computed against the targeted CD34+ cell count value of ≥ 2 million cells/kg of recipient’s body weight to obtain evaluable sensitivity and specificity of XN-HPC for an adequately harvested peripheral blood stem cell product. This analysis led us to an XN-HPC cut off value of ≥ 1.84 × 106 cells/kg of the recipient’s body weight with the sensitivity and specificity of 76% and 100% respectively. Of the evaluable harvested stem cell products, 74% had an XN-HPC of ≥ 1.84 × 106 cells/kg of the recipient’s body weight and all of them were found to have the CD34+ cell count value of ≥ 2 million cells/kg of recipient’s body weight.
In the phase II of this study, the computed XN-HPC cutoff, from the phase I study, for the targeted CD34+ cell count was validated in 112 harvested peripheral blood stem cell products. The positive predictive value and negative predictive value were calculated to be 100% and 17% respectively.
This study aimed to establish the functional equivalence of XN-HPC to CD34+ cells to establish the competency and adequacy of harvested peripheral blood stem cell products. The reasonable size of our data satisfactorily established the positive correlation between XN-HPC and CD34+ cell count. The study successfully established the highly specific role of XN-HPC as an alternative surrogate marker for the adequacy of hematopoietic stem cell dose in allogeneic PBSC setting.
Performing CD34+ cell count by flowcytometry is a mammoth task for the lower-middle income countries. In Pakistan, this task is further compounded by the need to import all the flourochromes as well as lack of trained personnel and limited availability of flowcytometers. This discovery has provided us with a major breakthrough to circumvent above mentioned problems and we safely rely on the XN-HPC count and MNC count in times when flourochromes are not available. Even if the financial convenience of XN-HPC is set aside, the simplicity, rapid turnaround time, ability to process multiple samples at a time and the convenience of performing intermediate product analysis to decide the collection time, makes it a highly preferred approach over CD34+ cell enumeration even for the developed countries.
Currently, we are working on the correlation and meaningful XN-HPC cutoff, for the target CD34+ cell count, on harvested bone marrow stem cell products and incipient results are highly encouraging. It is sufficient to say here that, for harvested bone marrow stem cell products, a different yet highly specific XN-HPC cutoff has been established for the optimal CD34+ cell count. The analyzed data will soon be shared in the coming paper after the completion of validation studies. We urge other institutes performing stem cell transplantation to further verify this important correlation so that significantly more convenient and financially feasible option of XN-HPC can be confidently used instead of the cumbersome and demanding procedure of flowcytometry for establishing the adequacy of harvested stem cell products. More studies on the utility of XN-HPC are required from different centers around the world to validate our findings.
One limitation of performing XN-HPC count is that it is only available on Sysmex XN-20 hematology analyzers. No other manufacturer or any other Sysmex model currently has the capability to enumerate XN-HPC data. On the positive side, there are an ever-increasing number of XN- 20 analyzers available in major hospitals worldwide.
References
2. Gratwohl A, Baldomero H, Passweg JF, Frassoni F, Niederwieser D, Schmitz N, et al. Hematopoietic stem cell transplantation for hematological malignancies in Europe. Leukemia. 2003 May;17(5):941-59.
3. Thomas ED. Bone marrow transplantation: a review. Seminars in Hematology. 1999;36(4 Suppl 7):95–103.
4. Schwartzberg L, Birch R, Blanco R, Wittlin F, Muscato J, Tauer K, et al. Rapid and sustained hematopoietic reconstitution by peripheral blood stem cell infusion alone following high-dose chemotherapy. Bone Marrow Transplantation. 1993 May 1;11(5):369-74.
5. Douay L, Gorin NC, Mary JY, Lemarie E, Lopez M, Najman A, et al. Recovery of CFU-GM from cryopreserved marrow and in vivo evaluation after autologous bone marrow transplantation are predictive of engraftment. Experimental Hematology. 1986 Jun 1;14(5):358-65.
6. Elias AD, Ayash L, Anderson KC, Hunt M, Wheeler C, Schwartz G. Mobilization of peripheral blood progenitor cells by chemotherapy and granulocyte-macrophage colony-stimulating factor for hematologic support after high-dose intensification for breast cancer. Blood. 1992;79(11):3036-3044.
7. Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86(10):3961–3969.
8. Peerschke EI, Moung C, Pessin MS, Maslak P. Evaluation of new automated hematopoietic progenitor cell analysis in the clinical management of peripheral blood stem cell collections. Transfusion. 2015 Aug;55(8):2001-9.
9. BENDER JG, To LB, WILLIAMS S, SCHWARTZBERG LS. Defining a therapeutic dose of peripheral blood stem cells. Journal of Hematotherapy. 1992;1(4):329-41.
10. Hequet O. Hematopoietic stem and progenitor cell harvesting: technical advances and clinical utility. Journal of blood medicine. 2015;6:55.
11. To LB, Haylock DN, Simmons PJ, Juttner CA. The biology and clinical uses of blood stem cells. Blood, The Journal of the American Society of Hematology. 1997 Apr 1;89(7):2233-58.
12. Letestu R, Marzac C, Audat F, Belhocine R, Tondeur S, Baccini V, et al. Use of hematopoietic progenitor cell count on the Sysmex XE-2100 for peripheral blood stem cell harvest monitoring. Leukemia & Lymphoma. 2007 Jan 1;48(1):89-96.
13. Zulkafli Z., Mustaffa R., Yusoff S.M. Hematopoietic progenitor cells as a predictive of CD34+enumeration prior to peripheral blood stem cells harvesting. Bali Medical Journal. 2014;3(3):112–115.
14. Oelschlaegel U, Bornhaeuser M, Thiede C, Ehninger G, Hoelig K. HPC enumeration with the Sysmex XE-2100 can guide further flow cytometric CD34+ measurements and timing of leukaphereses. Cytotherapy. 2003 Jan 1;5(5):414-9.
15. Park KU, Kim SH, Suh C, Kim S, Lee SJ, Park JS, et al. Correlation of hematopoietic progenitor cell count determined by the SE-9000™ automated hematology analyzer with CD34+ cell count by flow cytometry in leukapheresis products. American Journal of Hematology. 2001 May;67(1):42-7.
16. Steussy BW, Capper M, Krasowski MD, Rosenthal NS, Schlueter AJ. Algorithms utilizing peripheral blood hematopoietic progenitor cell counts in lieu of some CD 34+ cell counts predict successful peripheral blood stem cell collections with substantial time and cost savings. ISBT Science Series. 2016 Dec;11(3):153-62.
17. Yamane T. Possibility of identification of hematopoietic stem cells using a conventional blood cell counter. Enr J Haematol. 1995;55:207-8.
18. Takekawa K, Yamane T, Suzuki K, Hino M, Tatsumi N. Identification of hematopoietic stem cells by the SE- 9000TM automated hematology analyzer in peripheral blood stem cell harvest samples. Acta Haematologica. 1997;98(1):54-5.
19. Lefrère F, Zohar S, Beaudier S, Audat F, Ribeil JA, Ghez D, et al. Evaluation of an algorithm based on peripheral blood hematopoietic progenitor cell and CD34+ cell concentrations to optimize peripheral blood progenitor cell collection by apheresis. Transfusion. 2007 Oct;47(10):1851-7.
20. Jamal A, Khan MT, Parveen S, Rizvi Q, Farzana T, Zaidi U, et al. Peripheral Blood Stem Cell Harvest HPC Count Is an Effective Surrogate Marker for CD34+ Cell Count in Allogeneic Stem Cell Transplant Setting. Translational Oncology. 2020 Jul 1;13(7):100788.
21. Sutherland DR, ANDERSON L, KEENEY M, NAYAR R, Chin-Yee IA. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. Journal of Hematotherapy. 1996 Jun;5(3):213-26.