Abstract
External ventricular drain (EVD) is a standard way for both monitoring intracranial pressure (ICP) and draining cerebrospinal fluid (CSF) for aneurysmal subarachnoid hemorrhage (aSAH) patients. In our recent published paper, we found great variations in clinical practice of bedside ICP monitoring through EVD. This commentary describes the current problems with EVD practices and depicts future trends of cerebral monitoring for aSAH patients. We believe a monitoring system that meet the three criteria: continuous and precise; multimodal and noninvasive; personalized and predictive is promising in the advancement of neurointensive care of patients with aSAH.
Keywords
Aneurysmal subarachnoid hemorrhage (aSAH); Intracranial pressure (ICP); Multi-modality monitoring; Neural intensive care
ICP monitoring in aSAH
Since elevated ICP can cause herniation with high risk of irreversible brain damage and death, precise monitoring of ICP is extremely important in clinical practice for aSAH patients [4]. Different ICP monitoring techniques exist in intensive care unit (ICU) including epidural, subdural, intraparenchymal and intraventricular ICP monitoring [4]. For aSAH patients, external ventricular drain (EVD) is most commonly used due to its ability of both monitoring ICP and draining cerebral spinal fluid (CSF) [5,6].
ICP monitoring in aSAH
Since elevated ICP can cause herniation with high risk of irreversible brain damage and death, precise monitoring of ICP is extremely important in clinical practice for aSAH patients [4]. Different ICP monitoring techniques exist in intensive care unit (ICU) including epidural, subdural, intraparenchymal and intraventricular ICP monitoring [4]. For aSAH patients, external ventricular drain (EVD) is most commonly used due to its ability of both monitoring ICP and draining cerebral spinal fluid (CSF) [5,6].
Importance of ICP monitoring in aSAH
The annual incidence of aneurysmal subarachnoid hemorrhage (SAH) in the United States is 6-16 cases per 100,000 population, with approximately 30,000 cases occurring each year [1]. Elevated intracranial pressure (ICP) is a well-recognized phenomenon that can occur in an acute (within 24 hours), subacute (up to 7-10 days) and delayed (after 10 days) fashion following hemorrhage [2]. More than 50% of aSAH patients will develop elevated ICP (ICP>20 mmHg) during their stay in hospital [3], which is mostly caused by hydrocephalus, global cerebral edema, subdural hematoma, medication for vasospasm, etc [2].
Current challenges with EVD practices
Although EVD can provide precise ICP value while it is closed, it cannot monitor ICP while it is open to CSF drainage [6]. Documenting an accurate ICP value through EVD is important to assess the healthy status of the brain [7], however, this clinical practice still faces great challenges summarized below:
1. Which protocol should we use?
Currently, clinicians mainly adopt 2 different protocols for EVD treatment: one is ‘ICP monitor-first’ protocol which maintains continuous ICP monitoring with intermittent CSF drainage and the other is ‘CSF drainfirst’ protocol, which keeps continuous CSF drainage and clamping the tap for ICP intermittent checking (Figure 1) [7]. Studies have been conducted to investigate which protocol should be used for aSAH patients and they reported conflicting results. Some studies found no difference between the two protocols in patient outcome, while other studies reported that drain-first strategy is associated with morbidity or increased vasospasm and hydrocephalus [8-11].
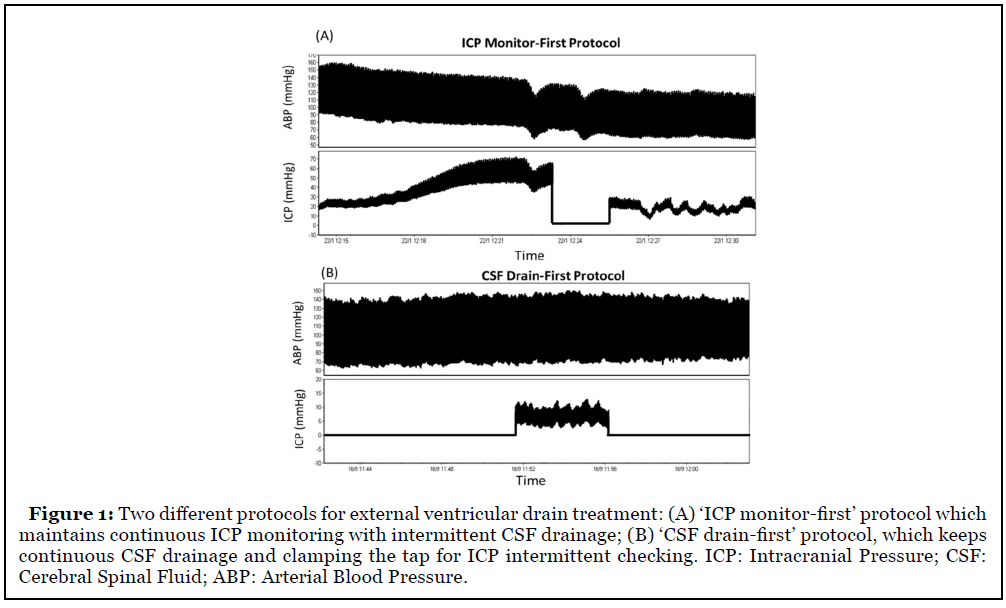
2. Is the current practice of clamping EVD to measure ICP good enough?
For drain-first protocol, Rogers et al. suggested a minimum period of 5-minute monitoring before documenting the ICP value, because ICP dynamics have inherent hysteresis and require a certain amount of time to reach a new equilibrium after closing the system to CSF drainage [12]. However, in our recent research, we found that 66% of EVD closures lasts less than one minute. Most cases do not provide sufficient time to reach the true ICP level [7]. A standard guideline and proper training to nurses are needed for ICP intermittent checking.
Future trends of cerebral monitoring for aSAH patient
Given the fast development of monitoring technology in ICU, the future trend of aSAH patient monitoring may need to fulfill the three criteria: continuous and precise; multimodal and noninvasive; personalized and predictive.
1. Continuous and precise: Current EVD cannot monitor ICP while it is open to CSF drainage, which may introduce additional risk of missing important information due to intermittent nature of the measurement [17]. In our previous study, we described a novel ventricular catheter (Integra® Camino® FLEX Ventricular Catheter, Integra Lifesciences, Ireland) which is a double-lumen catheter with one lumen for CSF diversion and the other one for ICP measurement. We compared the ICP measure by FLEX catheter and traditional EVD, the mean difference between the two ICPs was only 1.69 mmHg. This type of continuous and precise ICP monitoring system should be a future trend for aSAH patients at bedside.
2. Multi-modality and non-invasive: The emergence of technology that allows continuous realtime bedside monitoring of cerebral physiology facilitate assessment of brain status and provide important information for bedside treatment [18]. The international multidisciplinary consensus conference on multimodality monitoring in neurocritical care recommended ICP monitoring for patients at risk of high ICP [19]. For severe aSAH patients, EVD is proved to be valuable and often lifesaving [20]. Meanwhile, several other noninvasive technologies that provide information about cerebral hemodynamics were also recommended [18], including Transcranial Doppler for cerebral blood flow velocity detection; Near infrared spectroscopy (NIRS) for regional cortical oxygen saturation rSO2 monitoring (Figure 2); Continuous Electroencephalogram (EEG) for neural activity monitoring and for vasospasm and delayed cerebral ischemia detection; Multichannel amplitudeintegrated EEG (aEEG) for seizure or other abnormal brain activity detection in neonatal patients (Figure 3) [21-23]; Brain tissue oxygen monitoring for cerebral ischemia or hypoxia detection; Cerebral microdialysis for brain metabolism assessment, etc. [19]. Combining these monitors in a multimodal approach may provide comprehensive assessment about the brain and is promising in the advancement of ICU care of patients with aSAH [1].
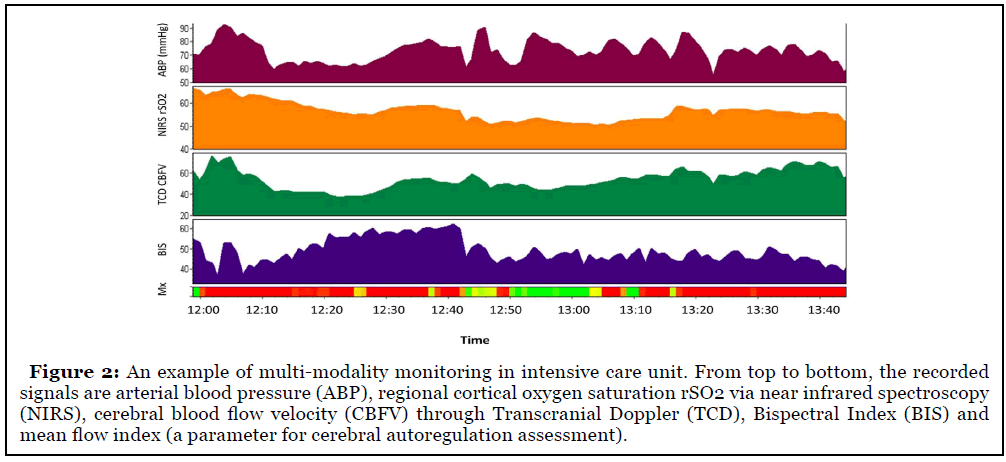
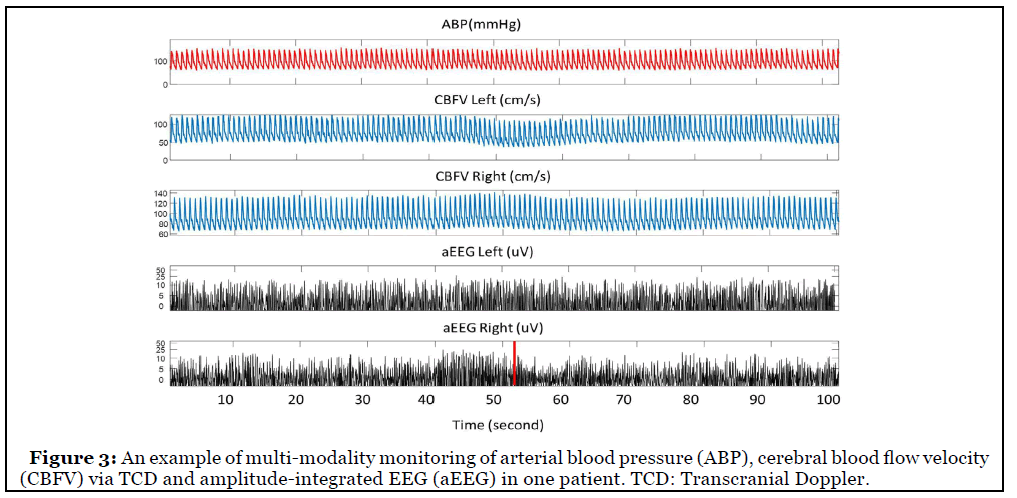
3. Personalized and predictive: With the concept of precision medicine, previous ‘one-to-all’ treatment policy needs to be improved. Different strategies have been introduced for personalized treatment that emphasizes the individual patient’s unique neurological and systemic physiology. For aSAH patients, a possible way for personalized treatment is through continuous cerebral autoregulation monitoring. The Cambridge group introduced a practical way of defining personalized optimal cerebral perfusion pressure (CPPopt) or arterial blood pressure (ABPopt) by identifying the CPP or ABP level at which autoregulatory vasoreactivity is most robust [24]. Targeting the optimal CPP/ABP may improve neurologic outcomes after adult traumatic brain injury [25]. Meanwhile, using continuous assessment of cerebral autoregulation, individualized lower limit (LLA) and upper limit of autoregulation (ULA) can be identified [26]. Studies demonstrated that keeping ABP greater than the individual patient’s LLA during cardiac surgery may reduce the incidence of post-operative delirium or acute kidney injury [27]. Further studies need to be conducted to test whether CPPopt or LLA is useful in improving aSAH patient outcome.
Moreover, in his review, Hu mentioned that future ICU monitoring will be predictive with the development of artificial intelligent technology [28]. Previous studies already demonstrated the possibility of using continuous EEG or aEEG to predict delayed cerebral ischemia, seizures or other neurological outcome [21-23,29-31] before clinical deterioration in ICU. For aSAH patients, how to combine lab results, electronic hospital records data and continuous digital monitoring to predict aSAH patient clinical outcome or shorten the duration of weaning trial would be of great help.
Advances in technology have made simultaneous multi-modality monitoring in ICU feasible and reliable. We believe a desired future monitoring system will be continuous and precise; multimodal and noninvasive; personalized and predictive.
3. How to standardize the EVD weaning trial and EVD removal protocol?
Many patients after aSAH require permanent CSF diversion with ventriculoperitoneal shunting (VPS) [13]. To determine which patient need VPS, weaning trials are performed with EVD clamped for 1-2 days [13]. If the patient passes the weaning trial, EVD will be removed or else a VPS will be implanted into the brain. It is not abnormal that a patient may experience several weaning trials before EVD removal. Moreover, a small subpopulation of patients may develop hydrocephalus in a delayed fashion after successful weaning trial and removal of EVD. Akinduro reported that among the patients who initially passed their EVD weaning trial and were discharged without a VPS, 15.4% of them subsequently develop delayed hydrocephalus and ultimately required VPS [14]. The mechanism of the failed weaning trials and delayed VPS after initial EVD removal remains poorly understood and still need further investigation [15,16].
References
2. Alotaibi NM, Wang JZ, Pasarikovski CR, Guha D, Al-Mufti F, Mamdani M, et al. Management of raised intracranial pressure in aneurysmal subarachnoid hemorrhage: time for a consensus?. Neurosurgical Focus. 2017 Nov 1;43(5):E13.
3. Heuer GG, Smith MJ, Elliott JP, Winn HR, Leroux PD. Relationship between intracranial pressure and other clinical variables in patients with aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery. 2004 Sep 1;101(3):408-16.
4. Raboel PH, Bartek J, Andresen M, Bellander BM, Romner B. Intracranial pressure monitoring: invasive versus non-invasive methods—a review. Critical Care Research and Practice. 2012;2012.
5. Slazinski T, Anderson T, Cattell E, Eigsti JE, Heimsoth S, Holleman J, et al. Nursing management of the patient undergoing intracranial pressure monitoring, external ventricular drainage, or lumbar drainage. Journal of Neuroscience Nursing. 2011 Aug 1;43(4):233-5.
6. Kim GS, Amato A, James ML, Britz GW, Zomorodi A, Graffagnino C, et al. Continuous and intermittent CSF diversion after subarachnoid hemorrhage: a pilot study. Neurocritical care. 2011 Feb 1;14(1):68-72.
7. Liu X, Griffith M, Jang HJ, Ko N, Pelter MM, Abba J, et al. Intracranial Pressure Monitoring via External Ventricular Drain: Are We Waiting Long Enough Before Recording the Real Value?. Journal of Neuroscience Nursing. 2020 Feb 1;52(1):37-42.
8. Amato A, Britz GW, James ML, Graffagnino C, Zomorodi AR, Zomorodi ME, et al. An observational pilot study of CSF diversion in subarachnoid haemorrhage. Nursing in Critical Care. 2011 Sep;16(5):252-60.
9. Olson DM, Zomorodi M, Britz GW, Zomorodi AR, Amato A, Graffagnino C. Continuous cerebral spinal fluid drainage associated with complications in patients admitted with subarachnoid hemorrhage. Journal of Neurosurgery. 2013 Oct 1;119(4):974-80.
10. Nwachuku EL, Puccio AM, Fetzick A, Scruggs B, Chang YF, Shutter LA, et al. Intermittent versus continuous cerebrospinal fluid drainage management in adult severe traumatic brain injury: assessment of intracranial pressure burden. Neurocritical Care. 2014 Feb 1;20(1):49-53.
11. Safavi-Abbasi S, Wilson CD, Ghafil C, Spetzler RF. Meta-analysis Comparing Continuous and Intermittent Cerebrospinal Fluid Drainage in Aneurysmal Subarachnoid Hemorrhage. Journal of Neurological Surgery Part B: Skull Base. 2016 Feb;77(S 01):A083.
12. Rogers M, Stutzman SE, Atem FD, Sengupta S, Welch B, Olson DM. Intracranial pressure values are highly variable after cerebral spinal fluid drainage. Journal of Neuroscience Nursing. 2017 Apr 1;49(2):85-9.
13. Gigante P, Hwang BY, Appelboom G, Kellner CP, Kellner MA, Connolly ES. External ventricular drainage following aneurysmal subarachnoid haemorrhage.British Journal of Neurosurgery. 2010 Dec 1;24(6):625- 32.
14. Akinduro OO, Vivas-Buitrago TG, Haranhalli N, Ganaha S, Mbabuike N, Turnbull MT, et al. Predictors of Ventriculoperitoneal shunting following Subarachnoid Hemorrhage treated with External Ventricular Drainage. Neurocritical Care. 2019 Aug 13:1-0.
15. Czorlich P, Ricklefs F, Reitz M, Vettorazzi E, Abboud T, Regelsberger J, et al. Impact of intraventricular hemorrhage measured by Graeb and LeRoux score on case fatality risk and chronic hydrocephalus in aneurysmal subarachnoid hemorrhage. Acta Neurochirurgica. 2015 Mar 1;157(3):409-15.
16. Wilson CD, Safavi-Abbasi S, Sun H, Kalani MY, Zhao YD, Levitt MR, et al. Meta-analysis and systematic review of risk factors for shunt dependency after aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery. 2017 Feb 1;126(2):586-95.
17. Berlin T, Murray-Krezan C, Yonas H. Comparison of parenchymal and ventricular intracranial pressure readings utilizing a novel multi-parameter intracranial access system. SpringerPlus. 2015 Dec 1;4(1):10.
18. Sandsmark DK, Kumar MA, Park S, Levine JM. Multimodal monitoring in subarachnoid hemorrhage. Stroke. 2012 May;43(5):1440-5
19. Lara LR, Püttgen HA. Multimodality Monitoring in the Neurocritical Care Unit. Continuum: Lifelong Learning in Neurology. 2018 Dec 1;24(6):1776-88.
20. Lovasik BP, McCracken DJ, McCracken CE, McDougal ME, Frerich JM, Samuels OB, et al. The effect of external ventricular drain use in intracerebral hemorrhage. World Neurosurgery. 2016 Oct 1;94:309-18.
21. Sun J, Ma D, Lv Y. Detection of seizure patterns with multichannel amplitude-integrated EEG and the color density spectral array in the adult neurology intensive care unit. Medicine. 2018 Sep;97(38).
22. Bruns N, Blumenthal S, Meyer I, Klose-Verschuur S, Felderhoff-Müser U, Müller H. Application of an amplitude-integrated EEG monitor (cerebral function monitor) to neonates. JoVE (Journal of Visualized Experiments). 2017 Sep 6(127):e55985.
23. Hellström-Westas L. Amplitude-integrated electroencephalography for seizure detection in newborn infants. InSeminars in Fetal and Neonatal Medicine 2018 Jun 1 (Vol. 23, No. 3, pp. 175-182). WB Saunders.
24. Hellström-Westas L. Amplitude-integrated electroencephalography for seizure detection in newborn infants. InSeminars in Fetal and Neonatal Medicine 2018 Jun 1 (Vol. 23, No. 3, pp. 175-182). WB Saunders.
25. Liu X, Donnelly J, Czosnyka M, Aries MJ, Brady K, Cardim D, et al. Cerebrovascular pressure reactivity monitoring using wavelet analysis in traumatic brain injury patients: A retrospective study. PLoS Medicine. 2017 Jul;14(7).
26. Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Czosnyka M, et al. The lower limit of cerebral blood flow autoregulation is increased with elevated intracranial pressure. Anesthesia & Analgesia. 2009 Apr 1;108(4):1278-83.
27. Brown CH, Neufeld KJ, Tian J, Probert J, LaFlam A, Max L, et al. Effect of targeting mean arterial pressure during cardiopulmonary bypass by monitoring cerebral autoregulation on postsurgical delirium among older patients: a nested randomized clinical trial. JAMA Surgery. 2019 Sep 1;154(9):819-26.
28. Hu X. An algorithm strategy for precise patient monitoring in a connected healthcare enterprise. NPJ Digital Medicine. 2019 Apr 30;2(1):1-5.
29. Rots ML, van Putten MJ, Hoedemaekers CW, Horn J. Continuous EEG monitoring for early detection of delayed cerebral ischemia in subarachnoid hemorrhage: a pilot study. Neurocritical Care. 2016 Apr 1;24(2):207- 16.
30. Guo Y, Fang S, Wang J, Wang C, Zhao J, Gai Y. Continuous EEG detection of DCI and seizures following aSAH: a systematic review. British Journal of Neurosurgery. 2019 Jun 17:1-6.
31. You W, Tang Q, Wu X, Feng J, Mao Q, Gao G, et al. Amplitude-integrated electroencephalography predicts outcome in patients with coma after acute brain injury. Neuroscience Bulletin. 2018 Aug 1;34(4):639-46.
