Abstract
The intestinal epithelium not only facilitates the absorption of nutrients, but also plays a pivotal role in guarding intestinal homeostasis and preventing opportunistic gut microbiome invasions. The intestinal epithelial cells have diverse and coordinated regulatory networks that provide intricate lines of defense, in order to maintain the integrity of the intestinal barrier. The epithelial defense comprises the anatomical structure of intestinal epithelial cells as a stout physical barrier and its covering mucus layer containing diverse antimicrobial peptides. In addition, epithelial cells are well-equipped with diverse microbial sensors, which upon activation induce the expression of downstream immunomodulatory networks of cytokines and chemokines. In this review, we summarize the intestinal epithelial cells’ defense mechanisms and their role in maintaining intestinal homeostasis.
Keywords
Cytokine biology, Epithelial cells, Immunomodulation, Inflammation
Introduction
The intestinal epithelial cells (IECs) form a semi-permeable barrier that allows the absorption of nutrients and electrolytes, meanwhile preventing harmful external environmental antigens from entering the host’s internal environment, in order to maintain the host’s homeostasis [1]. The mammalian intestine accommodates a dynamic community of trillions of microorganisms allowing the adaptation of diverse saccharolytic enzymes that complement the limited saccharolytic diversity encoded in the mammalian genome [2]. Although the host-microorganisms relationship is symbiotic in nature, such a dense bacterial community poses a serious threat to the host, and opportunistic invasion of the host’s internal environment by gut resident bacteria can lead to serious pathologies such as chronic inflammation or, in extreme cases, bacteremia. Taking into consideration the huge numbers and the high diversity of gut microbiome, and the large surface area of the intestine, the best defense strategy for the host is to prevent microorganisms from breaching the epithelial barrier. IECs play a central role in maintaining the epithelial barrier through shaping physical and chemical layers of defense to prevent the invasion of gut microorganisms into the internal environment of the host [3,4]. However, gut bacteria have evolved evasion strategies to escape from these layers of defense and breach epithelial barrier. Remarkably, epithelial cells utilize a next layer of defense against the invaded pathogens through cell-intrinsic microbial sensors and the activation of immune responses to eradicate invading pathogens [5,6]. In this review, we discuss the adaptations of IECs to limit opportunistic invasions of resident microorganisms, and how the intestinal epithelium minimizes the contact between luminal microbiota and the immune system, in order to prevent destructive immune responses and maintain intestinal homeostasis.
First Line of Defense: The Mucus Layer
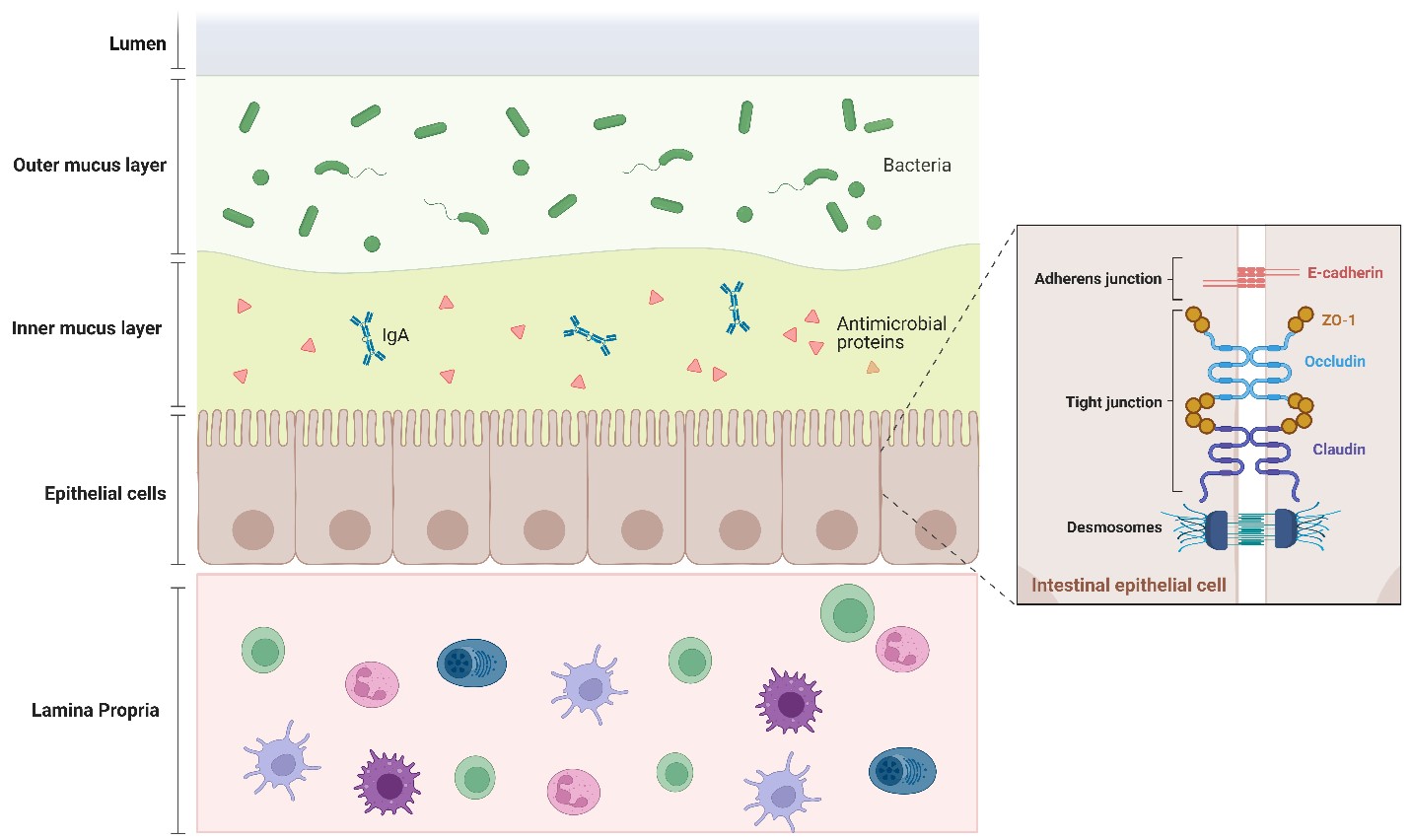
Goblet cells, which are specialized epithelial cells, secrete mucin glycoproteins. These mucins are arranged into a viscous gel-like layer covering the epithelial surface, forming inner and outer mucus layers [7]. Whereas the small intestine has only one loose mucus layer, the large intestine displays the two mucus layers to defend against trillions of inhabiting gut bacteria [8]. Mice deficient in Muc2, a key mucin glycoprotein, show bacterial translocation across the mucosal barrier and develop spontaneous colitis [9]. In addition, probiotic microbiota, such as lactobacilli, enhance the intestinal epithelium barrier function through the stimulation of mucin production [10]. On the other hand, colitogenic microbiota, such as Entamoeba histolytica, precipitate intestinal inflammation by degrading the C-terminal region of mucin [11].
Second Line of Defense: The Apical Junctional Complex (APC)
Epithelial cells exhibit an anatomical structure that separates the internal host environment from the external environmental stresses [12]. This separation is partly achieved by apical junctional complex (APC), which ensures the impermeability of both commensal and pathogenic bacteria inhabiting the gut. The APC consists of three types of junctional proteins. First, tight junction proteins such as claudins, occludin, junctional adhesion molecules, and zonula occludens (ZO). Second, adherens junction proteins such as E-cadherin. Lastly, the desmosomes [13]. Mice deficient in occludin expressed morphologically intact tight junction structures but exhibited elevated inflammation and a defective gut barrier [14]. Bacteria such as Clostridium difficile and Listeria monocytogenes target occludin and claudin, respectively, thus weakening the intestinal epithelial barrier and promoting their invasion leading to an increase in intestine permeability [15]. Moreover, epithelial cells display a constant turnover cycle every 3-5 and 5-7 days in the small and large intestines, respectively, which renews the epithelial barrier, and thus maintains intestinal homeostasis [16,17].
Third Line of Defense: Antimicrobial Peptides (AMPs) and Immunoglobulin A (IgA)
AMPs are secreted by gut epithelial cells and diffused through the mucus layer to prevent unwanted colonization of microbes. AMPs include defensins, cathelicidins, and C-type lectins, which have a wide spectrum of antibiotic activity [18,19]. Besides the microbicidal activity of AMPs, they have several other actions, like stimulating mucus secretion [20], expression of tight junction proteins [21], chemotaxis, cell proliferation, and enhancing the production of extracellular matrix proteins, confirming their roles in wound healing [22]. Defects in endogenous AMP expression and function have been linked with intestinal inflammation in mice. For example, Mice with a deficiency in Regenerating islet-derived protein 3 gamma (Reg IIIγ), a C-type lectin, exhibited increased mucosal bacterial burden and impaired spatial relationships between bacteria and their host tissues [23]. In addition, Ly6/Plaur domain-containing 8 (Lypd8) has been recently identified as an antimicrobial peptide, which contributes to the segregation of intestinal bacteria and intestinal epithelia in the large intestine [24]. Mice lacking Lypd8 demonstrate the disappearance of the bacteria-free space just above the epithelial layer of the colon [25].
IgA is secreted by plasma cells located in lamina propria. The secreted form of IgA (sIgA) is transcytosed across the epithelium to allow binding to luminal bacteria. The transcytosed IgA binds to bacteria on the luminal side of the epithelial barrier and prohibits their translocation [26]. The exact mechanisms by which IgA does these roles remain elusive but may include the ensnaring of bacteria in the mucus layer or enhancing fast phagocytic clearance of the pathogens that invade the epithelial cell barrier [27].
Fourth Line of Defense: Network of Microbial Sensors and Immunomodulatory Effectors
Despite the aforementioned defenses of IEC, pathogenic microorganisms have evolved escape strategies to escape. Remarkably, epithelial cells are well-equipped with intrinsic diverse and sophisticated regulatory networks to defend against the invaded pathogens. IECs utilize complex microbial sensors called pattern recognition receptors (PRRs) [28], which upon activation orchestrate the secretion of immunomodulatory molecules such as chemokines and cytokines to culminate in an appropriate immune response towards invading pathogens [29,30]
PRRs recognize conserved bacterial structures called pathogen-associated molecular patterns (PAMPs) that are not found in the host’s cells. Additionally, PRR recognizes danger-associated molecular patterns (DAMPs) released from stressed or damaged cells [31]. PRRs comprise two main categories of receptors: membrane-bound toll-like receptors (TLRs), and intracellular nucleotide oligomerization domain (NOD)-like receptors (NLRs). Upon microbial recognition, activation of PRRs activates a signaling cascade culminating in the expression of pro-inflammatory cytokines and antimicrobial mediators, and the recruitment of immune cells to aid in the eradication of the bacterial threat and protect the epithelium from pathogenic invasion [32]. Further, it has been shown that TLR signaling is involved in epithelial cell proliferation [33,34], IgA production [35], maintenance of tight junctions [36], and antimicrobial peptide expression which are essential for maintaining a healthy epithelial barrier [37]. TLR2 activation efficiently maintains the tight junction-associated barrier assembly in intestinal epithelial cells against stress-induced damage and inhibits mucosal inflammation [38]. High expression levels of TLR3 in intestinal epithelial cells correlate with resistance against rotavirus infection [39]. Mice that have deficiencies either in Tlr4 or Tlr5 exhibit impaired innate immune responses and are more vulnerable to dextran sodium sulfate (DSS)-induced colitis [40,41]. The best-characterized NLRs are NOD1 and NOD2, both of which identify PAMPs by leucine-rich repeats (LRRs) at the C-terminus like TLRs [42]. In accordance with their intracellular localization, NODs are specific for the detection of pathogens that invade the intestinal epithelial cells such as Shigella and Salmonella [43]. Consistently, Nod2-deficient mice exhibited severe colitis with increased bacterial invasion [44].
IECs present luminal antigens to intraepithelial lymphocytes (IELs) to modulate the adaptive immune system [45]. The bidirectional interactions between IELs and IECs are important to maintain immune homeostasis at the intestinal barrier [46]. Following bacterial invasion, IECs secrete chemokines such as CXCL8, CXCL1, CXCL3, and CXCL5 that recruit neutrophils. In turn, neutrophils secrete interleukin-22 (IL-22), which stimulates the expression of antimicrobial peptides by the colon epithelium and protects the epithelium from chemically induced damage [47,48]. Besides, IECs recruit dendritic cells (DCs) and T helper 17 (Th17) cells by secreting CCL20. In turn, Th17 secretes IL-17 which increases intestinal epithelial cell proliferation and reduces barrier permeability [49]. In addition, DCs cells secrete IL-28A, which induces intestinal epithelial proliferation [50].
Figure. 1. Structure of the Intestinal epithelial barrier: The intestinal epithelium is equipped with three layers of defense, chemical, physical, and cellular, which ensure the spatial segregation between the luminal microbes and the underlying immune system. The chemical barrier is the first line of defense formed by a double mucus layer including secreted antimicrobial peptides and IgA. Epithelial cells ensure microbes do not enter our tissues by forming a continuous, almost impregnable physical barrier that surrounds the gut lumen. This barrier is maintained by Junctional complexes between the cells formed by the tight proteins (zonula occludens (ZO), claudin, occludin), adherens junctions (E-cadherin), and desmosomes. The third layer of defense is the basal layer beneath the epithelial cells is the lamina propria, which is the immune barrier composed of gut-associated lymphocytes.
Conclusion and Future Remarks
IECs are key players in shaping and maintaining intestinal homeostasis, which is critical for the host’s metabolism and survival. IECs utilize diverse and complex networks to shape physical and chemical layers of defense against opportunistic gut microbiome invasions. Additionally, the cross-talk between IEC and the immune compartment strengthens the host defense against possible pathogenic invasions from gut microbiome. It is thus of particular interest to understand how the IECs regulatory signaling networks operate, and how these sophisticated pathways shape cell-intrinsic and cell-cell responses. Harnessing such knowledge will eventually offer more opportunities for better and more specific therapies targeting IECs to strengthen the intestinal barrier and/or induce immune tolerance with minimal side effects. Besides, nutritional support to enrich certain microbiome and promote a gut anti-inflammatory environment may prevent provoking detrimental immune response. Also, stem cell therapies to regenerate damaged IECs may be potentially helpful for treating severe cases of intestinal inflammation. Of note, choosing the best therapeutic strategy will likely depend on the comprehensive assessment of the etiology and the severity of intestinal inflammation.
Author Contributions
M.M.E wrote the manuscript draft. H.M. and T.K edited the manuscript and supervised the work.
Funding
This work was supported by Kishimoto Foundation. M.M. E. is supported by a full scholarship (ID: 69) from the Ministry of Higher Education of the Arab Republic of Egypt.
References
2. Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011 Jun 16;474(7351):298-306.
3. Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature Reviews Immunology. 2010 Mar;10(3):159-69.
4. Turner JR. Intestinal mucosal barrier function in health and disease. Nature Reviews immunology. 2009 Nov;9(11):799-809.
5. Constant DA, Nice TJ, Rauch I. Innate immune sensing by epithelial barriers. Current Opinion in Immunology. 2021 Dec 1; 73:1-8.
6. Oswald IP. Role of intestinal epithelial cells in the innate immune defence of the pig intestine. Veterinary Research. 2006;37(3):359-68.
7. Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2008 Sep 30;105(39):15064-9.
8. Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proceedings of the National Academy of Sciences of the United States of America. 2011 Mar 15;108(supplement_1):4659-65.
9. Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006 Jul 1;131(1):117-29.
10. Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1999 Apr 1;276(4):G941-50.
11. Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proceedings of the National Academy of Sciences of the United States of America. 2006 Jun 13;103(24):9298-303.
12. Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterology. 2014 Dec; 14:1-25.
13. Farquhar MG, Palade GE. Junctional complexes in various epithelia. The Journal of Cell Biology. 1963 May 1;17(2):375-412.
14. Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Molecular Biology of the Cell. 2000 Dec 1;11(12):4131-42.
15. Paradis T, Bègue H, Basmaciyan L, Dalle F, Bon F. Tight junctions as a key for pathogens invasion in intestinal epithelial cells. International Journal of Molecular Sciences. 2021 Mar 2;22(5):2506.
16. Tian J, Li Y, Bao X, Yang F, Tang X, Jiang Q, et al. Glutamine boosts intestinal stem cell-mediated small intestinal epithelial development during early weaning: Involvement of WNT signaling. Stem Cell Reports. 2023 Jul 11;18(7):1451-67.
17. Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, Vallance BA. The intestinal epithelium: central coordinator of mucosal immunity. Trends in Immunology. 2018 Sep 1;39(9):677-96.
18. Liévin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clinical Microbiology Reviews. 2006 Apr;19(2):315-37.
19. Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host–microbiota mutualism. Nature Reviews Microbiology. 2011 Apr;9(4):233-43.
20. Tai EK, Wong HP, Lam EK, Wu WK, Yu L, Koo MW, et al. Cathelicidin stimulates colonic mucus synthesis by up‐regulating MUC1 and MUC2 expression through a mitogen‐activated protein kinase pathway. Journal of Cellular Biochemistry. 2008 May 1;104(1):251-8.
21. Goto H, Hongo M, Ohshima H, Kurasawa M, Hirakawa S, Kitajima Y. Human beta defensin-1 regulates the development of tight junctions in cultured human epidermal keratinocytes. Journal of Dermatological Science. 2013 Aug 1;71(2):145-8.
22. Pound LD, Patrick C, Eberhard CE, Mottawea W, Wang GS, Abujamel T, et al. Cathelicidin antimicrobial peptide: a novel regulator of islet function, islet regeneration, and selected gut bacteria. Diabetes. 2015 Dec 1;64(12):4135-47.
23. Loonen LM, Stolte EH, Jaklofsky MT, Meijerink M, Dekker J, Van Baarlen P, et al. REG3γ-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal Immunology. 2014 Jul;7(4):939-47.
24. Kayama H, Okumura R, Takeda K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annual Review of Immunology. 2020 Apr 26; 38:23-48.
25. Okumura R, Kurakawa T, Nakano T, Kayama H, Kinoshita M, Motooka D, et al. Lypd8 promotes the segregation of flagellated microbiota and colonic epithelia. Nature. 2016 Apr 7;532(7597):117-21.
26. Yu T, Yang W, Yao S, Yu Y, Wakamiya M, Golovko G, et al. STING promotes intestinal IgA production by regulating Acetate-producing bacteria to maintain host-microbiota Mutualism. Inflammatory Bowel Diseases. 2023 Jun 1;29(6):946-59.
27. Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nature Reviews Immunology. 2003 Jan 1;3(1):63-72.
28. Gourbeyre P, Berri M, Lippi Y, Meurens F, Vincent‐Naulleau S, Laffitte J, et al. Pattern recognition receptors in the gut: analysis of their expression along the intestinal tract and the crypt/villus axis. Physiological Reports. 2015 Feb;3(2):e12225.
29. McCormick BA, Hofman PM, Kim J, Carnes DK, Miller SI, Madara JL. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. The Journal of Cell Biology. 1995 Dec 15;131(6):1599-608.
30. Eckmann L, Jung HC, Schürer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff MF. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology. 1993 Dec 1;105(6):1689-97.
31. Hansen JD, Vojtech LN, Laing KJ. Sensing disease and danger: a survey of vertebrate PRRs and their origins. Developmental & Comparative Immunology. 2011 Sep 1;35(9):886-97.
32. Maldonado-Contreras AL, McCormick BA. Intestinal epithelial cells and their role in innate mucosal immunity. Cell and Tissue Research. 2011 Jan; 343:5-12.
33. Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006 Sep 1;131(3):862-77.
34. Fukata M, Hernandez Y, Conduah D, Cohen J, Chen A, Breglio K, et al. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflammatory Bowel Diseases. 2009 Jul 1;15(7):997-1006.
35. Shang L, Fukata M, Thirunarayanan N, Martin AP, Arnaboldi P, Maussang D, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008 Aug 1;135(2):529-38.
36. Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004 Jul 1;127(1):224-38.
37. Santaolalla R, Fukata M, Abreu MT. Innate immunity in the small intestine. Current Opinion in Gastroenterology. 2011 Mar;27(2):125.
38. Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007 Apr 1;132(4):1359-74.
39. Pott J, Stockinger S, Torow N, Smoczek A, Lindner C, McInerney G, et al. Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathogens. 2012 May 3;8(5):e1002670.
40. Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2005 May;288(5): G1055-65.
41. Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD, et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host & Microbe. 2012 Aug 16;12(2):139-52.
42. Hurley BP, McCormick BA. Intestinal epithelial defense systems protect against bacterial threats. Current Gastroenterology Reports. 2004 Oct;6(5):355-61.
43. Carneiro LA, Travassos LH, Philpott DJ. Innate immune recognition of microbes through Nod1 and Nod2: implications for disease. Microbes and Infection. 2004 May 1;6(6):609-16.
44. Amendola A, Butera A, Sanchez M, Strober W, Boirivant M. Nod2 deficiency is associated with an increased mucosal immunoregulatory response to commensal microorganisms. Mucosal Immunology. 2014 Mar;7(2):391-404.
45. Creagh Bhlthsc J. Targeting Intestinal Epithelial Cell-Derived Interleukin-23 (p19) To Improve Intestinal Barrier Integrity in Inflammatory Bowel Disease. Thesis 2022.
46. Goto Y. Epithelial cells as a transmitter of signals from commensal bacteria and host immune cells. Frontiers in Immunology. 2019 Aug 28;10:2057.
47. Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology. 1999 Aug 1;117(2):359-67.
48. Yang SK, Eckmann LA, Panja AS, Kagnoff MF. Differential and regulated expression of CXC, CC, and C-chemokines by human colon epithelial cells. Gastroenterology. 1997 Oct 1;113(4):1214-23.
49. Song X, Dai D, He X, Zhu S, Yao Y, Gao H, et al. Growth factor FGF2 cooperates with interleukin-17 to repair intestinal epithelial damage. Immunity. 2015 Sep 15;43(3):488-501.
50. Chiriac MT, Buchen B, Wandersee A, Hundorfean G, Günther C, Bourjau Y, et al. Activation of epithelial signal transducer and activator of transcription 1 by interleukin 28 controls mucosal healing in mice with colitis and is increased in mucosa of patients with inflammatory bowel disease. Gastroenterology. 2017 Jul 1;153(1):123-38.