Abstract
Glial cells play a critical role in the development and function of the mammalian central nervous system (CNS). Among other roles, these cells provide the myelin sheath needed for the efficient propagation of impulses along nerve fibers, provide trophic support for neuronal cells, and remove toxins and excess neurotransmitters from the interstitial space. Transplantation of glial cells or glial progenitors into the diseased or injured CNS can provide therapeutic benefits. However, generation of therapeutically useful quantities of glia, in particular oligodendrocytes, is technically challenging. Furthermore, generation of glial precursors from sources such as embryonic stem (ES) cells and induced pluripotent stem (iPS) cells poses potential safety risks due to the tumorigenic potential of undifferentiated cells. Here we report a method that enables the efficient generation and expansion of glial precursors from tissue-restricted neural stem cells (NSC). NSC-derived glial precursors can be expanded extensively in culture and retain the capacity to differentiate into oligodendrocytes and astrocytes in vitro and in vivo. Upon transplantation into different animal models of demyelination a substantial proportion of these cells become oligodendrocytes with the capacity to myelinate host axons. These results demonstrate that tissue-restricted human neural stem cells can serve as an efficient source for myelinating oligodendrocytes with therapeutic potential.
Keywords
Experimental autoimmune encephalomyelitis, EAE, Remyelination, Stem cell therapy, Regeneration, Oligodendrocyte progenitors
Introduction
Neurodegenerative diseases have a profound societal impact and pose significant healthcare costs. Diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Multiple Sclerosis (MS) affect millions of individuals in the US at a cost of hundreds of billions of dollars annually. Although in some cases the symptoms can be treated, there is currently no cure for the vast majority of neurodegenerative disorders. This unmet medical need is a primary driver for the development of therapies based on transplantation of neural stem cells that have the potential to replace cells of the central nervous system (CNS) lost to disease or injury [1].
One area of intense interest involves the transplantation of myelin-forming oligodendrocytes to reverse demyelination arising from disease or injury (reviewed in [2]). Acute and chronic disorders such as MS, neuromyelitis optica spectrum disorder, transverse myelitis, and optic neuritis result in progressive demyelination of nerve axons in the brain, spinal cord, or optic nerve. Similarly, spinal cord injury is typically accompanied by progressive demyelination of axons and concomitant neuronal atrophy [3]. The consequences of axonal demyelination include impaired conduction of nerve signals by affected neurons leading to a decrease in sensation and motor function. Furthermore, demyelination is frequently accompanied by axonal degeneration (reviewed in [4]).
The potential therapeutic value of oligodendrocytes has been demonstrated in animal studies by transplantation of oligodendrocyte precursor cells purified directly from neural tissue. One group demonstrated that cells dissected from fetal human forebrain or adult white matter and selected using immunosorting methods could be transplanted into myelin-deficient animals and provide significant myelination of host neurons [5-8]. This strategy was capable of extending the lifespan of hypomyelinated mutant mice [8]. Expanded neural progenitors derived from regions of human fetal forebrain have been shown to have significant capacity to remyelinate rodent spinal cord upon grafting into areas of focal demyelination [9], and can myelinate host axons in a mouse model Pelizaeus-Merzbacher disease, which is characterized by severe hypomyelination [10]. Similarly, recovery of locomotor function after grafting of neural stem cells (NSC) in animal models of spinal cord injury has been demonstrated to be due at least in part to myelinating oligodendrocytes [11].
Human trials using stem cells for remyelination have begun. There have been several trials of mesenchymal stem cells injected intravenously, intrathecally or both in relapsing remitting and in progressive MS. Results suggest that mesenchymal stem cells can slow the progression of disease and might improve neurological function. Larger studies are being planned, which will also focus on the mechanism by which mesenchymal stem cells might be promoting endogenous remyelination. In progressive MS, a phase 1 study using intrathecally injected neural precursor cells reached its primary safety outcome with no severe adverse events in 2 years of follow up. In exploratory analyses, they showed that at high doses, the stem cells slowed brain atrophy by creating a pro-regeneration environment of anti-inflammatory and neuroprotective molecules [12]. Pre-clinical models using induced pluripotent stem cells (iPS) offer the promise of neural progenitor transplantation of an autologous product without the need for immunosuppression. Studies with iPS cells have not yet entered human clinical trials in demyelinating disease.
In order to provide sufficient material for therapeutic purposes, it is necessary to develop and implement methods for efficient and large-scale generation of oligodendrocytes that could successfully engraft the host CNS and myelinate host axons. Here we describe the development of a human fetal neural stem cell line that can be expanded through many passages, while retaining the ability to generate a high proportion of oligodendrocytes upon transplantation into white matter in rodents. We show that these cells act as highly enriched oligodendrocyte progenitor populations and can replace lost host oligodendrocytes and remyelinate dys- or hypo-myelinated areas following transplantation into rodents.
Materials and Methods
Isolation and propagation of precursors
Precursor populations were obtained from tissue isolated post-mortem from aborted human fetuses. Each of the two cell lines described herein was prepared from a single contiguous segment of hindbrain tissue from a different donor. Tissue donations were made with informed consent of the donor following guidelines that were established and monitored by an independent review board and compliant with the guidelines of the National Institutes of Health (NIH) and the Food and Drug Administration (FDA). Independent Review Consulting, Inc. was the Institutional Review Board that provided oversight. Cells were dissociated and cultured in serum-free growth medium (consisting of DMEM/F12 base media with glucose, glutamine, sodium bicarbonate, HEPES, 25 µg/mL insulin, apo-transferrin, progesterone, putrescine, sodium selenite, and 10ng/ml bFGF). Media was completely replaced every other day and bFGF was added fresh daily.
Optimization of insulin concentration in this media for generation of the oligodendrocyte lineage was performed by switching cells from the conditions described above into media with the components described above and containing various concentrations of insulin (from 1.5 μg/mL to 25 μg/mL) and evaluating the proliferative and differentiative capacity of resulting cells for 16 days in culture. Upon establishing that 5 μg/mL provided the ideal conditions, this condition and the effect of including PDGF-AA, was characterized in more detail as described in the Results. Ultimately, a standard media formulation was established that consisted of DMEM/F12 base media with glucose, glutamine, sodium bicarbonate, HEPES, 5 µg/mL insulin, apo-transferrin, progesterone, putrescine, sodium selenite, 10 ng/mL bFGF, and 20 ng/mL PDGF-AA. Under these conditions cells were expanded for up to 6 months without exhibiting changes in growth properties.
Cells used in transplantation experiments had been maintained for a range of 8-12 passages, or approximately 2-3 months in culture. Some transplantation experiments used NSI-777 cells that had been infected with a lentiviral vector driving GFP expression under control of the ubiquitin promoter to facilitate tracking of the grafted cells.
Animals
All animal and experimental protocols were approved and performed according to the Johns Hopkins Animal Care and Use Committee guidelines (# RA15M189). The study was carried out in compliance with the ARRIVE guidelines. Adult male athymic rats (Charles River) were used for all rat studies and were approximately 220-280 g at time of study initiation. Animals were housed in standard cages, with 12/12h light cycle and had free access to food and water.
Spinal contusion: Rats were anesthetized with isoflurane (2% maintenance) and mounted in a stereotaxic frame with spinal clamps (Stoelting). A laminectomy was performed to expose the lumbar spinal cord [13], and using a New York Impactor, a 10 g rod (2 mm tip diameter) was dropped 6.25 mm on to the dorsal surface of the spinal cord, targeting the left lateral column of the L2/3 spinal segment. This resulted in a mild unilateral contusion injury to the lateral white matter tracts. After hemostasis was confirmed, all incisions were closed using standard techniques. Cell transplantation was performed 6-8 days after injury. The original laminectomy was re-opened, and cells were injected using standard techniques targeting the lateral column in a rostral/caudal line, centered at the lesion site. Six injections per animal were placed approximately 1 mm apart. Cell density was 20,000 cells/µl and each injection was 1 µl in volume. Animals survived for 3 months after cell grafting and were perfusion-fixed upon sacrifice.
Focal spinal cord chemical demyelination: Under isoflurane anesthesia and while spinal-clamped in a stereotaxic frame, a laminectomy was performed on the T11 vertebra, and lysolecithin (1%, 1 µl per injection) was injected into the dorsal column to initiate demyelination. Six injections were made in the rostral/caudal direction approximately 2 mm apart targeting the dorsal white matter of the T9/10 spinal segments. Transplantation of undifferentiated NSI-777 was performed 3-4 days after injury and involved placement of 6 cell injections/animal in the same locations as lysolecithin injections. Cell density was 20,000 cells/µl and each injection was 1 µl. Animals survived for 3 months after cell grafting and were perfusion-fixed upon sacrifice.
Shiverer mice: Shiverer mice of both sexes were transplanted as neonates (P1-P2). Cells were injected bilaterally into each brain hemisphere, with two injections made per hemisphere. Cell density was 100,000 cells/μl and the volume of each injection was 1 μl. Tissue was processed for analysis 120-135 days after cell grafting.
Athymic rat: Under isoflurane anesthesia, animals were fixed in a stereotaxic frame to immobilize the head. Burr holes were made with a dental drill and cells were injected into the corpus callosum and/or striatum using a microinjector and a 33G steel needle attached to a 100 μL glass syringe. A total of either 1 million, 3 million, or 10.8 million cells were injected to each animal. Incisions were closed by steel clips. Tissue was processed for analysis 6 months after cell grafting.
Immunohistochemistry
All animals were perfusion-fixed by standard protocols using heparinized-saline followed by 4% paraformaldehyde. Tissue was post-fixed overnight in 4% paraformaldehyde at 4°C and then stored in 30% sucrose-PBS. Sections (30 µm thick) were cut by cryostat and stained using standard methods. Antibodies to HuNu (human-specific nuclear antigen; 1:400), myelin basic protein, NeuN (a neuronal nuclear antigen identified as Fox-3; 1:500), and Olig2 (a bHLH transcription factor associated with oligodendrocyte lineage specification; 1:500) were purchased from Millipore. Antibodies to Ki67 (a nuclear protein associated with proliferating cells) were purchased from Abcam. Antibody to human GFAP (astrocyte marker) was purchased from Origene. Imaging was performed with a Leica DMLB fluorescent microscope. Confocal imaging was performed with an Olympus FV1000 or Zeiss 710 confocal microscope. Image editing was done with Photoshop CS5. Quantification was done using Image-Pro Plus 6.2. Migration quantification was performed on 2 sagittal sections per animal ensuring sections were taken from a similar medial-lateral level.
Electron microscopy
Brain tissue was fixed in 3% glutaraldehyde and 4% formaldehyde in 0.1 M Sorensen’s buffer pH 7.2. Post-fixation was done with 2% osmium tetroxide. The tissue was then dehydrated in graded ethanol. Embed 812 (EMS) was used as the embedding media. 1 μm sections were stained with 1% toluidine blue. For electron microscopy, thin sections (70-80 nm) were cut on a Reichert Jung Ultracut E microtome and placed on formvar coated copper 100 mesh grids. The sections were stained with uranyl acetate and followed by lead citrate. All imaging was performed on a Zeiss Libra 120 with a Veleta (Olympus) camera.
Statistics
Cell migration data described in Figure 6 were derived from analyzing 2 brain sections per animal, with 3 animals per group; a mean value was calculated for each animal and then a mean for the group was generated. Data are presented as mean ± SEM.
Results
Generation of glial precursors
As previously reported, we have successfully used a modified culture medium based on the N2 formulation originally described by Bottenstein [14] to expand neural stem cells derived from fetal human spinal cord [15]. One observation made in the course of these studies is that the expanded neural stem cell population can efficiently generate neurons and astrocytes, but not oligodendrocytes, upon differentiation in vitro or after transplantation in vivo [16]. There is a great deal of literature supporting the role of platelet-derived growth factor (PDGF) and insulin or insulin-like growth factors (IGFs) in regulating the generation of oligodendrocytes and oligodendrocyte precursors. We therefore sought to determine if modifying these components of the culture could have an impact on the differentiation potential of fetal human neural stem cells (hNSC).
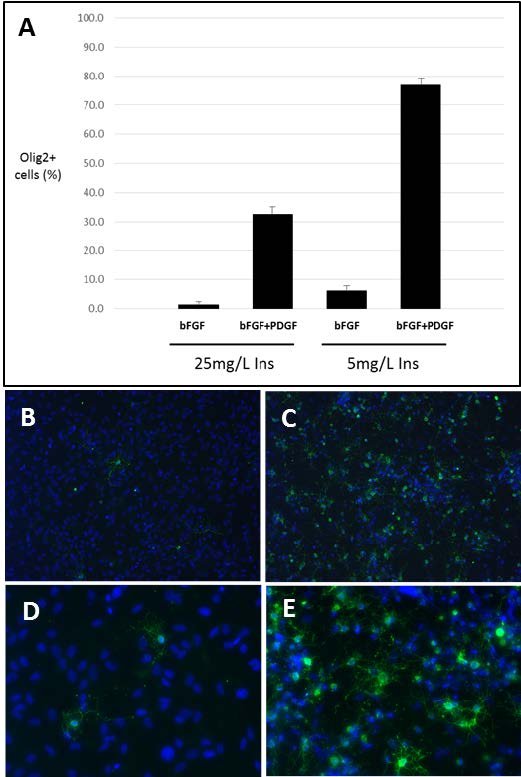
We exposed neural stem cells derived from human fetal hindbrain tissue from a single donor (12.5 weeks gestational age) to N2 media containing low (5 μg/mL) or high (25 μg/mL) concentrations of insulin in the presence of basic fibroblast growth factor (bFGF) alone or with the addition of PDGF. After approximately 6 weeks in culture with multiple passages the cells were evaluated for expression of the oligodendrocyte lineage marker Olig2 (Figure 1A). Only 1.4% of cells grown in the presence of bFGF in media containing high insulin expressed Olig2. Reduction of insulin concentration or addition of PDGF to the culture each had the effect of increasing Olig2 expression in expanded hNSC (to 6.1% and 32.6%, respectively). Combining the reduction in insulin and addition of PDGF had a synergistic effect, resulting in Olig2 expression in 76.9% of cells in the expanded population (Figure 1A). Consistent with this observation, when expanded cells were induced to differentiate by withdrawal of mitogen, a much higher proportion of cells grown in low insulin in the combined presence of bFGF and PDGF were found to be immunopositive for oligodendrocyte markers O4 and GalC compared to those expanded in high insulin and bFGF (37.6% vs 0.6%). Differentiated cells were costained for both markers to enable detection of cells at early and later stages of oligodendrocyte differentiation. Cells expanded in low insulin in the presence of bFGF and PDGF maintained the capacity to generate GalC-immunopositive oligodendrocytes upon differentiation at 30-40% of total cells through at least passage 13, approximately 3-4 months in culture (data not shown). These cells demonstrated stereotypical arborized oligodendrocyte morphology (Figures 1B-1E). Taken together, these data demonstrate that insulin concentration and presence of PDGF can impact the ability of hNSC to generate cells of the oligodendrocyte lineage upon differentiation.
Figure 1. Human hindbrain NSC expanded under differing conditions demonstrate distinct potential for differentiating into oligodendrocytes. (A) Cells expanded in the presence of or absence of PDGF and in the presence of 5 µg/mL or 25 µg/mL insulin were immunostained for Olig2 before differentiation. The percentage of total cells that were Olig2 immunopositive was determined by counting at least 6 fields per condition and is shown with error bars indicating standard deviation. (B-E) Cells expanded under these conditions were allowed to differentiate for 6 days and stained for antibodies to GalC and O4 (green) and Hoechst to label nuclei (blue). Cells expanded in 25 µg/mL insulin in the absence of PDGF (B and D) or in 5 µg/mL insulin in the presence of PDGF (C and E) demonstrate typical stellate morphologies upon differentiation (B and C, 20x objective; D and E, 40x objective).
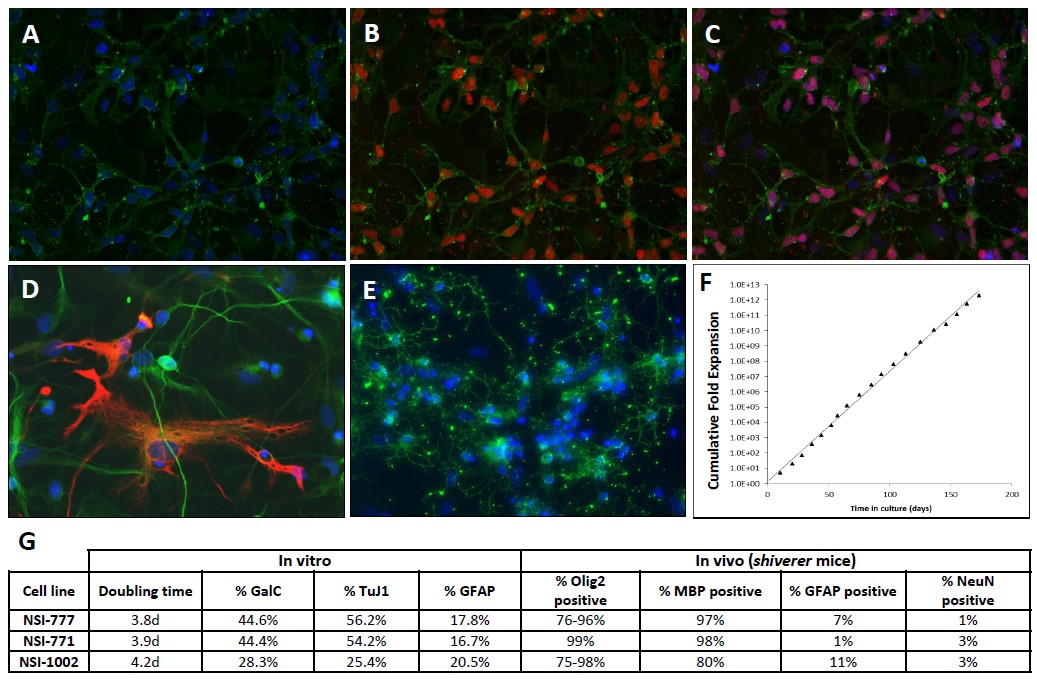
We then used these conditions to generate a cell line, called NSI-777, from fetal hindbrain tissue from a different donor (12 weeks gestational age). Cells were expanded for three consecutive passages in medium containing 25 μg/mL insulin using only bFGF as the mitogen and were expanded from this point forward using low insulin media containing bFGF and PDGF as mitogens. NSI-777 expanded under these conditions were immunopositive for oligodendrocyte precursor lineage markers A2B5 and Olig2 (Figures 2A-2C), and upon differentiation generated neurons, astrocytes, and oligodendrocytes as confirmed by immunostaining for cell type specific markers neuron-specific β-tubulin, GFAP, and GalC, respectively (Figures 2D and 2E). NSI-777 showed consistent growth kinetics during a six-month period in culture (Figure 2F), and retained the capacity to differentiate into neurons, astrocytes, and oligodendrocytes in reproducible proportions (data not shown).
In order to demonstrate the reproducibility of this method, we used these conditions to generate and characterize three distinct cell lines from different donor tissues. Upon establishment, the proliferative and differentiative properties of each line was evaluated in vitro and following grafting into shiverer mice (Figure 2G). Cells were evaluated for rate of proliferation in culture and for their ability to generate neuronal, astrocytic, and oligodendrocytic cells following differentiation in vitro or grafting. Each line showed the capacity to generate all lineages in vitro at high proportions but were found to almost exclusively generate cells of the oligodendrocyte lineage upon transplantation. Though the relative proportions of each lineage differed somewhat from one cell line to another, they were comparable and demonstrate that this method is sufficiently robust that it can be applied to multiple donor tissues with similar effect.
Figure 2. In vitro characterization of NSI-777 cells. (A-C) Undifferentiated NSI-777 expanded in vitro in the presence of bFGF and PDGF are immunopositive for markers of the oligodendrocyte lineage. Cells were stained for the ganglioside epitope A2B5 (A-C, green) and the bHLH transcription factor Olig2 (B and C, red). (D, E) Following differentiation cells were stained for TuJ1 (D, green), GFAP (D, red), and GalC (E, green) to identify neuronal, astrocytic, and oligodendrocytic lineages, respectively. Nuclei in A-E are stained using Hoechst dye (blue). (F) Growth kinetics of NSI-777 were monitored for 6 months in culture by recording expansion at each passage to determine cumulative fold expansion over time in culture. (G) Comparison of the proliferative and differentiative properties of three distinct cell lines derived from three different donor hindbrain tissues using the optimized conditions.
Transplantation of glial precursors into animal models of focal demyelinating disease
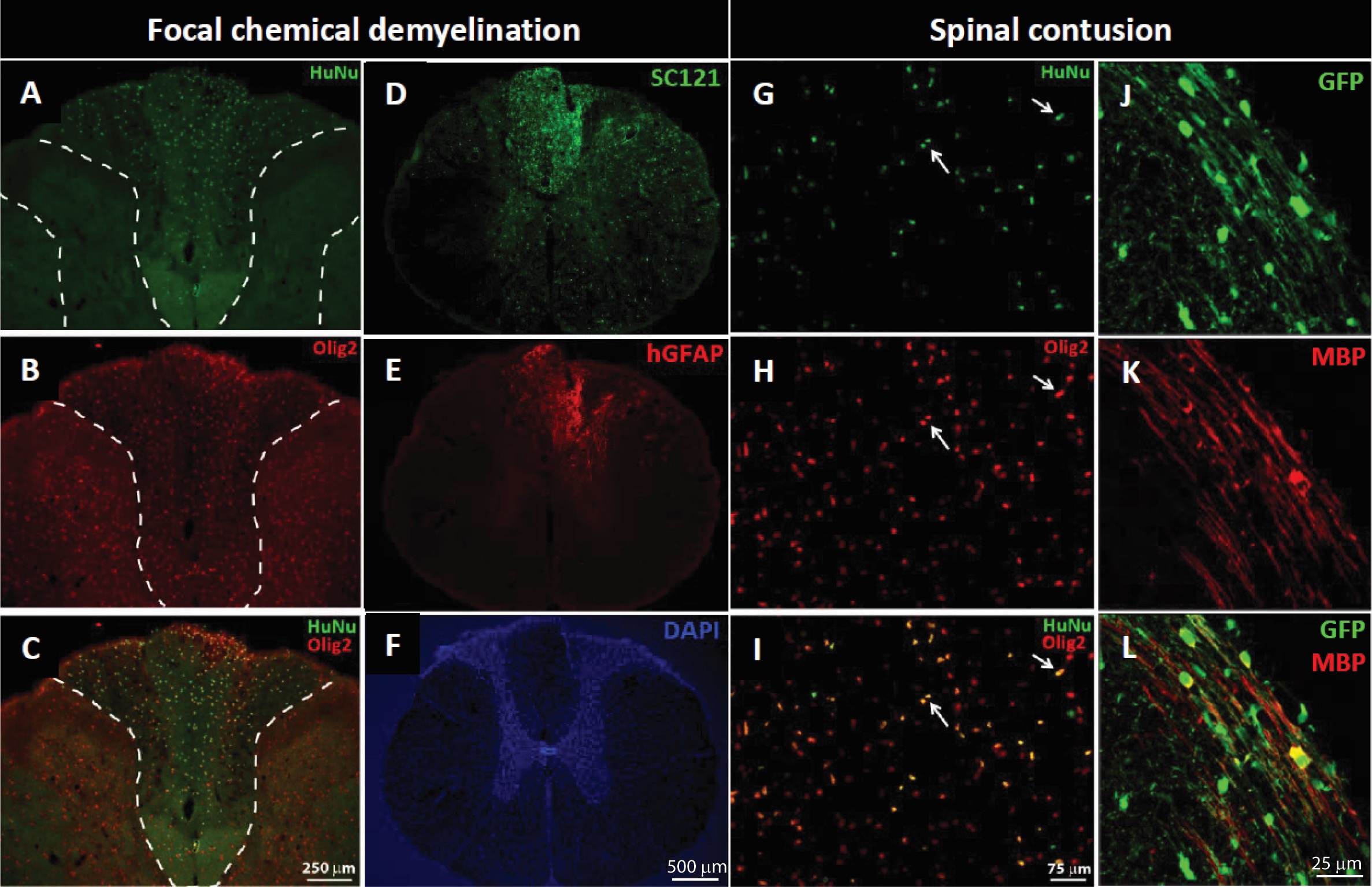
In order to demonstrate the capacity of NSI-777 to produce myelin we transplanted expanded cells into animal models for demyelinating diseases. In the first model, NSI-777 was grafted into adult athymic rats that had been treated by injection of lysolecithin into the spinal cord to induce transient focal demyelination. Lysolecithinin was injected into the dorsal column, targeting the dorsal white matter of the T9/10 spinal segments. After 3-4 days NSI-777 cells were injected to the same locations as the lysolecithin and after an additional 3 months, cell grafts were evaluated by immunostaining (Figure 3). Grafted cells showed evidence of survival and migration away from the graft site, as demonstrated by staining with the human-specific antibodies HuNu and SC121 (Figures 3A, 3C, and 3D).
NSI-777 cells were routinely found within the dorsal column and were typically Olig2-immunopositive (Figures 3B and 3C). While graft cores were evident at the injection sites (not shown), large numbers of cells were found away from the injection site and scattered throughout the lateral and ventral white matter, at least 1 mm away from the injection site. Cells remaining in the graft core appeared more progenitor-like, with basic round cell morphology, and were often nestin-immunopositive (not shown). In addition to Olig2-immunopositive cells, NSI-777 generated a small proportion of neurons and astrocytes, as demonstrated by the presence of cells costaining for NeuN/SC121 (not shown) or human-specific GFAP antibody (Figures 3E and 3F).
NSI-777 was also grafted into the mechanically injured rat spinal cord 7 days after injury. Spinal contusion was generated via weight-drop injury targeting the left lateral column of L2/3 spinal segments and resulted in a mild unilateral contusion injury to the lateral white matter tracts. Cell transplantation was performed 6-8 days after injury, with NSI-777 grafts targeting the lateral column in a rostral/caudal line centered at the lesion site. Cell grafts evaluated 3 months after transplantation were readily identified in all animals by immunohistochemistry and were consistently found in the lateral column ipsilateral to injury. Graft cores were evident at the injection sites, and a considerable population of cells had migrated away from the injection site and were scattered throughout the ipsilateral dorsal, lateral and ventral white matter. As in the chemical demyelination model NSI-777 produced a small population of astrocytes positive for hGFAP-immunoreactivity, typically only found within the graft core, and a small population of NeuN-immunopositive cells (not shown). The mitotic rate as evidenced by Ki67 expression was only 3% of the total HuNu-immunoreactive population (not shown). The majority of NSI-777 cells were Olig2-immunopositive (Figures 3G-3I). In this case, the engrafted cells displayed a clear propensity towards the oligodendrocyte lineage, expressing Olig2. Grafted cells in the lateral column expressed myelin basic protein (MBP), as demonstrated by co-immunostaining for GFP and MBP (Figures 3J-3L).
Figure 3. Survival and integration of NSI-777 in animal models of focal demyelination. Demyelination was induced by lysolecithin injection (A-F) or contusion injury (G-L). NSI-777 cells were injected into the lesion site and grafts were evaluated after 3 months. Human cells were identified by staining for HuNu (A, C, G, and I), SC121 (D), or GFP (J and L). Cells of the oligodendrocyte lineage were identified by staining with Olig2 (B, C, H, and I) or MBP (K and L) and cells of the astrocyte lineage were identified by staining with GFAP (E). Panels A-C show the dorsal column, and both left and right dorsal horns; HuNu-immunopositive nuclei are found throughout the dorsal column (A) with most double labeled for Olig2 (B, C). Human GFAP-immunoreactive cells were also identified (D-F). Similar expression of Olig2 was noted in grafted cells following transplantation into a spinal contusion lesion (G-I; arrows). Utilizing a GFP-expressing version of NSI-777 enabled identification of graft-derived MBP (J-L). White dashed lines (A) outline the left and right dorsal horns; similar lines in B and C enclose the dorsal column.
Transplantation of glial precursors into myelin-deficient mice
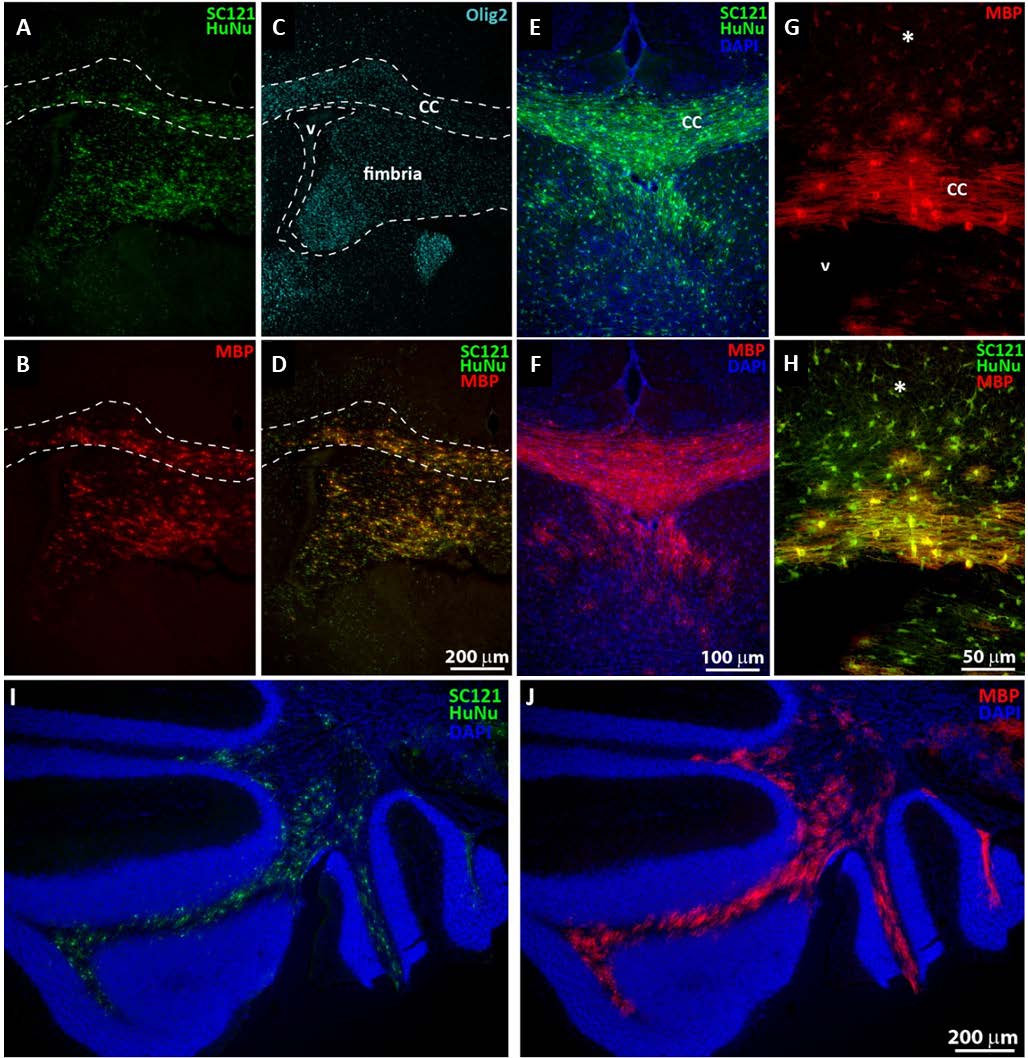
The ability of NSI-777 to generate myelinating oligodendrocytes was evaluated by grafting cells bilaterally into the cortex of neonatal shiverer mice and evaluating properties of the grafted cells after 4-4.5 months. Shiverer mice carry a mutation in the MBP gene, resulting in hypomyelination. Because of the mutation, any MBP detectable by immunohistochemistry must be produced by the grafted cells. Cells were evaluated by staining for human-specific markers (SC121, HuNu) and phenotypic markers (MBP, Olig2, NeuN, GFAP). Cell migration from the graft site was extensive and grafted cells were often found concentrated in or near the corpus callosa (Figures 4A-4D) and fimbria (Figure 4C). Corpus callosa were frequently densely packed with human-derived cells that strongly expressed MBP, appearing to be actively myelinating host axons located in the vicinity (Figures 4E and 4F). Human cells in areas adjacent to the corpus callosa (asterisks in Figures 4G and 4H) were usually weakly MBP-immunoreactive and were less complex in morphology compared to those within the corpus callosa. Migration patterns to the cerebellum were seen as well (Figures 4I and 4J).
Figure 4. Integration of NSI-777 in myelin-deficient hosts. NSI-777 was grafted into neonatal shiverer mice and evaluated after ~4 months for survival and differentiation. Grafted cells were identified by staining for HuNu and SC121 (A, D, E, H, and I) and oligodendrocyte lineage was confirmed by staining for MBP (B, D, F, G, H, and J) or Olig2 (C). Corpus callosum (‘CC’ in C, E, and G) is delineated by dashed lines in A-D. Fimbria is delineated with dashed lines in C and lateral ventricle is indicated in C and G with ‘V’. Asterisk in G and H indicates cells outside the boundary of the corpus callosum. Panel A-D illustrate the corpus callosum and fimbria areas, while E-H show a higher magnification of the corpus callosum. Panel I-J are captured from the cerebellum.
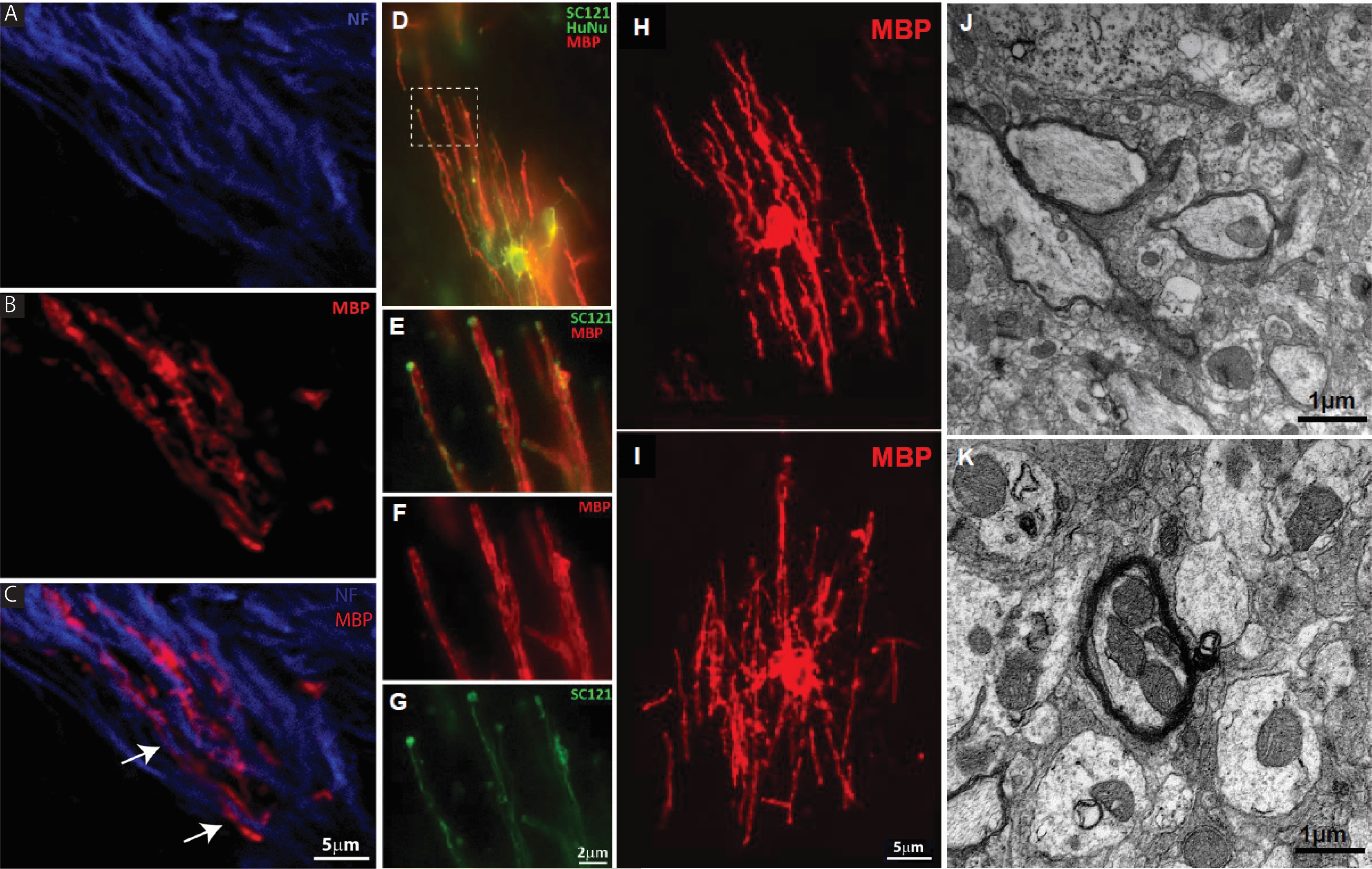
Close examination of cells within corpus callosum in shiverer mice reveals that grafted cells appear to be actively myelinating host axons (Figure 5). Host neurofilament-immunoreactive axons appear encapsulated in graft-derived MBP (Figures 5A-5C, arrows in C). High power confocal imaging shows graft-derived MBP-immunoreactive structures form tube-like processes in association with host neurons (Figures 5D-5G, with inset in Figure 5D shown in greater detail in Figures 5E-5G). NSI-777 found in axon tracts of the corpus callosum were strongly MBP-immunoreactive and developed thick parallel processes (Figures 5H and 5I).
Electron microscopy of brains from shiverer mice grafted with NSI-777 demonstrated the presence of myelin sheath in the area of NSI-777 grafts (Figures 5J and 5K). Quantitation of myelination was estimated using g-ratios [17]. G-ratios were calculated by dividing the inner diameter of the axon by the outer diameter of the axon + myelin. Ratios of 0.6 – 0.85 are considered normal, depending on the particular tract, where the lower numbers represent greater myelination. Bare axons with no myelin are closer to 1.0. For this study, we identified axons that showed myelination and measured g-ratios of a variety of myelinated fibers to evaluate the spectrum observed, revealing g-ratios averaging 0.8355 (n=24 myelinated axons from 12 sections).
Figure 5. NSI-777 ensheath host axons. NSI-777 was grafted into neonatal shiverer mice and evaluated after ~4 months for survival and differentiation. (A-C) Grafted cells developed MBP-positive processes (B and C, red) that appear to be closely associated with host axons (arrows in C). Host axons were identified with antibody to neurofilament (A and C, blue). (D-I) Grafted cells identified by staining for SC121 and HuNu (D, E, and G, green) form tube-like MBP-positive processes and characteristic oligodendrocyte morphologies (D-F, H, I, red) when grafted into white matter. The dashed box in (D) is enlarged in panels E-G to highlight the apparent association of graft-derived MBP and host neurons (J and K). Examples of graft-derived remyelination was found by EM to ensheath host axons (arrows) among demyelinated axons (arrow heads).
Migration of NSI-777 after transplantation
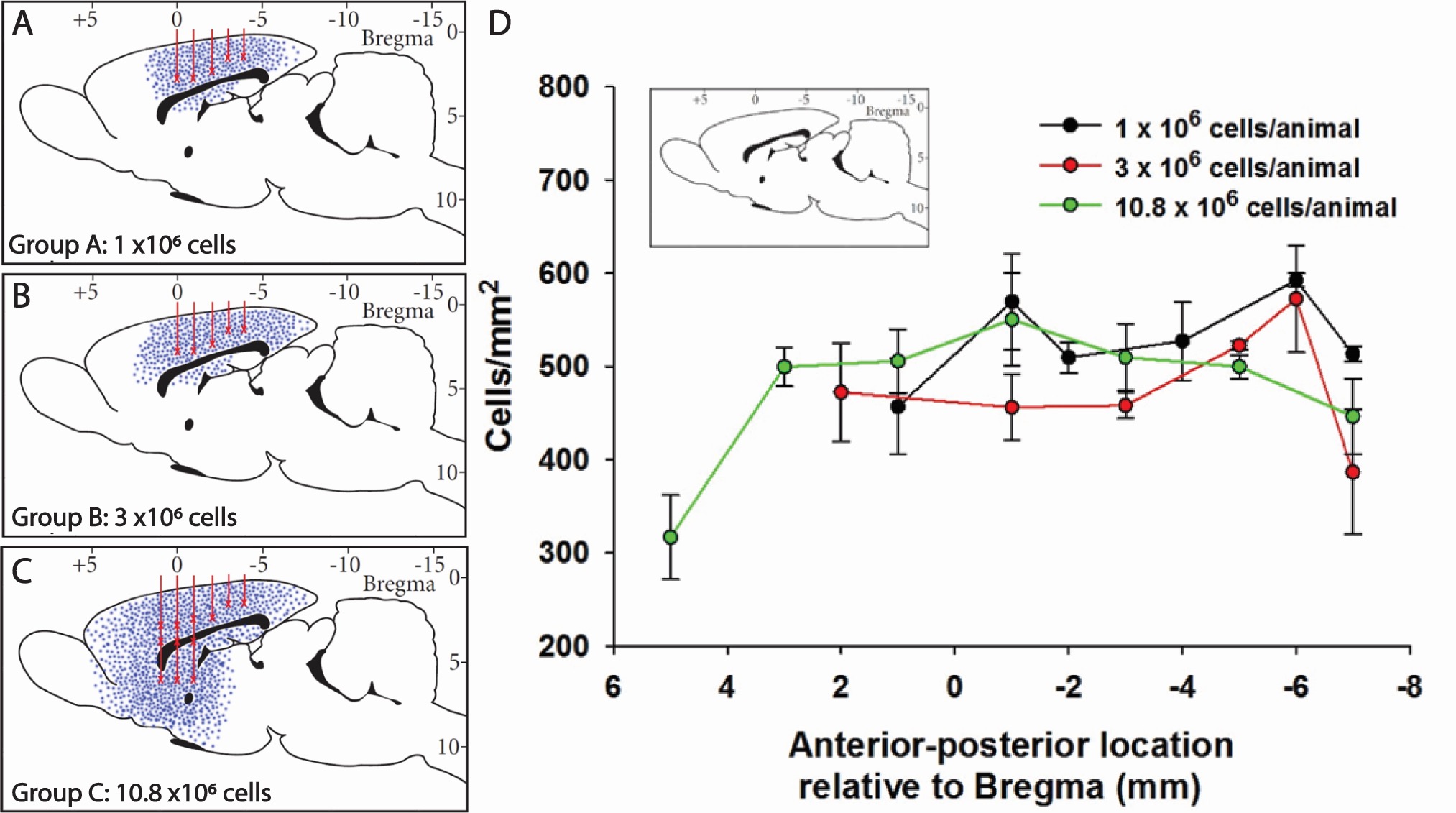
In order to assess the long-term survival and migratory properties of NSI-777 we grafted cells into corpus callosum alone or corpus callosum and striatum of athymic nude rats and evaluated the presence of human cells in the grafted animals after 6 months (Figure 6). Animals received from 1 million to 10.8 million cells and were observed to remain healthy throughout the study, with no apparent effects of the widespread grafting. At the conclusion of the study animals had persisting grafts as identified by diffuse HuNu-immunoreactivity, and no evidence of tissue disruption was noted.
Cell distribution was assessed by two methods: i) linear migration in the rostral-caudal direction, and ii) cell density (per area). Linear migration measurements indicated only minor differences between groups that received differing numbers of grafts, however grafting of cells to the striatum in addition to the corpus callosum resulted in widespread migration throughout the caudate putamen.
Figures 6A-6C show a representative sagittal projection of graft location for animals receiving a total of 1 million, 3 million, or 10.8 million cells (Figures 6A, 6B, and 6C, respectively). Blue dots indicate relative location of grafted cells in each group, red lines signify injection tracts, and ‘X’ designates injection sites. Figure 6D shows an assessment of graft cell density relative to rostral-caudal direction, with density of grafted cells calculated within each identified contiguous graft. It is striking to note that the cells delivered at the highest dose extended approximately 1.2 cm in the anterior-posterior axis within the host brain (Figure 6D).
Figure 6. Migratory potential of NSI-777 in the host brain. NSI-777 were grafted into corpus callosum alone or corpus callosum and striatum of athymic nude rats and evaluated after 6 months. Animals received 1 million (A), 3 million (B), or 10.8 million cells (C) into corpus callosum alone (A and B) or corpus callosum and striatum (C). (A-C) Representative sagittal projection of graft location. Blue dots indicate relative location of grafted cells in each group, red lines signify injection tracts, and ‘X’ designates injection sites. An assessment of graft cell density relative to the rostral-caudal direction is shown in D, with density of grafted cells calculated within each identified contiguous graft. (D) Summary of cell density and location in each group. The x-axis represents stereotaxic coordinates (anterior-posterior) with zero being Bregma. Data are presented as mean ± SEM.
Discussion
The generation of oligodendrocytes from multipotent precursors is a complex process involving a host of different factors in instructive or selective capacities. Early experiments that pursued the mechanisms of oligodendrocyte generation used progenitors such as O-2A cells that are not multipotent but instead are committed exclusively to the generation of glial cells [18]. These studies were instrumental in defining the role of specific factors in the expansion, differentiation, and survival of oligodendrocytes and their precursors. Such factors include bFGF, PDGF, Insulin-like growth factor 1 (IGF1), ciliary neurotrophic factor (CNTF), bone morphogenetic protein 2 (BMP2), neurotrophin 3 (NT3), retinoic acid, and thyroid hormone (T3) [19-24].
Additional studies have evaluated the roles of such factors in generating oligodendrocytes and oligodendrocyte precursors from multipotent NSC or pluripotent embryonic stem (ES) cells or induced pluripotent stem (iPS) cells. Researchers have had success developing methods for generating oligodendrocyte precursor cells from embryonic stem cells [25-32] and induced pluripotent stem cell populations [33-35] using growth factor cocktails containing factors known to be involved in CNS patterning, including bFGF, PDGF, IGF1, SHH, CNTF, RA, and T3. Transplantation of these expanded precursor cells shows successful engraftment and survival of oligodendrocyte populations, and in some cases myelin formation and functional improvement in animal models of injury [25,26,30,31,33,36-38].
Despite the role played by numerous factors in oligodendrocyte generation, it is clear that three factors are key: PDGF, bFGF, and insulin. PDGF and bFGF are well-known mitogens for O-2A cells and other oligodendrocyte precursors and can cooperate to promote O-2A proliferation [39-41]. bFGF may also have a role in induction of the oligodendrocyte lineage [42]. PDGF receptors are present on the surface of oligodendrocyte progenitors and can be used as a method for purifying such cells from cultured cells or dissociated tissues [5,6]. A principal role of insulin signaling in oligodendrocyte development is to promote survival of oligodendrocyte progenitors [43] and/or augment differentiation [44,45]. However, the role of insulin is complicated by the fact that it is a member of the family of peptide growth factors which includes IGF-1 and IGF-2. This family of factors act through related receptors to activate similar intracellular pathways, and IGF-1 has been shown to have a complex role in oligodendrocyte development including lineage commitment, proliferation and survival of oligodendrocyte precursors, and differentiation and survival of oligodendrocytes [46-50], and in some cases insulin has the same effect [51].
Our study demonstrates that the presence of PDGF is critical in order for human neural stem cells to maintain the capacity to generate Olig2+ oligodendrocyte progenitors. Furthermore, we show that the presence of high concentrations of insulin can suppress the generation of Olig2+ oligodendrocyte progenitors from human neural stem cells, even in the presence of PDGF. The expression of Olig2 in a high proportion of proliferating cells is reflective of their capacity to generate oligodendrocytes, as shown by the differentiation of these cells into GalC and MBP expressing oligodendrocytes in high proportions in vitro and after grafting.
One key observation relates to the differentiation potential of human neural stem cells expanded in low insulin conditions in the presence of bFGF and PDGF. When the cells were induced to differentiate in vitro by withdrawal of growth factors we observed the generation of neurons, astrocytes, and oligodendrocytes in proportions of approximately 1 : 0.2 : 1. Upon transplantation into myelin deficient mice or into demyelinated or naïve rats we observed that the differentiated population almost exclusively comprised cells of the oligodendrocyte lineage. This suggests that the environment in which the cells are placed may have a significant impact on their differentiative potential. Numerous cues from the local environment may influence this process, including p27Kip1, fibroblast growth factor-2, anosmin-1, retinoic acid, sonic hedgehog and Notch1 signaling, among others [52-54] – reviewed here [55]. Consistent with this is our observation in this study that grafted cells residing in the corpus callosum take on a more mature differentiated oligodendrocyte morphology than those taking up residence outside of white matter tracts.
Regardless of the placement of NSI-777, only a small proportion of cells continued to express Ki67 three months after grafting. Only 2-7% of cells grafted into myelin-deficient, demyelinated, or naïve animals were Ki67 positive upon analysis after 3-6 months. Ki67-positive cells were dispersed and there were no signs of hyperplastic growth or pathology, even after grafting of up to 10.8 million cells into naïve rat brains suggesting that this approach is generally safe and well tolerated by rodents.
Based on immunologic and morphological analysis NSI-777-derived oligodendrocytes appear to be capable of producing myelin that effectively ensheaths host neurons. High power confocal imaging revealed a tight association of MBP-immunoreactive structures and host axons, and human-derived myelin appeared to be ensheathing host neurofilament-immunoreactive axons (Figure 5). Indeed, the MBP-immunoreactive structures frequently displayed a tube-like appearance and numerous examples were identified showing a thin human oligodendrocyte extension/process (SC121-positive) contacting a thick MBP-immunoreactive axon-like fiber. Electron microscopy of grafted cells in myelin-deficient shiverer mice enabled us to determine that g-ratios of myelinated fibers averaged 0.8355, within the normal range for myelinated axons. Taken together, these data support the notion that the human neural stem cell line NSI-777 can be expanded extensively while maintaining the capacity to generate myelinating oligodendrocytes after transplantation.
Stem cell transplantation promises to be a useful therapeutic tool for regeneration of damaged tissue in demyelinating diseases. In peripherally demyelinating syndromes where Schwann cells have the potential to regenerate and remyelinate, focus has been on suppressing the underlying autoimmune disease. In the central nervous system, there is the compounded effect of the underlying immune disease in the context of limited healing from myelin producing cells. Exogenous stem cell transplantation can provide the necessary substrate to generate myelin producing cells and also provide a pro-regenerative environment that may be helpful in the healing process.
Disclosures
ML received research support from National Institutes of Health, Maryland Technology Development Corporation, Sanofi, Genzyme, Alexion, Alnylam, Shire, Acorda and Apopharma. He also received personal compensation for consultation with Alexion, Acorda, and Genzyme and he serves on the scientific advisory boards for Alexion, Acorda and Quest Diagnostics. For this study, NeuralStem supported this work through Johns Hopkins University.
References
2. Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, et al. Remyelination after spinal cord injury: is it a target for repair?. Progress in Neurobiology. 2014 Jun 1;117:54-72.
3. Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. Journal of Comparative Neurology. 2005 Jun 13;486(4):373-83.
4. Bjartmar C, Yin X, Trapp BD. Axonal pathology in myelin disorders. Journal of Neurocytology. 1999 Apr;28:383-95.
5. Sim FJ, McClain CR, Schanz SJ, Protack TL, Windrem MS, Goldman SA. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nature Biotechnology. 2011 Oct;29(10):934-41.
6. Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, et al. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nature Medicine. 2004 Jan 1;10(1):93-7.
7. Windrem MS, Roy NS, Wang J, Nunes M, Benraiss A, Goodman R, et al. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. Journal of Neuroscience Research. 2002 Sep 15;69(6):966-75.
8. Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008 Jun 5;2(6):553-65.
9. Buchet D, Garcia C, Deboux C, Nait-Oumesmar B, Baron-Van Evercooren A. Human neural progenitors from different foetal forebrain regions remyelinate the adult mouse spinal cord. Brain. 2011 Apr 1;134(4):1168-83.
10. Marteyn A, Sarrazin N, Yan J, Bachelin C, Deboux C, Santin MD, et al. Modulation of the innate immune response by human neural precursors prevails over oligodendrocyte progenitor remyelination to rescue a severe model of Pelizaeus-Merzbacher disease. Stem Cells. 2016 Apr;34(4):984-96.
11. Yasuda A, Tsuji O, Shibata S, Nori S, Takano M, Kobayashi Y, et al. Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord. Stem Cells. 2011 Dec;29(12):1983-94.
12. Genchi A, Brambilla E, Sangalli F, Radaelli M, Bacigaluppi M, Furlan R, et al. Neural stem cell transplantation in patients with progressive multiple sclerosis: an open-label, phase 1 study. Nature Medicine. 2023 Jan 9:75-85.
13. Hefferan MP, Galik J, Kakinohana O, Sekerkova G, Santucci C, Marsala S, et al. Human neural stem cell replacement therapy for amyotrophic lateral sclerosis by spinal transplantation. PLoS One, 2012. 7(8): p. e42614.
14. Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proceedings of the National Academy of Sciences. 1979 Jan;76(1):514-7.
15. Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes & Development. 1996 Dec 15;10(24):3129-40.
16. Yan J, Xu L, Welsh AM, Hatfield G, Hazel T, Johe K, et al. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Medicine. 2007 Feb;4(2):e39.
17. Guy JO, Ellis EA, Hope GM, Emerson SC. Maintenance of myelinated fibre g ratio in acute experimental allergic encephalomyelitis. Brain. 1991 Feb 1;114(1):281-94.
18. Raff MC, Abney ER, Fok-Seang J. Reconstitution of a developmental clock in vitro: a critical role for astrocytes in the timing of oligodendrocyte differentiation. Cell. 1985 Aug 1;42(1):61-9.
19. Bansal R, Miyake H, Nakamura I, Eto H, Gotoh A, Fujisawa M, et al. Fibroblast growth factors and their receptors in oligodendrocyte development: implications for demyelination and remyelination. Developmental Neuroscience. 2002;24(1):35-46.
20. Barres A, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994 May;120(5):1097-108.
21. Barres BA, Raff MC, Gaese F, Bartke I, Dechant G, Barde YA. A crucial role for neurotrophin-3 in oligodendrocyte development. Nature. 1994 Jan 27;367(6461):371-5.
22. Orentas DM, Miller RH. Regulation of oligodendrocyte development. Molecular Neurobiology. 1998 Dec;18:247-59.
23. Raff MC, Durand B, Gao FB. Cell number control and timing in animal development: the oligodendrocyte cell lineage. International Journal of Developmental Biology. 2002 May 1;42(3):263-7.
24. Rodríguez‐Peña A. Oligodendrocyte development and thyroid hormone. Journal of Neurobiology. 1999 Sep 15;40(4):497-512.
25. All AH, Bazley FA, Gupta S, Pashai N, Hu C, Pourmorteza A, et al. Human embryonic stem cell-derived oligodendrocyte progenitors aid in functional recovery of sensory pathways following contusive spinal cord injury. PLoS One, 2012. 7(10): p. e47645.
26. Faulkner J, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitors for the treatment of spinal cord injury. Transplant Immunology. 2005 Dec 1;15(2):131-42.
27. Glaser T, Perez‐Bouza A, Klein K, Brüstle O. Generation of purified oligodendrocyte progenitors from embryonic stem cells. The FASEB Journal. 2005 Jan;19(1):112-4.
28. Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nature Protocols. 2009 Nov;4(11):1614-22.
29. Kang SM, Cho MS, Seo H, Yoon CJ, Oh SK, Choi YM, et al. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells. 2007 Feb;25(2):419-24.
30. Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. Journal of Neuroscience. 2005 May 11;25(19):4694-705.
31. Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010 Jan;28(1):152-63.
32. Sundberg M, Hyysalo A, Skottman H, Shin S, Vemuri M, Suuronen R, et al. A xeno-free culturing protocol for pluripotent stem cell-derived oligodendrocyte precursor cell production. Regenerative Medicine. 2011 Jul;6(4):449-60.
33. Douvaras P, Wang J, Zimmer M, Hanchuk S, O’Bara MA, Sadiq S, et al. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Reports. 2014 Aug 12;3(2):250-9.
34. Khazaei M, Ahuja CS, Fehlings MG. Generation of oligodendrogenic spinal neural progenitor cells from human induced pluripotent stem cells. Current Protocols in Stem Cell Biology. 2017 Aug;42(1):2D-0.
35. Numasawa-Kuroiwa Y, Okada Y, Shibata S, Kishi N, Akamatsu W, Shoji M, et al. Involvement of ER stress in dysmyelination of Pelizaeus-Merzbacher Disease with PLP1 missense mutations shown by iPSC-derived oligodendrocytes. Stem Cell Reports. 2014 May 6;2(5):648-61.
36. Kawabata S, Takano M, Numasawa-Kuroiwa Y, Itakura G, Kobayashi Y, Nishiyama Y, et al. Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Reports. 2016 Jan 12;6(1):1-8.
37. Kim DS, Jung SJ, Lee JS, Lim BY, Kim HA, Yoo JE, et al. Rapid generation of OPC-like cells from human pluripotent stem cells for treating spinal cord injury. Experimental & Molecular Medicine. 2017 Jul;49(7):e361.
38. Salewski RP, Mitchell RA, Li L, Shen C, Milekovskaia M, Nagy A, et al. Transplantation of induced pluripotent stem cell-derived neural stem cells mediate functional recovery following thoracic spinal cord injury through remyelination of axons. Stem Cells Translational Medicine. 2015 Jul;4(7):743-54.
39. Behar T, McMorris FA, Novotný EA, Barker JL, Dubois‐Dalcq M. Growth and differentiation properties of O‐2A progenitors purified from rat cerebral hemispheres. Journal of Neuroscience Research. 1988 Oct;21(2‐4):168-80.
40. Bögler O, Wren D, Barnett SC, Land H, Noble M. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proceedings of the National Academy of Sciences. 1990 Aug;87(16):6368-72.
41. McKinnon RD, Matsui T, Dubois-Dalcq M, Aaronsont SA. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990 Nov 1;5(5):603-14.
42. Naruse M, Nakahira E, Miyata T, Hitoshi S, Ikenaka K, Bansal R. Induction of oligodendrocyte progenitors in dorsal forebrain by intraventricular microinjection of FGF-2. Developmental Biology. 2006 Sep 1;297(1):262-73.
43. Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, et al. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992 Jul 10;70(1):31-46.
44. Wilson HC, Onischke C, Raine CS. Human oligodendrocyte precursor cells in vitro: phenotypic analysis and differential response to growth factors. Glia. 2003 Nov;44(2):153-65.
45. Ye P, D'Ercole AJ. Insulin-like growth factor actions during development of neural stem cells and progenitors in the central nervous system. Journal of Neuroscience Research. 2006 Jan;83(1):1-6.
46. Åberg ND, Johansson UE, Åberg MA, Hellström NA, Lind J, Bull C, et al. Peripheral infusion of insulin-like growth factor-I increases the number of newborn oligodendrocytes in the cerebral cortex of adult hypophysectomized rats. Endocrinology. 2007 Aug 1;148(8):3765-72.
47. Frederick TJ, Wood TL. IGF-I and FGF-2 coordinately enhance cyclin D1 and cyclin E–cdk2 association and activity to promote G1 progression in oligodendrocyte progenitor cells. Molecular and Cellular Neuroscience. 2004 Mar 1;25(3):480-92.
48. Jiang F, Frederick TJ, Wood TL. IGF-I synergizes with FGF-2 to stimulate oligodendrocyte progenitor entry into the cell cycle. Developmental Biology. 2001 Apr 15;232(2):414-23.
49. McMorris FA, Dubois‐Dalcq M. Insulin‐like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. Journal of Neuroscience Research. 1988 Oct;21(2‐4):199-209
50. Zeger M, Popken G, Zhang J, Xuan S, Lu QR, Schwab MH, et al. Insulin‐like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007 Mar;55(4):400-11.
51. Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. The Journal of Cell Biology. 2004 Jan 5;164(1):111-22.
52. Bribián A, Medina-Rodríguez EM, Josa-Prado F, García-Álvarez I, Machín-Díaz I, Esteban PF, et al. Functional heterogeneity of mouse and human brain OPCs: relevance for preclinical studies in multiple sclerosis. Journal of Clinical Medicine. 2020 Jun 2;9(6):1681.
53. Casaccia-Bonnefil P, Tikoo R, Kiyokawa H, Friedrich V, Chao MV, Koff A. Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27Kip1. Genes & Development. 1997 Sep 15;11(18):2335-46.
54. Ortega JA, Radonjić NV, Zecevic N. Sonic hedgehog promotes generation and maintenance of human forebrain Olig2 progenitors. Frontiers in Cellular Neuroscience. 2013 Dec 13;7:254.
55. Baydyuk M, Morrison VE, Gross PS, Huang JK. Extrinsic factors driving oligodendrocyte lineage cell progression in CNS development and injury. Neurochemical Research. 2020 Mar;45:630-42.