Keywords
Central nervous systems, Glial scar, Neuronal cell
Commentary
We recently reported that glypican-2, a neuronal cell surface glycoprotein, is involved in age-dependent differences in axonal regenerative capacity in the mammalian central nervous systems (CNS) [1]. While several extrinsic inhibitory factors expressed or deposited in the lesion after trauma hinder axonal regeneration, our understanding of intrinsic factors expressed in neurons that regulate axonal regeneration is still limited. In this commentary, we will briefly overview the current information on neuronal intrinsic mechanisms for axonal regeneration, along with commenting on our recent achievements.
Trauma to the adult CNS results in axonal dissection and functional dysfunction due to the disconnection of neural circuits. Regeneration of neuronal axons and reconstruction of neural circuits are almost hopeless, especially in the adult CNS. Extrinsic inhibitors of axon regeneration associated with the glial scar, including myelin inhibitors such as Nogo [2], MAG (myelin-associated glycoprotein) [3], and OMgp (oligodendrocyte-myelin glycoprotein] [4], CSPG (chondroitin sulfate proteoglycan) [5] and Sema3 [6] have been the focus of attention for understanding these mechanisms [7]. In contrast, embryonic or juvenile neuronal axons are known to have relatively better regenerative potential. It is also well recognized that pediatric spinal cord injury patients have better recovery prospects compared to adult ones. Indeed, embryonic neurons are able to extend their axons even on the mature CNS tissue [8]. These facts strongly suggest that there are significant differences in the intrinsic ability of axon regeneration across ages and that age-dependent pro-regenerative gene expression determines the response of axons to injury [9,10]. Interestingly, it has also been reported that injured adult neurons are reprogrammed back to an embryonic state at the transcriptional level to facilitate axonal regeneration [11].
Another interesting phenomenon regarding axonal regeneration is "pre-conditioning." Dorsal root ganglion (DRG) neurons have both peripheral and central axons. Primary damage to central axons does not result in their regeneration, but pre-lesioning of peripheral axons followed by central axon damage causes regeneration of central axons [12]. Although the situation is slightly different, pre-conditioning to the lens promotes axonal regeneration after subsequent optic nerve injury [13]. This pre-conditioning is known to drastically change the transcriptional state of injured neurons, partially through epigenetic reprogramming mediated by the calcium-cAMP axis [13-15] and regulates specific pro-regenerative transcription factors such as KLF family members [16], c-Jun [17] and CREB [18]. In turn, these transcription factors activate several regeneration-associated genes (RAGs) and enhance axonal regeneration.
So far, several RAGs, including growth-associated protein 43 (Gap43), small proline-rich protein 1A (Sprr1a), Arginase 1 (Arg1), and galanin, have been identified along with pro-regenerative transcription factors [17]. There is significant overlap to ignore between RAGs and age-dependent pro-regenerative genes. For instance, the expression of the alpha2delta2 subunit of voltage-gated calcium channels (VGCCs) increases with neuronal development. Conversely, it is downregulated by pre-conditioning of the peripheral branch of DRGs. Pharmaceutical blockade of the alpha2delta2 subunit by Pregabalin, which is already approved by the United States Food and Drug Administration (FDA) to treat neuropathic pain, promoted axonal regeneration after spinal cord injury in adult mice [19].
PI3P (phosphatidylinositol 3,4,5-trisphosphate)/Akt/mTOR (mammalian target of rapamycin) axis is indispensable for protein translation. The pathway is negatively regulated by PTEN (phosphatase and tensin homolog deleted on chromosome 10) through dephosphorylation of 3-phosphate group of PI3P. The expression of PTEN in retinal ganglion neurons (RGCs) is lower in young and higher in adults, indicating that de novo protein synthesis is limited in adult neurons. Deletion of PTEN by Cre-loxP systems in adult RGCs resulted in long-distance regeneration of optic nerve after its injury [20].
To study mechanisms of axonal regeneration and its inhibition, the in vitro glial scar model in which increased concentration of CSPG aggrecan was prepared on culture vessels was frequently utilized [21,22]. The system mimics the lesion center of spinal cord injury, where inhibitory CSPGs form an increasing concentration gradient from the epicenter to the core of the lesion. Primary cultured DRG neurons stop extending their axons on the CSPG gradient, forming so-called dystrophic endbulbs on their axonal terminals. Intriguingly, this response is specific to adult neurons. Embryonic neurons never form dystrophic endbulbs, even on the CSPG gradient, and continue extending their axons and breaking through the gradient. The receptor-type protein tyrosine phosphatase sigma (PTPσ) has been reported as a receptor for CSPG, mediating inhibitory signaling for axon regeneration in neurons [22-24]. Heparan sulfate proteoglycans (HSPG) are antagonistic ligands for PTPσ and rescue the inhibitory pathway through the CS-PTPσ axis [25,26]. Therefore, we hypothesized that some members of HSPG works as RAGs and found that glypican-2 was specifically expressed in embryonic neurons (Figure 1) [1]. Heparan sulfate was also enriched in embryonic neurons, and its removal resulted in the transformation of the growth cone into dystrophic endbulbs. Conversely, overexpression of glypican-2 in adult neurons restored the formation of dystrophic endbulbs on the CSPG gradient. Unfortunately, glypican-2 alone was not sufficient to induce full axonal elongation on inhibitory substrates. It is also unclear whether glypican-2 is induced by pre-conditioning, but glypican-2 is considered as promising RAG.
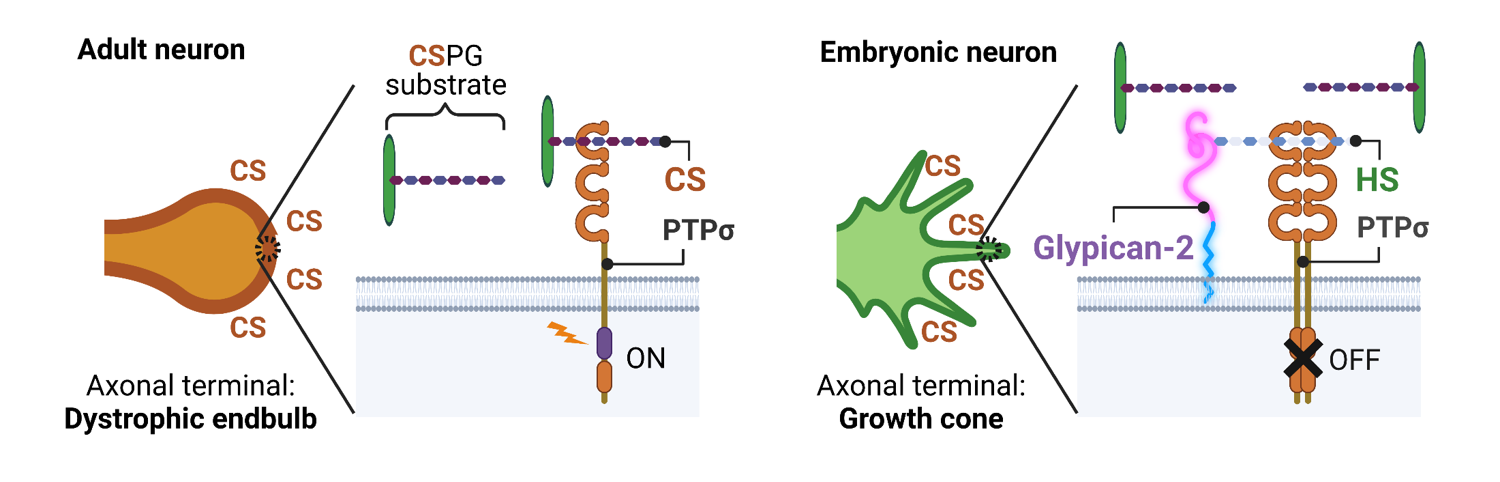
Figure 1. Glypican-2 works as RAG in embryonic neuron. In adult neuron, CSPG activates its receptor PTPσ and induces dystrophic endball. Glypican-2, a cell-surface HSPG specifically expressed in embryonic neuron, antagonizes CS-PTPσ interaction and rescues dystrophic endball formation. Created by BioRender.
In order to achieve successful axonal regeneration in the adult CNS, a combinatorial strategy to modify both intrinsic and extrinsic mechanisms, along with the regulation of inflammation, remyelination, and synaptic integration, seems to be the most effective approach. Identifying controllable RAGs and establishing methods to manipulate their function will be particularly important in future research.
Acknowledgments
We wish to acknowledge all the members of Human Glycome Atlas Project (HGA). This research was supported by AMED-CREST under Grant Number JP23gm1410011 to K.K. This work was supported by JSPS KAKENHI Grant Numbers 23K05987 to K.S. and 21K18251 to K.K.
Author Contributions
K.S. and K.K. have accepted responsibility for the entire content of the manuscript and approved its submission.
Conflict of Interest Statement
The authors declare no competing interests.
References
2. GrandPré T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403(6768):439-44.
3. McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13(4):805-11.
4. Kottis V, Thibault P, Mikol D, Xiao ZC, Zhang R, Dergham P, et al. Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J Neurochem. 2002;82(6):1566-9.
5. Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416(6881):636-40.
6. He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90(4):739-51.
7. Silver J, and Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146-56.
8. Shewan D, Berry M, Cohen J. Extensive regeneration in vitro by early embryonic neurons on immature and adult CNS tissue. J Neurosci. 1995;15(3 Pt 1):2057-62.
9. Koseki H, Donegá M, Lam BY, Petrova V, van Erp S, Yeo GS, et al. Selective rab11 transport and the intrinsic regenerative ability of CNS axons. Elife. 2017;6.
10. Geoffroy CG, Meves JM, Zheng B. The age factor in axonal repair after spinal cord injury: A focus on neuron-intrinsic mechanisms. Neurosci Lett. 2017;652:41-9.
11. Poplawski GHD, Kawaguchi R, Van Niekerk E, Lu P, Mehta N, Canete P, et al. Injured adult neurons regress to an embryonic transcriptional growth state. Nature. 2020;581(7806):77-82.
12. Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309(5971):791-3.
13. Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20(12):4615-26.
14. Cho Y, Sloutsky R, Naegle KM, Cavalli V. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013;155(4):894-908.
15. Finelli MJ, Wong JK, Zou H. Epigenetic regulation of sensory axon regeneration after spinal cord injury. J Neurosci. 2013;33(50):19664-76.
16. Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326(5950):298-301.
17. Lerch JK, Martínez-Ondaro YR, Bixby JL, Lemmon VP. cJun promotes CNS axon growth. Mol Cell Neurosci. 2014;59:97-105.
18. Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, et al. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44(4):609-21.
19. Tedeschi A, Dupraz S, Laskowski CJ, Xue J, Ulas T, Beyer M, et al. The Calcium Channel Subunit Alpha2delta2 Suppresses Axon Regeneration in the Adult CNS. Neuron. 2016;92(2):419-34.
20. Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322(5903):963-6.
21. Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J Neurosci. 2004;24(29):6531-9.
22. Sakamoto K, Ozaki T, Ko YC, Tsai CF, Gong Y, Morozumi M, et al. Glycan sulfation patterns define autophagy flux at axon tip via PTPRsigma-cortactin axis. Nat Chem Biol. 2019;15(7):699-709.
23. Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326(5952):592-6.
24. Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, et al. Proteoglycan-specific molecular switch for RPTPσ clustering and neuronal extension. Science. 2011;332(6028):484-8.
25. Sakamoto K, Ozaki T, Kadomatsu K. Axonal Regeneration by Glycosaminoglycan. Front Cell Dev Biol. 2021;9:702179.
26. Sakamoto K, Ozaki T, Suzuki Y, Kadomatsu K. Type IIa RPTPs and Glycans: Roles in Axon Regeneration and Synaptogenesis. Int J Mol Sci. 2021;22(11).
