Abstract
Introduction: From December 2019, the first cases of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection were reported and established a crisis for global health with a pandemic of coronavirus disease-19 (COVID-19). The SARS-CoV-2 is a ß-coronavirus and until October 2020, more than 46 million confirmed cases and one million deaths were notified. The predominant clinical condition involves fever, dry cough and shortness of breath. However, gastrointestinal (GI) manifestations have been shown as an important clinical finding in the course of the disease. Moreover, the pathophysiology of these presentations is still the subject of studies but shows the interaction between the SARS-CoV-2 and Angiotensin-Converting Enzyme type 2 (ACE2), expressed in both the respiratory and gastrointestinal tracts, as viral pathogenesis involved.
Methods: We surveyed relevant articles published in English and Portuguese in the PubMed and BVS databases.
Results: This review included 55 articles. The main GI manifestations of COVID-19 related are diarrhea, nausea, vomiting, abdominal pain and GI bleeding. Yet, fecal-oral transmission appears as a possible route of transmission of SARS-CoV-2 and evaluation of these patients, as well as biomarkers hepatics have been described in severe cases and should be investigated.
Conclusion: Our work concludes that the SARS-CoV-2 infection can involve digestive symptoms, being valid a special attention to patients who present them. Thus, the aim of this review is to describe the relations between COVID-19 and gastrointestinal tract and its main findings as well.
Keywords
COVID-19; Pandemic; Gastrointestinal Manifestations; Fecal-Oral Transmission
Introduction
The Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the new coronavirus of severe acute respiratory syndrome 2 (SARS-CoV-2), single-stranded, positive sense, spherical RNA virus with spikes protein that protrude on its surface giving the appearance of a crown, from the Latin corona. It belongs to the large family of coronaviruses (CoVs) and the genus β-coronavirus [1]. COVID-19 can involve manifestations in the respiratory system, as well as other biological systems, as a intestinal [ 2]. Since its identification in late December 2019, the number of cases of SARS-CoV-2 infection has increased, reaching to date October 3, 2020 the worldwide mark of 46.591.622 confirmed cases and 1.201.200 reported deaths [ 3]. The main symptoms evidenced in patients were fever (83% - 98%) and cough (68% - 82%) [4], fatigue (44% -75%) [5], headache (8%), myalgia (11%) [6], shortness of breath (31% -55%), sore throat (5%) and chest pain (2%) [7], being the SARS-CoV-2 transmitted by direct or indirect contact. Respiratory transmission propagates, in direct contact, through saliva, respiratory secretions or in respiratory droplets, being expelled when the individual coughs, sneezes, sings or speaks. The indirect transmission is given by the contact between host and contaminated surfaces and objects [8-10]. Angiotensin- Converting Enzyme type 2 (ACE2), highly expressed on lung alveolar epithelial cells and small intestinal epithelial cells, may play a crucial role in the pathogenesis of COVID-19 [11]. The viral spike (S) protein of SARS-CoV-2 binds to ACE2 as a cellular receptor, leading to entry of the vírus in the host cell [12], bringing the gastrointestinal tract as a potential route of virus through of the fecaloral transmission and emphasizing the importance of maintaining hygiene [13,14]. The first confirmed case in the USA presented diarrhea and the fecal sample positive on the 7th day. Thus, gastrointestinal (GI) symptoms are commonly observed in patients with COVID-19, such as diarrhea, anorexia, nausea, vomiting, abdominal pain and GI bleeding. [14,15]. As the pandemic evolves, there is a regular influx of new insights about the COVID-19 pathogenesis and possible routes of transmission and we herein discuss the gastrointestinal manifestations of SARS-CoV-2 infection and the related aspects.
Methodology
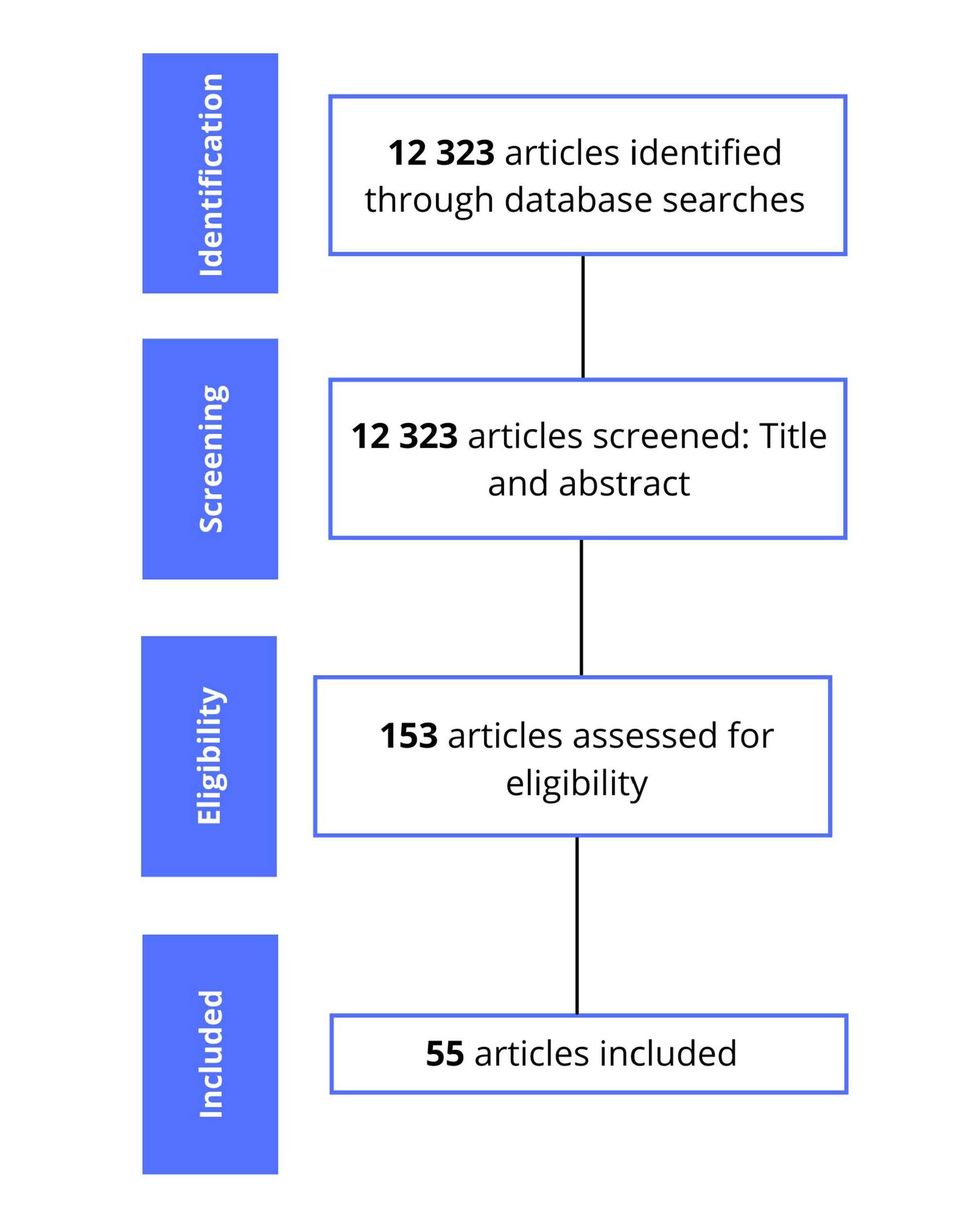
For this review, we surveyed relevant articles published in English and Portuguese in the United States National Library of Medicine (PubMed) and Virtual Health Library (BVS) databases. The descriptors used were COVID-19; SARS-CoV-2; coronavirus; Angiotensin-Converting Enzyme 2; as well as the terms symptoms, transmission, gastrointestinal pathogenicity, gastrointestinal manifestations, hepatic manifestations, liver injury and fecal-oral transmission associated with COVID-19. Articles that did not address the topics in the title and / or abstract and written in languages other than English and Portuguese were excluded. Eligibility criteria was based on articles that brought information on the researched topics: pathophysiology, gastrointestinal manifestations, laboratory findings and fecal-oral transmission of the SARS-CoV-2 infection. Thus, 12323 articles were found, in which 55 are included in this review. The summary of the articles selection process is shown in Figure 1.
Figure 1. Summary of the articles selection process.
Results
Pathophysiology of gastrointestinal manifestations
There is no consensus about how SARS-CoV-2 reaches the gastrointestinal tract. Some studies showed the similarities of SARS-CoV-2 with SARS-CoV and MERSCoV, such as genetic sequence, which may explain the similar epidemiologic and clinical features [16,17]. The pathophysiological mechanisms of this are multifactorial, although many points remain uncertain [18], and includes the virus attachment, receptor recognition, protease cleaving and membrane fusion of its transmembrane spike glycoprotein (S-protein) receptor-binding domain, specific cell receptors (ACE2), and host cellular transmembrane serine protease (TMPRSS) [19].
The main pathway that SARS-CoV-2 enters cells is through binding of protein S to Angiotensin-Converting Enzyme type 2 (ACE2) [17,20]. ACE2 receptors are expressed in various human cells, including epithelial cells in the lungs, neuronal and glial cells in the brain and cardiomyocytes [21]. Moreover, ACE2 has a high expressiveness in glandular cells of the gastric, duodenal and rectal epithelia and also on endothelial cells and enterocytes of the small intestine [17,20].
SARS-CoV-2 also enters cells using the receptors for transmembrane protease serine 2 (TMRPSS2), a cell surface protein that is expressed by epithelial cells of specific tissues, such as small intestinal epithelial cells [22], with co-expression of ACE2 and TMPRSS2 detected in enterocytes and esophagus, mainly in the small intestine [21].
There may be several events resulting from the interaction of SARS-CoV-2 and ACE2. The diarrhea may be due the direct aggression, alteration of the ACE2 function and, consequently, interference in the balance of the gastrointestinal tract, due to the change in the intestinal microbiota, for example [13]. The negative regulation of ACE2 by the virus also may reduce the production of important metabolites in the regulation of intestinal homeostasis. Thus, there is a reduction of bacterias with physiological function, while pathological bacterias multiply [23].
Another possible cause of diarrhea is the host immune response, which is capable of causing damage to the intestinal epithelium, due the effects to the cytokine storm. [13,24]. Several studies have shown that cytokines and chemokines, mainly interleukin IL-2, IL-6, IL-7 and IL-10, in patients with COVID-19 are elevated. Besides, in patients with severe disease, there is a considerable increase in TNF-α, when compared to non-severe patients [6,21,25]. Furthermore, as a consequence of the alteration of the intestinal microbiota, activation of the Th17 response with the production of IL-17A may occur, which acts in the recruitment of neutrophils and can cause immune damage in the GI tract, with the presence of diarrhea and other gastrointestinal symptoms, for example [24,25]. Among the other changes noted, there is an increase in fecal calprotectin due to the intestinal inflammatory response and directly correlated with IL-6 [26]. Among patients with COVID-19, levels of calprotectin and IL-6 were higher among those who experienced diarrhea [21].
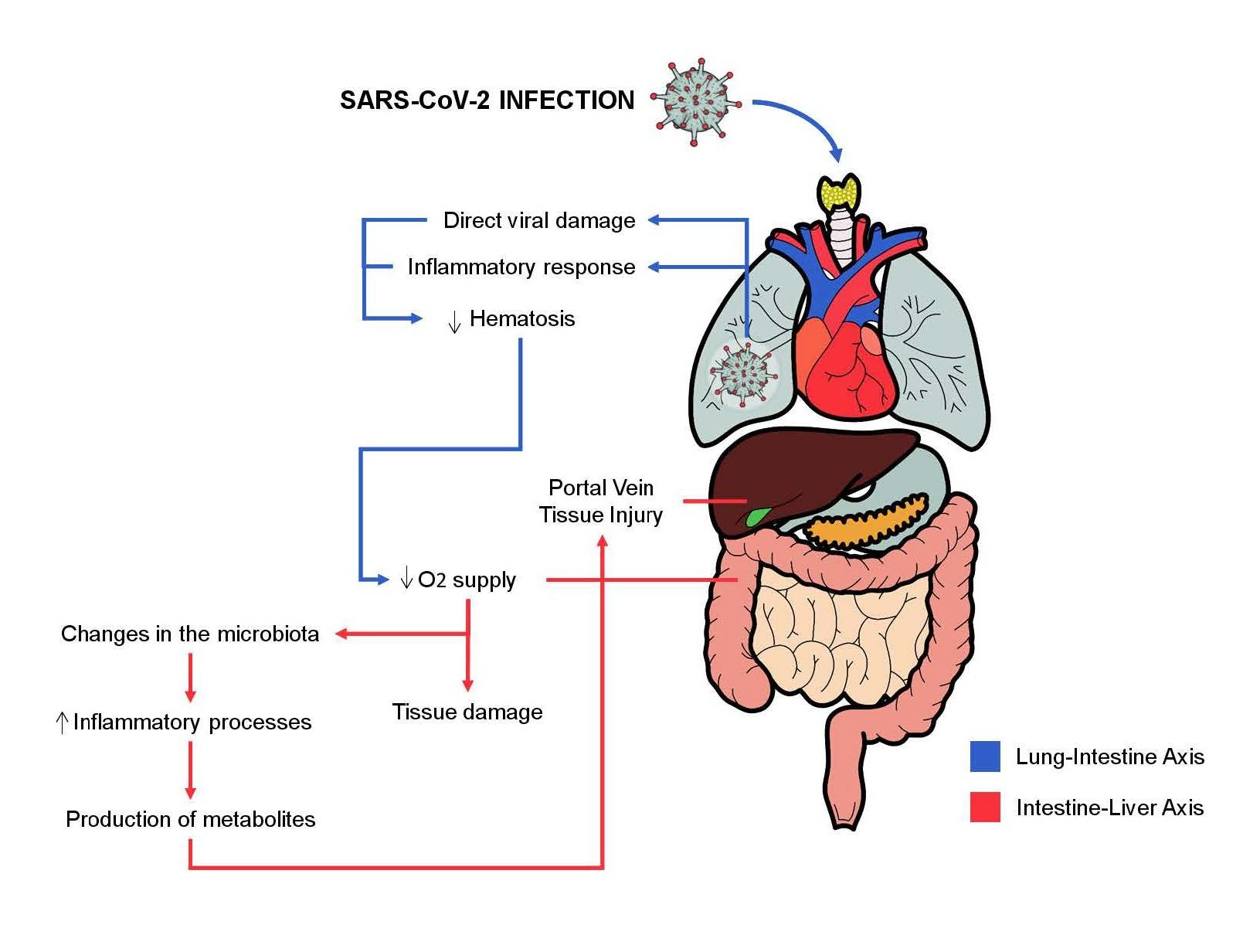
Several hepatic changes are also observed in some cases of SARS-CoV-2 infection and can be caused by direct damage to cholangiocytes and hepatocytes, as well as the cytokine storm causes systemic involvement of the organism and is identified as a possible cause [25,27]. The so-called gut-lung axis is also of great importance in intestinal symptoms during COVID-19. Pneumonia caused by the virus causes systemic tissue hypoxia that can cause changes in the intestinal microbiota, tissue damage (intestinal and hepatic, for example), in addition to being an important indicator of severity [23,27]. The high expression of ACE2 in bile duct cells and liver biopsies confirmed the potential viral route in liver tissues [28,29], and liver function abnormalities could be indicated by abnormal Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) concentrations, accompanied by slightly increased bilirubin concentrations [28]. In addition to the intestine-lung axis, there is the intestineliver axis. Thus, the alteration of the intestinal microbiota and the inflammatory process produce metabolites that are directed to the liver, through the portal vein [25]. Cholangiocytes have significant amounts of ACE2 and are targets of SARS-CoV-2 infection, their impairment represents another possibility of injury and impairment of the liver system [18,30,31]. The intestine-lung axis and intestine-liver axis and their relationship with SARSCoV- 2 are illustrated in Figure 2.
Figure 2. The intestine-lung axis and intestine-liver axis and their relationship with SARS-CoV-2.
Older adults and people with comorbidities are more vulnerable to present severe conditions of COVID-19 [32,33]. One of the hypotheses is that the loss of microbial diversity is associated with aging and causes higher susceptibility to inflammation [21].
Gastrointestinal manifestations
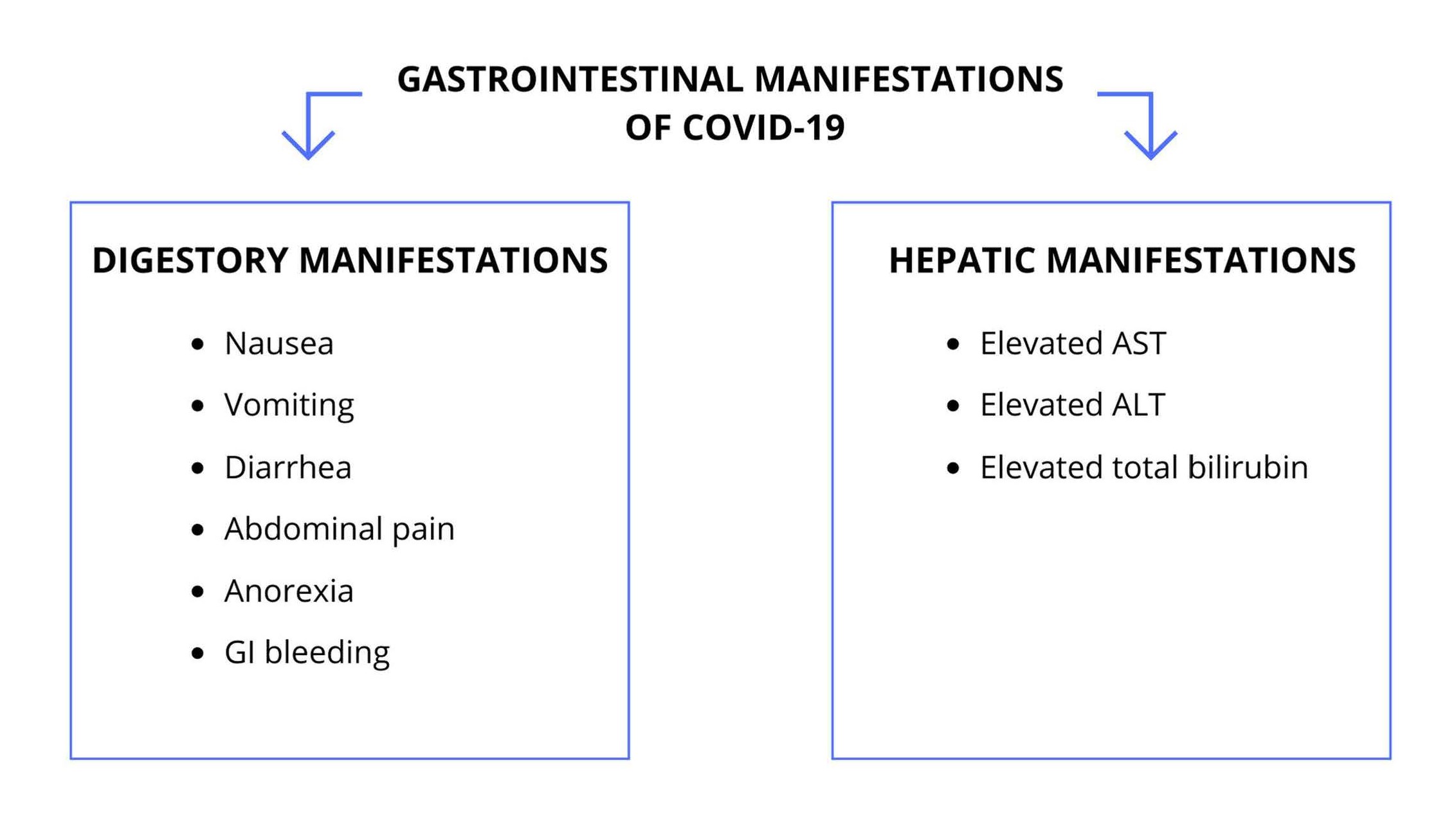
SARS-CoV-2 is a β-coronavirus that relates to Angiotensin- Converting Enzyme 2 (ACE2), expressed in the esophageal epithelial cells and in the ileum and colon absorptocytes, which may demonstrate the digestive manifestation of COVID-19 [25,34] and the capacity of this coronavirus to infect and actively replicate in the gastrointestinal tract [13,35]. The mains gastrointestinal manifestations of SARS-CoV-2 infection are shown in Figure 3.
Figure 3. Gastrointestinal Manifestations of COVID-19. GI: Gastrointestinal; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase.
A study published in February 2020 with patients from Wuhan, China, examined 41 subjects positive for COVID-19 with major initial symptoms of fever, cough and myalgia, in addition to GI symptoms, such as diarrhea [6]. Interestingly, the first case of the disease in the United States reported nausea, vomiting and diarrhea on admission, after the patient returned from a trip to Wuhan, with RT-PCR of respiratory samples positive for SARSCoV- 2 and, subsequently, the virus was also detected in fecal samples [36].
Therefore, it should be noted that the most common gastrointestinal manifestations associated with SARSCoV- 2 include nausea, vomiting, abdominal pain and diarrhea. Gastrointestinal bleeding and changes in liver enzymes alanine transferase (ALT) and aspartate transferase (AST) have also been reported [37]. A study in California revealed that GI symptoms affect approximately 31.9% of patients, with anorexia, nausea / vomiting and diarrhea being the most common and mildly described [38]. Other studies show reports of patients who presented with gastrointestinal symptoms as the only manifestation of COVID-19 [37,39], a fact that stands out the importance of their understanding. Furthermore, in the province of Zhejiang, China, it was observed that 11% of the cases of COVID-19 had gastrointestinal manifestations, the most common being diarrhea affecting about 70% of the patients with gastrointestinal symptoms and 8% of the total cases. Another important factor was the 10.8% rate for liver disorders with an increased level of aspartate aminotransferase (AST) and / or alanine aminotransferase (ALT) [40].
When comparing adults and children, it was noted that GI symptoms are present regardless of age. Yet, vomiting happens to be more frequent in children, while diarrhea and abdominal pain is found most often in adults. Moreover, diarrhea may be the first symptom before the diagnosis of SARS-CoV-2 infection [25,41]. Furthermore, it was observed that critically ill patients were more likely to have digestive symptoms when compared to non-severe patients [37].
In view of this, even if gastrointestinal manifestations, simultaneous or isolated, are not prominent, they are still useful for the identification of the spectrum of COVID-19. It is also useful as an alternative way of prevention and surveillance strategy, which may lead to a faster and efficient management, reducing spread, possible complications and mortality rate.
Laboratory findings
Studies show that in addition to lung and gastrointestinal injuries caused by SARS-CoV-2 infection, liver damage has also been observed [42,43]. Despite the low expression in liver cells of the angiotensin II-converting enzyme (ACE2), about 2.6% of the total number, there is a relevant quantity of this receptor in the bile duct cells, about 59.7% of the total [44]. This percentage is similar to that of type 2 alveolar cells, which means that infection and viral replication in these structures are possible [42]. The bile duct is responsible for the regeneration and immune response of the liver. Thus, the virus may possibly infect the bile duct cells, leading to liver dysfunction and damage [ 41].
Increased numbers of Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST), in addition to the slight increase in total bilirubin and the decrease in albumin levels suggest these alterations [41]. Viral infection in the hepatic duct also leads to a storm of pro-inflammatory cytokines at the site, which, in laboratory findings, mean the continuous decrease in the lymphocytes count and increase in the neutrophil count [44]. In addition, there was an increase in the serum concentrations of IL-1β, IL-6 and TNF -α. The use of medications during the treatment of COVID-19, such as antibiotics, antivirals and antipyretics, may also be related to liver damage, with changes in AST / ALT levels [43]. All these findings were found in patients with advanced stages of infection.
Fecal-Oral transmission
The resistant envelope of SARS-CoV-2, that makes able to resist a higher variability of adverse conditions, and the prolonged excretion of viral RNA in feces have been found in infected patients raising a possible route of fecal-oral transmission and retransmission through the formation of aerosols from infected feces, besides the chance of indirect contamination [45,46].
A study with 4,243 patients verified the presence of the SARS-CoV-2 RNA in 48.1% (95% CI, 38.3-57.9) of the individuals during the manifestation of the disease. Among fecal samples collected after a negative respiratory test, 70.3% (95% CI, 49.6-85.1) were found to be positive for the viral RNA [15]. Moreover, was detected SARS-CoV-2 RNA in fecal samples of 39 (53.42%) among 73 patients included in their studies, and the viral RNA continued to be detected in feces of 17 (23.29%) individuals even after the viral clearance in the respiratory tract [47]. Another study including 4805 individuals detected the elimination of the viral genetic material in feces of 40.5% (95% IC, 27.4- 55.1) of patients[52]48. Complementarily, a meta-analysis embracing 95 studies observed a prevalence of 51.8% (95% CI, 43.8-59.7) of positivity for SARS-CoV-2 RNA in feces from infected patients, and the mean permanence time was of 12.5 days after the obtainment of negative results in samples from the respiratory tract. Yet, the presence and persistence of the viral genetic material in feces does not mean that the virus has a potentially infectious status.
However, studies have isolated viable SARS-CoV-2 from cultures obtained from GIT, supporting the idea of a viral fecal-oral transmission [50]. Other findings that favor the hypothesis of a fecal-oral transmission are the observance of positive samples from toilets and sinks by some studies [12].
Some studies have shown that the SARS-CoV-2 RNA load in feces, right after symptoms onset, is lower than that observed in swabs of the respiratory tract [49]. However, on the respiratory site, those values decrease quicker than in the stool. As observed by Wu et al. patients’ fecal samples remained positive on an average of 27.9 after the onset of symptoms, 11.2 days longer than as respiratory samples [19]. Regarding the concentration of viral RNA in feces, the results of Wolfel et al. indicate a variable viral load, according to the day the material was collected, between 10³-10? copies of RNA/g of fecal sample [50]. Nevertheless, the presence of viral RNA in feces alone does not point to the existence of viable viruses, a fact observed by several studies that, despite the detection of RNA, did not obtain positive cultures, except for Wang et al who of his 44 fecal samples with SARS-CoV-2 RNA, found viable viruses in 2 of the 4 samples he performed culture [51].
If oral-fecal transmission of SARS-CoV-2 is confirmed new concerns will arise such as the spread of this virus through wastewater, especially in developing countries that do not have an adequate sanitation system [52]. Is worth mentioning that the presence of the virus in sewers enables the epidemiology based on wastewater to function as a complementary approach to stipulate the presence and prevalence of COVID-19 in communities [53]. Furthermore, caution in handling the feces of infected patients and correct disinfection of the sewage in hospitals should be strongly recommended [54], along with appropriate paramentation for exams such as colonoscopy and physical examination itself given the risk to the health of professionals involved. The prolonged time of viral elimination in feces also demands greater attention to hand and sanitary disinfection, even with the respiratory samples being tested negative, besides the expansion of the isolation and discharge criteria that currently take only the respiratory site into account [55].
Conclusion
Therefore, it is evident that SARS-CoV-2 infection may present, in addition to respiratory symptoms, with gastrointestinal manifestations previously or during the course of the disease. The ACE2 receptor seems to play an important role in the pathogenesis of the infection, which could justify the gastric and intestinal symptoms as well as possible liver damage, bringing such findings to the list of manifestations of COVID-19. Yet, the fecal-oral route has excelled as a possibility of transmission of SARS-CoV-2, especially in developing countries without adequate sanitation systems, being needed additional studies about their real role on the disease course.
References
2. Wang L, Wang Y, Ye D, Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current. International Journal of Antimicrobial Agents. 2020 Jun;55(6):105948
3. World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Geneva: World Health Organization; 2020.
4. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine. 2020 Apr 30;382(18):1708-20.
5. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 Jul;75(7):1730-1741.
6. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020 Feb 15;395(10223):497-506.
7. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020 Feb 15;395(10223):507-13.
8. Qu G, Li X, Hu L, Jiang G. An imperative need for research on the role of environmental factors in transmission of novel coronavirus (COVID-19). Environmental Science & Technology. 2020 Apr 7;54(7):3730-3732.
9. Mohan SV, Hemalatha M, Kopperi H, Ranjith I, Kumar AK. SARS-CoV-2 in environmental perspective: Occurrence, persistence, surveillance, inactivation and challenges. Chemical Engineering Journal. 2021 Jul; 405:126893.
10. Morawska L, Cao J. Airborne transmission of SARSCoV- 2: The world should face the reality. Environment International. 2020 Apr 10:105730.
11. Kumar VC, Mukherjee S, Harne PS, Subedi A, Ganapathy MK, Patthipati VS, et al. Novelty in the gut: a systematic review and meta-analysis of the gastrointestinal manifestations of COVID-19. BMJ Open Gastroenterology. 2020 May 1;7(1): e000417.
12. Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19). The Journal of Pathology. 2020 Jul;251(3):228-248
13. Ong SW, Tan YK, Chia PY, Lee TH, Ng OT, Wong MS, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020 Apr 28;323(16):1610-2.
14. Dhar J, Samanta J, Kochhar R. Corona Virus Disease-19 pandemic: The gastroenterologists’ perspective. Indian Journal of Gastroenterology. 2020 Jun;39(3):220-231.
15. Cheung KS, Hung IF, Chan PP, Lung KC, Tso E, Liu R, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020 Jul;159(1):81-95.
16. Rabaan AA, Al-Ahmed SH, Haque S, Sah R, Tiwari R, Malik YS, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV: a comparative overview. Infez Med. 2020 Jun 1;28(2):174- 84.
17. Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. Journal of Gastroenterology and Hepatology. 2020 May;35(5):744-8.
18. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nature Medicine. 2020 Jul;26(7):1017-32.
19. Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020 May 1;158(6):1518-9.
20. Lai CC, Ko WC, Lee PI, Jean SS, Hsueh PR. Extrarespiratory manifestations of COVID-19. International Journal of Antimicrobial Agents. 2020 May 22:106024.
21. Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Translational Research. 2020; 226:57-69.
22. Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: Serendipity or Opportunity for Intervention?. Cancer Discovery. 2020 Jun 1;10(6):779-82.
23. Trottein F, Sokol H. Potential causes and consequences of gastrointestinal disorders during a SARS-CoV-2 infection. Cell Reports. 2020 Jul 3:107915.
24. Tian Y, Rong L, Nian W, He Y. gastrointestinal features in COVID-19 and the possibility of faecal transmission. Alimentary Pharmacology & Therapeutics. 2020 May;51(9):843-51.
25. Ye Q, Wang B, Zhang T, Xu J, Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. American Journal of Physiology- Gastrointestinal and Liver Physiology. 2020 Aug 1;319(2): G245-52.
26. Ouali SE, Achkar JP, Lashner B, Regueiro M. Gastrointestinal manifestations of COVID-19. Cleveland Clinic Journal of Medicine. 2020 Jun 18.
27. Lee IC, Huo TI, Huang YH. Gastrointestinal and liver manifestations in patients with COVID-19. Journal of the Chinese Medical Association. 2020 83(6):521-523.
28. Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology. 2020 Jul;5(7):667-678.
29. Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): What do we know till now? Arab J Gastroenterol. 2020; 21 (1): 3-8.
30. Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterology Journal. 2020 Jun;8(5):509-19.
31. Cheong J, Bartell N, Peeraphatdit T, Mosli M, Al- Judaibi B. Gastrointestinal and liver manifestations of COVID-19. Saudi journal of gastroenterology: Official Journal of the Saudi Gastroenterology Association. 2020 Sep;26(5):226-32.
32. Galanopoulos M, Gkeros F, Doukatas A, Karianakis G, Pontas C, Tsoukalas N, et al. COVID-19 pandemic: Pathophysiology and manifestations from the gastrointestinal tract. World Journal of Gastroenterology. 2020 Aug 21;26(31):4579-4588.
33. Esteve A, Permanyer I, Boertien D, Vaupel JW. National age and co-residence patterns shape covid-19 vulnerability. Proc Natl Acad Sci U S A. 2020. 117 (28) 16118-16120.
34. Li LY, Wu W, Chen S, Gu JW, Li XL, Song HJ, et al. Digestive system involvement of novel coronavirus infection: prevention and control infection from a gastroenterology perspective. Journal of Digestive Diseases. 2020 Apr;21(4):199-204.
35. Kopel J, Perisetti A, Gajendran M, Boregowda U, Goyal H. Clinical Insights into the Gastrointestinal Manifestations of COVID-19. Digestive Diseases and Sciences. 2020 ;65(7):1932-1939.
36. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. Washington State 2019- nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. The New England Journal of Medicine. 2020 Mar 5;382(10):929-936.
37. Chen T, Yang Q, Duan H. A severe coronavirus disease 2019 patient with high-risk predisposing factors died from massive gastrointestinal bleeding: a case report. BMC Gastroenterology. 2020 Sep 29;20(1):318.
38. Cholankeril G, Podboy A, Aivaliotis VI, Tarlow B, Pham EA, Spencer SP, et al. High Prevalence of Concurrent Gastrointestinal Manifestations in Patients with Severe Acute Respiratory Syndrome Coronavirus 2: Early Experience from California. Gastroenterology. 2020 Aug 1;159(2):775-7.
39. Remes-Troche JM, Ramos-de-la-Medina A, Manríquez-Reyes M, Martínez-Pérez-Maldonado L, Lara EL, Solís-González MA. Initial Gastrointestinal Manifestations in Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection in 112 Patients from Veracruz in Southeastern Mexico. Gastroenterology. 2020 Sep 1;159(3):1179-81.
40. Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020 Jun 1;69(6):1002-9.
41. Cha MH, Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID-19: A comprehensive review. World Journal of Gastroenterology. 2020 May 21;26(19):2323-32.
42. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver International. 2020 May;40(5):998-1004.
43. Parohan M, Yaghoubi S, Seraj A. Liver injury is associated with severe Coronavirus disease 2019 (COVID-19) infection: a systematic review and metaanalysis of retrospective studies. Hepatology Research. 2020 Aug;50(8):924-935.
44. Wu J, Song S, Cao HC, Li LJ. Liver diseases in COVID-19: Etiology, treatment and prognosis. World Journal of Gastroenterology. 2020 May 21;26(19):2286-93.
45. Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. The Lancet Gastroenterology & Hepatology. 2020 May 1;5(5):434-5.
46. van Doorn AS, Meijer B, Frampton CM, Barclay ML, de Boer NK. Systematic review with meta-analysis: SARS-CoV-2 stool testing and the potential for faecal-oral transmission. Alimentary Pharmacology & Therapeutics. 2020 Oct;52(8):1276-88.
47. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 May 1;158(6):1831-3.
48. Parasa S, Desai M, Chandrasekar VT, Patel HK, Kennedy KF, Roesch T, et al. Prevalence of Gastrointestinal Symptoms and Fecal Viral Shedding in Patients with Coronavirus Disease 2019: A Systematic Review and Meta-analysis. JAMA Network Open. 2020 Jun 1;3(6): e2011335.
49. Collivignarelli MC, Collivignarelli C, Miino MC, Abbà A, Pedrazzani R, Bertanza G. SARS-CoV-2 in sewer systems and connected facilities. Process safety and environmental protection. 2020 Nov;143: 196-203.
50. Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 May;581(7809):465-9.
51. Gupta S, Parker J, Smits S, Underwood J, Dolwani S. Persistent viral shedding of SARS-CoV-2 in faeces-a rapid review. Colorectal Disease. 2020 Jun;22(6):611-620.
52. Odih EE, Afolayan AO, Akintayo I, Okeke IN. Could Water and Sanitation Shortfalls Exacerbate SARS-CoV-2 Transmission Risks?. The American Journal of Tropical Medicine and Hygiene. 2020 Aug;103(2):554-557.
53. Daughton CG. Wastewater surveillance for populationwide Covid-19: The present and future. Science of The Total Environment. 2020 May 23:139631.
54. Smyk W, Janik MK, Portincasa P, Milkiewicz P, Lammert F, Krawczyk M. COVID-19: focus on the lungs but do not forget the gastrointestinal tract. European Journal of Clinical Investigation. 2020 May 14: e13276.
55. Chen C, Gao G, Xu Y, Pu L, Wang Q, Wang L, Wang W, et al. SARS-CoV-2–positive sputum and feces after conversion of pharyngeal samples in patients with COVID-19. Annals of internal medicine. 2020 Jun 16;172(12):832-834.