Abstract
Introduction: Gastrointestinal stromal tumors (GIST) account for 85% of all mesenchymal neoplasms of the digestive tract. Of these, two thirds are found in the stomach. GISTs usually present as small lesions, often incidentally discovered. On the other hand, the presence of an abdominal mass, a very infrequent finding, can produce symptoms: Abdominal pain, digestive bleeding, nausea, vomiting, in addition to weight loss and intestinal constipation.
Case Presentation: Male patient, 52 years old, asymptomatic, with progressive increase in abdominal volume for 5 years, attributed to beer ingestion. Eating normally, usual bowel rhythm and performing daily work activities. Studied by abdominal tomography that showed expansive formation, with some calcifications and hypodense areas of necrosis, lobulated, measuring 33.1 x 28.6 x 21.8 cm and occupying almost the entire abdominal cavity.
Conclusion: Giant gastric GIST are rare neoplasms and surgical R0 resection is the best therapeutic proposal.
Keywords
Giant GIST, Surgical management, Gastrointestinal Stromal Tumors, Mitotic index
Introduction
Although gastrointestinal stromal tumors, GISTs, are the most common neoplasms arising from the gastrointestinal mesenchyme, they represent less than 1% of all digestive tumors [1]. Its incidence has increased in recent years, probably due to improved diagnostic methods [2]. It is currently known that GIST is the most common sarcoma [3]. It originates from interstitial cells of Cajal and depends on the transcription factor ETV-1. These are neoplasms associated with molecular alterations and some mutations: In more than 75% of cases there is the KIT mutation (CD 117) [3], 10% have mutation in platelet-derived growth factor receptor alpha (PDGFRA) and wild-type GISTs have a mutation in the SDH pathway [3]. There does not seem to be a predilection between the sexes. They affect a wide age range, but in around 75% of cases, it is more common over 50 years of age [4]. Any organ of the digestive tract can be affected by GIST, however more than 50% of cases are seen in the stomach, 25% in the small intestine and a minority in the colon, rectum, retroperitoneum, omentum, among others [5-9]. Its size can vary from millimetric lesions to the so-called giant GISTs, a definition in the literature for those with more than 10 cm in diameter [10].
Case Report
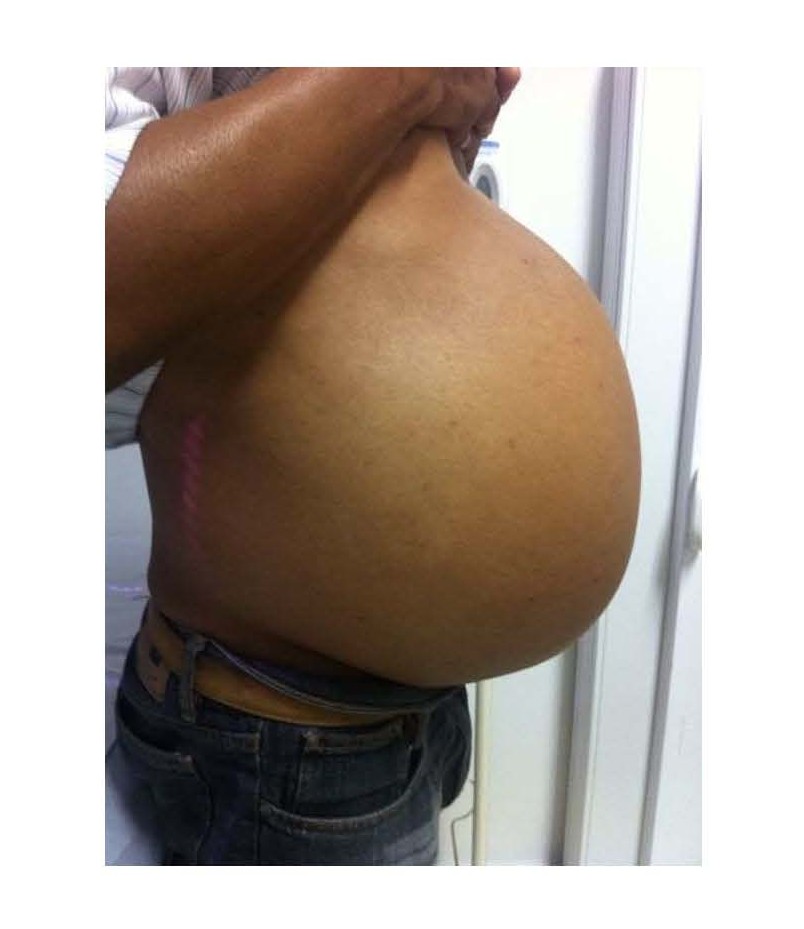
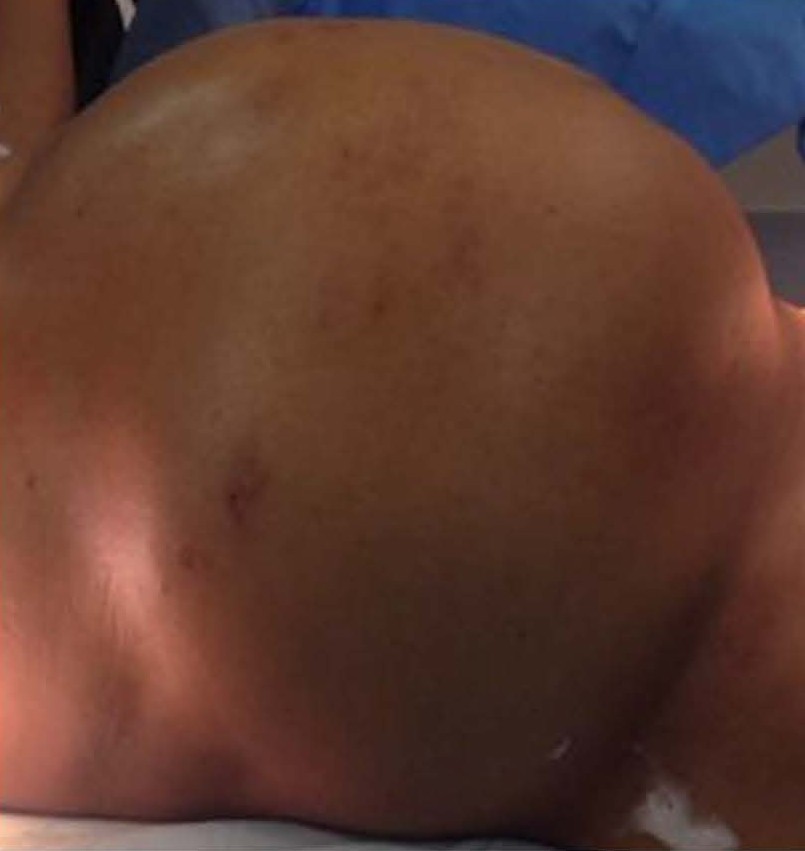
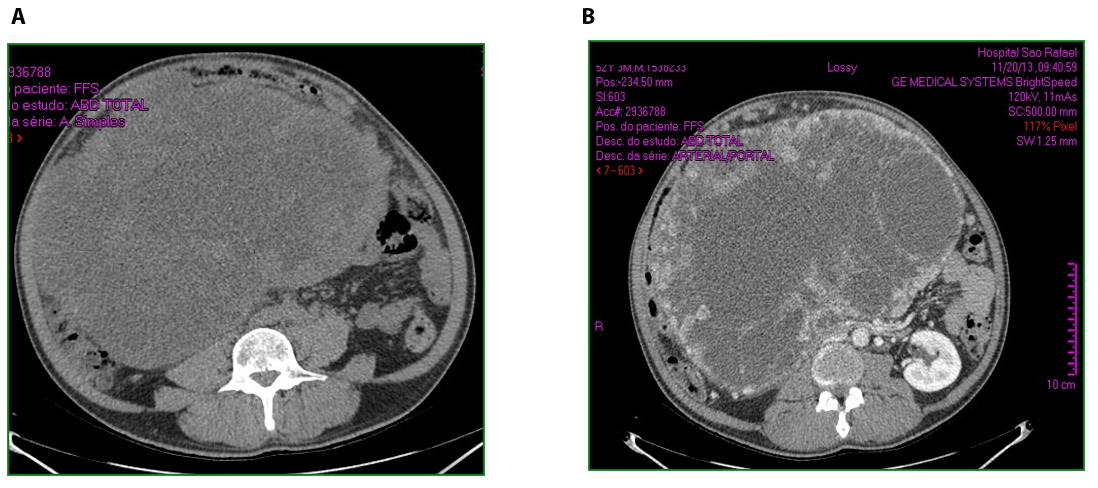
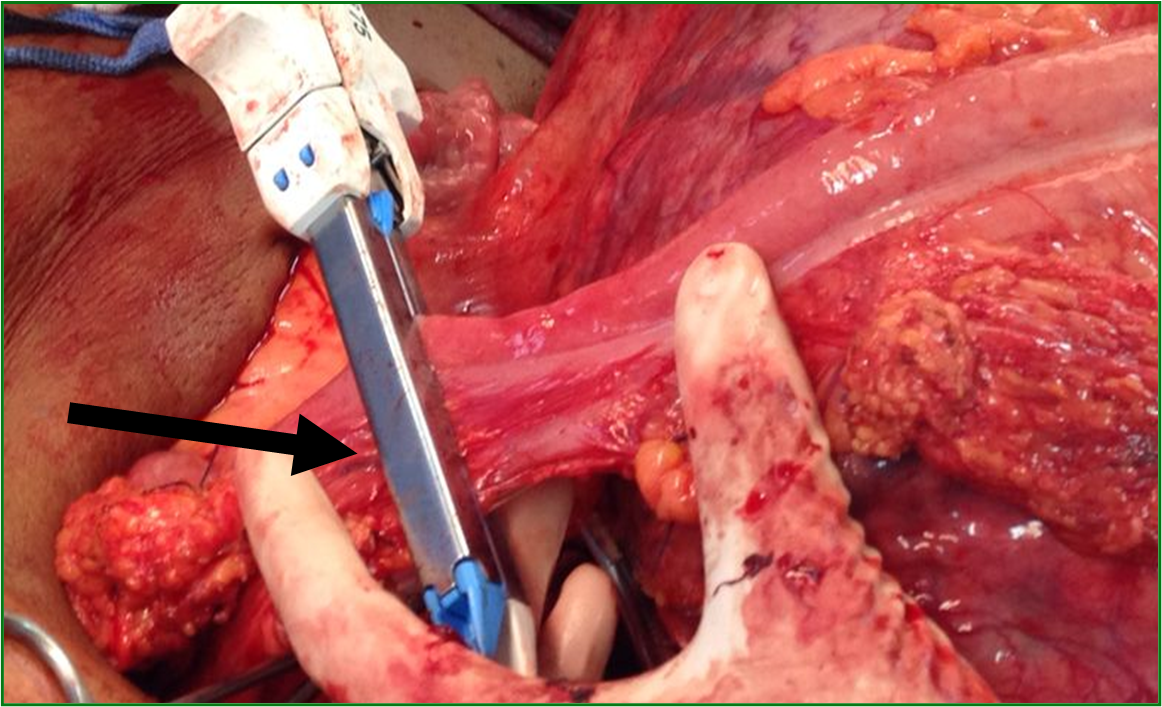
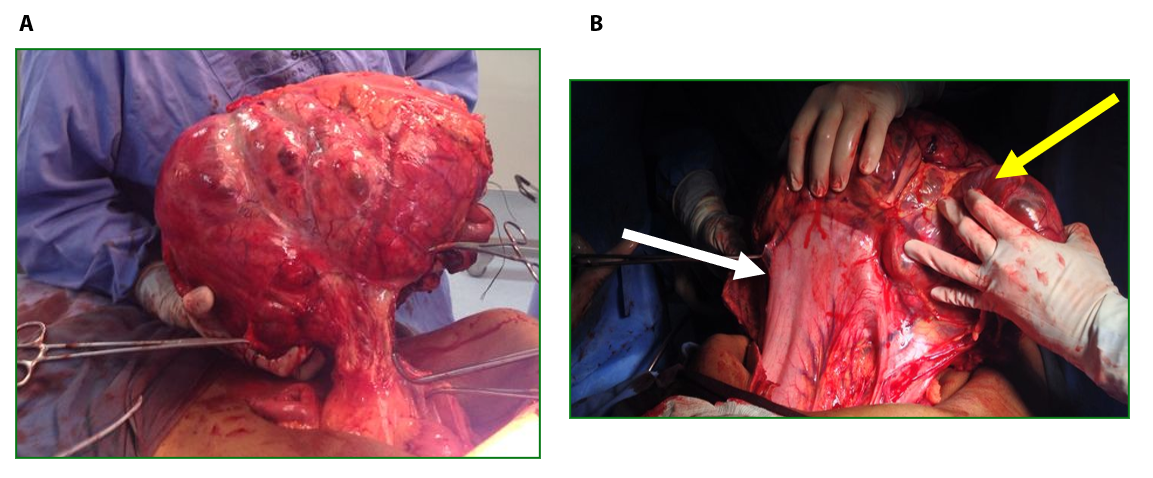
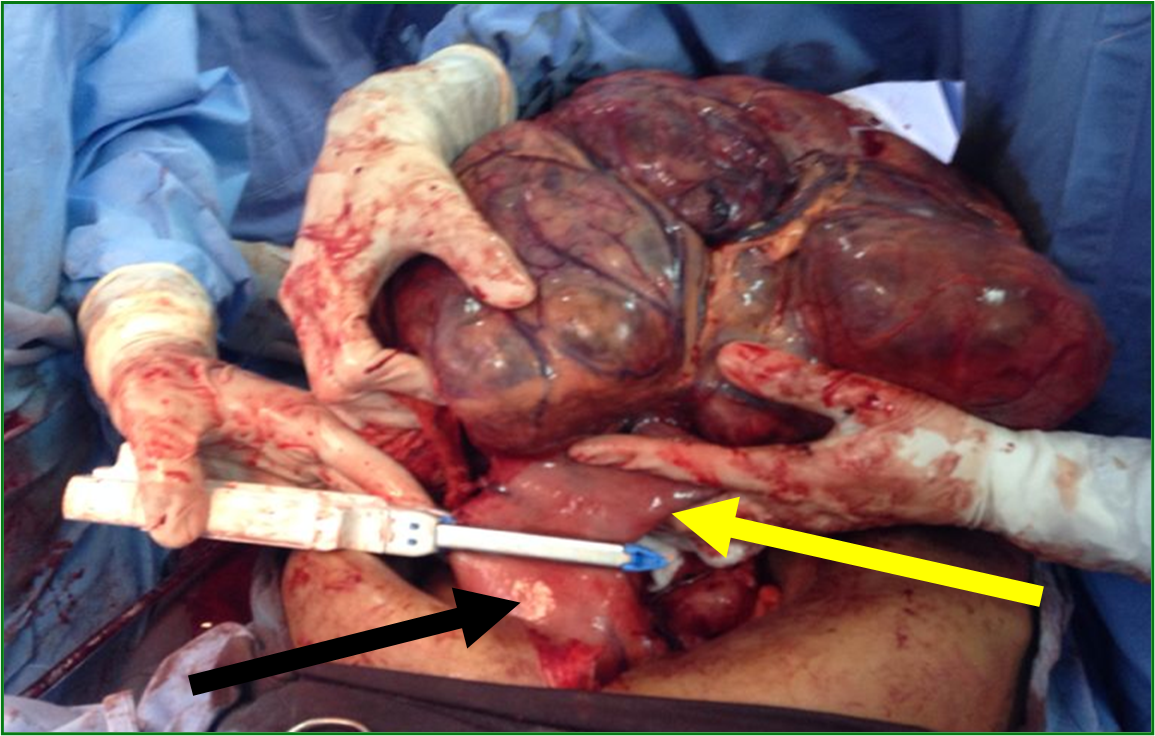
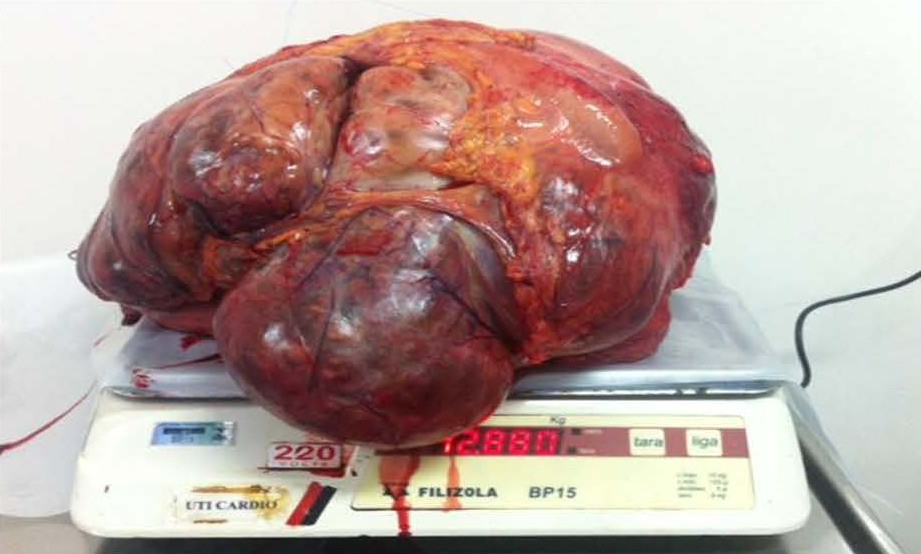
Male patient, 52 years old, asymptomatic, coming from the outpatient clinic of the upper digestive tract surgery service at Hospital São Rafael / Rede D'Or, Salvador – Bahia – Brazil, with a report of slow and progressive increase in abdominal volume over 5 years, attributed by him to an increase in the body mass index (BMI) resulting from a high intake of alcoholic beverages and foods rich in fat. The patient ate normally, had normal bowel rhythm and denied: postprandial fullness, nausea, vomiting or abdominal pain. There was no weight loss in the period, patient performed normal work activities and Karnofsky performance status was (KPS) 100%. On physical examination, we observed: Significant increase in abdominal volume (Figure 1), due to a solid mass, adhered to deep planes, painless ó even in the supine position, he did not refer associated respiratory discomfort (Figure 2). There were no cervical, axillary or inguinal lymph node enlargements or stigmata of liver disease. Tomography of the total abdomen showed an expansive formation, with some calcifications, lobulated, hypodense areas of necrosis in the periphery of the lesion, measuring 33.1 x 28.6 x 21.8 cm, occupying almost the entire abdominal cavity and inseparable from the viscera, making it difficult to have the interpretation of its origin (Figures 3A and 3B). GIST and other types of sarcoma were the main radiological suspicions. No preoperative biopsy was performed because there was a lot of necrosis in the mass, especially peripheral. The staging was complemented with a chest tomography, which was without alterations. Undergoing exploratory laparotomy, we observed multiple adhesions, one of them firm to the transverse colon, requiring transversectomy to release the tumor mass (Figure 4) with primary colon-colonial anastomosis. After complete dissection of the tumor, we observed its origin in the gastric body (Figures 5A and 5B). We opted for partial en bloc gastrectomy (R0) (Figure 6), with reconstruction of the Roux-y transit with the fundus of the stomach. Surgical time of 3 hours, without the use of blood products and abdominal drain. Tumor mass alone weighing approximately 13 kg (Figure 7). The first 24 hours after surgery (PO) were spent in the intensive care unit (ICU). A liquid oral diet was started on the 2nd PO and progressed according to clinical evolution to a mild oral diet. There was surgical re-approach on the 8th PO Day due to sepsis of an abdominal focus, secondary to colon-colonic anastomosis dehiscence. A right hemicolectomy and ileostomy with mucous fistula were performed. Hospital discharge on the 35th day of hospital admission.
Figure 1. Patient in orthostasis with large increase in abdominal volume.
Figure 2. Patient in dorsal decubitus with a large solid mass in the abdomen, without systemic repercussion of venous return and without respiratory discomfort.
Figure 3. A. Large mass occupying almost the entire abdominal cavity. B. Peripheral calcification white areas.
Figure 4. Tumor strongly adhered to the transverse colon. Resection of the transversus in bloc together with the tumor was performed using a linear stapler (Black arrow).
Figure 5. A. Tumor mass dissected and completely outside the abdominal cavity. B. Tumor of gastric origin. Tumor (yellow arrow). Stomach (white arrow).
Figure 6. Partial gastrectomy using a linear cutting stapler. Part of the body and gastric antrum (yellow arrow). Residual body and gastric fundus (black arrow).
Figure 7. Massive tumor mass weighing almost 13 kg.
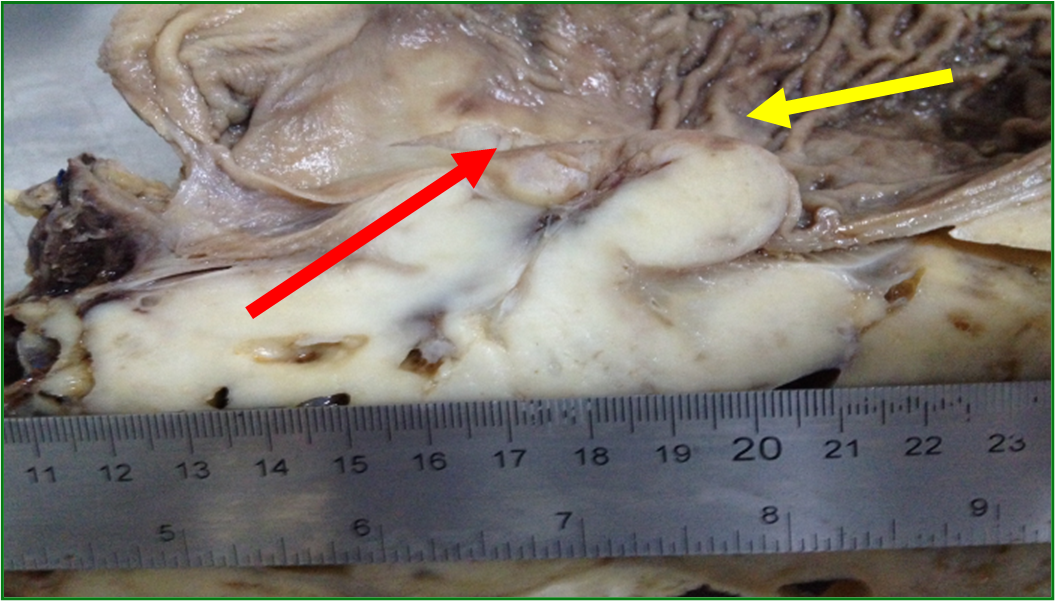
An anatomopathological analysis showed a mass measuring 35 x 30 x 3.0 cm located in the gastric wall with integral mucosa (Figure 8), margins and free lymph nodes, mitotic index of 0/50 high-power fields. An immunohistochemistry confirmed gastric GIST (Table 1). Postoperatively, Imatinib Mesylate was prescribed for 3 years based on the size of the lesion. The digestive transit was reconstructed 6 months after hospital discharge. In outpatient follow-up for 7 years, he has no disease recurrence and good quality of life.
Figure 8. Gastric mucosa (yellow arrow). Red arrow showing line with lesion below mucosa.
| Size | Mitotic count | |
| Very low risk | <2cm | <5/50 HPF |
| Low risk | 2 – 5 cm | <5/50 HPF |
| Intermediate | <5 cm 5 – 10 cm |
6 – 10/50 HPF <5/50 HPF |
| High risk | >5 cm >10 cm Any size |
>5/50 HPF Any mitotic rate >10/50 HPF |
| GIST: Gastrointestinal Stromal tumors. HPF: High-Power Field. | ||
Discussion: Gastric GISTs
The North American database (Surveillance, Epidemiology, and End Results – SEER) shows an annual incidence of GISTs in that country of 0.78/100,000 [11]. The importance of gastric GISTs lies in the fact that they are borderline stromal tumors with malignant potential in 20 to 25% of cases, with the liver being their main site of metastasis, followed by the peritoneum, 65% and 21% respectively [12-14].
Risk Stratification: Primary Site, Size and Mitotic Index
The natural evolution of this pathology can be challenging and complex. There is a lot of discussion in the literature about criteria that can predict which tumors have the most aggressive potential and, therefore, should be resected and which patients benefit from adjuvant therapy. The primary site, tumor size and mitotic index are the main prognostic factors for aggressiveness [10,15,16]. Fletcher et al. [17] (Table 1) created the first risk classification for malignancy of GISTs 19 years ago. They considered size and mitotic index the two most important prognostic factors and classified GISTs into: very low risk, low risk, medium risk, and high risk. Currently, the National Comprehensive Cancer Network (NCCN) [11] correlates lesion size and mitotic index with a metastasis rate ranging from 0% to 86% (Table 2).
| Tumor Size | Mitotic Rate | Predicted Biologic Behavior |
| £ 2 cm | £ 5 mitoses/50 HPFs | Metastasis rate: 0% |
| >5 mitoses/50 HPFs | Metastasis rate: 0% | |
| >2 cm to £ 5 cm | £ 5 mitoses/50 HPFs | Metastasis rate: 1.9% |
| >5 mitoses/50 HPFs | Metastasis rate: 16% | |
| >5 cm to £ 10 cm | £ 5 mitoses/50 HPFs | Metastasis rate: 3.6% |
| >5 mitoses/50 HPFs | Metastasis rate: 55% | |
| >10 cm |
£ 5 mitoses/50 HPFs | Metastasis rate: 12% |
| >5 mitoses/50 HPFs | Metastasis rate: 86% | |
| GISTs: Gastrointestinal Stromal Tumors. HPF: High-Power Fields. | ||
Primary site
Arguably, the stomach is the organ of the gastrointestinal tract with the highest prevalence of this pathology, around 60% of cases [3,10,11,15,18,19]. Less frequently, it can affect the small intestine, esophagus, colon or even the omentum [10,15]. Despite of that fact, stomach GISTs generally have the best prognosis in terms of overall and disease-free survival when compared to intestinal GISTs, for example [20]. Our patient presented a tumor that occupied the abdominal cavity in almost its entirety, making information such as: the origin of the neoplasm and what other structures could be involved as unfeasible. During exploratory laparotomy, we were able to identify the gastric body as the primary site, corroborating literature data regarding the main organ of origin for GISTs. There were no metastatic implants in liver or peritoneum.
Mitotic rate
Patients at low risk for metastasis and disease recurrence have a mitotic rate <5/50 hpb and consequently those with a rate of >5/50 hpb are at higher risk [20]. In the literature reviewed, the mitotic rate as an isolated criterion is presented in this way. Nevertheless, an isolated assessment of a single data should not be made, since the primary site, size, and mitotic index have an influence on the natural history of this pathology. In controversy, Chairat Supsamutchai et al. retrospectively analyzed prognostic factors associated with metastases or disease recurrence in 68 patients undergoing surgical resection of gastric GIST [21]. In that study they observed that lesions <5 cm and mitotic rate £ 6 HPF had longer survival than those with mitotic index >6HPF, but without statistical significance (P=0.08). Patients with lesions between 6 and 10 cm had longer survival when the mitotic rate £ 6 HPF than those with an index >6 HPF (P=0.014). For patients with tumors >10 cm, there was no statistical difference between mitotic rates £ 6 HPF and >6 HPF (P=0.42). For these authors, only the high mitotic index (>6 HPF) showed statistical significance (p=0.004) as an important predictor for metastases and/or disease recurrence.
Tumor size and symptoms
Miettinen et al. [22] retrospectively analyzed 1765 patients with gastric GIST with lesion size ranging from 0.5 to 44 cm (mean of 6 cm), being: lesions £ 2 cm in 7.5% of cases, in 38.2% of the patients had lesions between 2 and 5 cm, 29.7% had a size between 5 and 10 cm and 24.6% were >10 cm. The latter are defined in the literature as giant GISTs [10]. The size of the gastric GIST is an important prognostic factor and will be related to: the presence or absence of symptoms, potential for aggressiveness of the tumor and management. Very small GISTs, £ 2 cm, may not produce symptoms, and in 12% of cases are incidentalomas in upper digestive endoscopy (EDA) [1,22]. On the other hand, the largest ones can cause: dyspepsia, early satiety, feeling of abdominal bloating, fatigue due to anemia or even obstruction of the digestive tract [10], with digestive bleeding being the most prevalent finding in the literature [22]. Regarding giant GISTs, in several case reports found in the literature review (Table 3), the most common symptom was abdominal pain. Probably a clinical finding directly related to the size of the tumors. Our patient had a lesion measuring 35 cm in its largest diameter, weighing 12.88 kg (Figure 7) and presenting no clinical symptoms or laboratory abnormalities. We believe that the slow, indolent growth of the tumor has caused an adaptation and, therefore, it is in the 5% of patients who have giant GIST (abdominal mass) and who are described in the literature as asymptomatic [14].
| References | Year | Anatomic location | Symptoms | Size (cm) | Weight (Kg) |
| Kitabayashi et al. [23] | 2001 | Stomach | Hemoperitoneum | 15 × 11 × 4.4 | |
| Kimura et al. [24] | 2004 | Stomach | Constipation | 20 | |
| Mehta et al. [25] | 2005 | Stomach | Abdominal pain | 13 x 10 | |
| Dal Corso et al. [26] | 2007 | Stomach | Anemia | 17 x 13 x 9 | 1.630 |
| Cruz Jr et al. [27] | 2008 | Stomach | Abdominal pain | 32 × 25 × 21 | 3.750 |
| Funahashi et al. [28] | 2008 | Stomach | Abdominal distension | 25 × 18 × 11 | |
| Cappellani et al. [29] | 2013 | Stomach | Abdominal distension | 37 × 24 × 13 | 8.5 |
| Colovic et al. [13] | 2013 | Stomach | Abdominal fullness | 20.5 × 16 | |
| Notani et al. [30] | 2013 | Stomach | Abdominal fullness | 22 | |
| Skandalos et al. [31] | 2013 | Stomach | Abdominal pain | 10.77 × 9.67 | |
| Schneider et al. [32] | 2014 | Stomach | Abdominal pain | 19 × 18 × 16 | 2.6 |
| A. Koyuncuer et al. [10] | 2015 | Stomach | Abdominal pain | 39 × 27 × 14 | 6.109 |
| Silano F. et al | 2014 | Stomach | Asymptomatic | 33 x 28 x 21 | 12.880 |
| kg: kilogram, cm: centimeter | |||||
Treatment
Gastric GISTs £ 2 cm in asymptomatic patients, is a safe companion?
Long-term North American observational study [33] with 1055 patients not applicable to GISTs no risk progression, even with mitotic index >5 mitoses / 50HPFs [11] metastasis rate to 0%. The European Sarcoma Group (ESMO) [34] and the Asian consensus on the diagnosis and management of gastrointestinal stromal tumors [2] classify their segment as very low risk and feasible lesions. In our clinical practice, we annually follow up with upper digestive endoscopy (EDA) for growth monitoring. Endoscopic ultrasonography (EUS) is used only at the beginning for diagnosis by biopsy and immunohistochemistry. Gastric GISTs > 2cm in asymptomatic patients, is follow-up safe? For GISTs larger than 2 cm, the literature is definitive: resection. They are considered to be at high risk for malignancy [2,11,34]. In this group of GISTs, the risk rate for metastasis increases progressively as a result of size (Table 2). In our case, the GIST was gigantic, over 30 cm, with a risk rate for metastasis in the literature between 12 and 86% [11], undoubtedly surgical. However, after extensive histopathological analysis, there was no mitotic figure in the surgical specimen.
Is there room for neoadjuvant therapy?
It is important to understand that when there is space for adjuvant there will also be space for neoadjuvant therapy and gastric GIST is no exception. However, neoadjuvant chemotherapy with Imatinib Mesylate, or other secondary drug lines, is preoperatively indicated for downstage, in large tumors for which immediate surgical resection would be impossible [35-37]. Therefore, lesion biopsy and histological confirmation need to be performed. In our patient, we did not do downstage because the periphery of the lesion had several foci of necrosis with a high chance of not being successful with the biopsy and assuming the risk of rupture of the tumor capsule with the procedure. Surgical resection: Surgical resection is the main pillar for the treatment of non-metastatic gastric GIST, where R0 margins are essential for healing and prognostic criteria. GISTs ³ 2 cm, symptomatic and those with growth or predictive signs of malignancy such as: ulceration, bleeding, necrosis and heterogeneous echogenicity on endoscopic ultrasonography (EUS), form the group with formal indication for resectability [38]. The choice of method for resection takes into account the characteristics inherent to the tumor, elective or urgent surgery and trained staff. In urgent scenarios such as bleeding and mechanical obstruction of the digestive tract or elective surgery for large tumors, these are the basic indications for conventional surgery, exploratory laparotomy.
Laparoscopic surgery
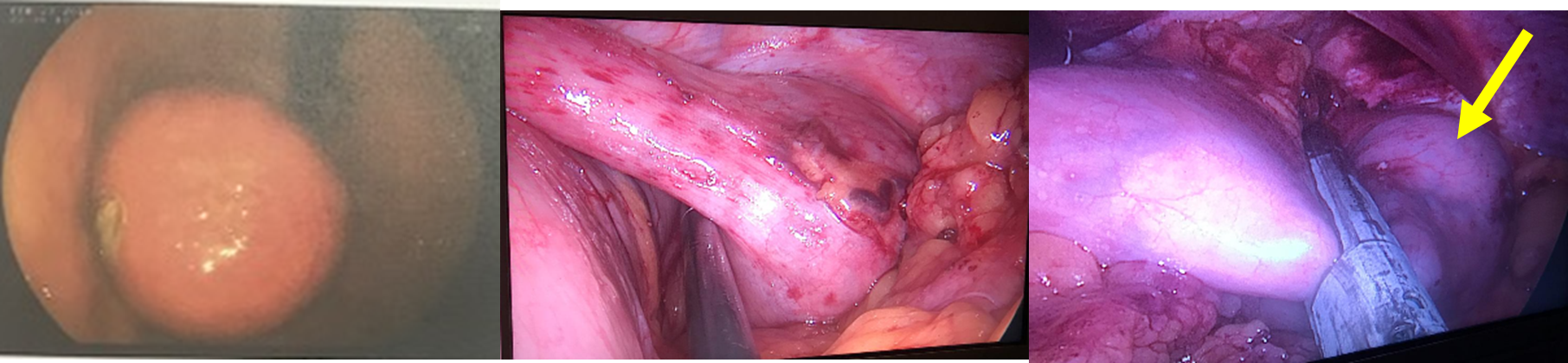
There is a discouragement in the literature for laparoscopic resections of major gastric GISTs [39]. Western consensus recommends laparoscopic resections in lesions £ 2 cm and in Asia, Japan, <5 cm [39]. Our digestive surgery service has resected several gastric GISTs using this method. In our opinion, laparoscopic surgery can be performed whenever a partial gastrectomy (wedge) with complete removal of the tumor without rupture or fragmentation is feasible, especially because there is no formal indication for lymphadenectomy in GISTs because its main dissemination is hematogenous, this being reserved if there is a suspicious ganglion in the preoperative period. We understand that the size of the lesion is a factor to always be considered, but not as a cutoff point that contraindicates the method per se. For our strategy, two points are important: type of lesion growth and the association of intraoperative endoscopy. In exophytic growth of the GIST into the abdominal cavity, even in larger lesions without invasion of other structures, wedge gastrectomy using a laparoscopic stapler and endoscopy is feasible, ensuring that the gastric wall has been fully stapled. In lesions with intraluminal growth, some scenarios are also feasible for this method of resectability (Figure 9). However, in addition to technical expertise, knowing that not only the size, but the location of the tumor can modify the approach, making wedge resection unfeasible and requiring a partial or total gastrectomy.
Figure 9. A. Lesion seen on digestive endoscopy measuring approximately 7 cm. B. Ligation and section of short vessels and greater release from the gastric fundus. C. Partial wedge transection of the gastric fundus with the entire enveloped lesion (yellow arrow).
Association between Laparoscopy and Endoscopy – LECS (Laparoscopic Endoscopic Cooperative Surgery)
LECS is a minimally invasive method widespread in Asian countries in which endoscopy plays an active role together with laparoscopy to dissect and resect the tumor. Its indication is for lesions up to 5 cm and is based on the difficulties that laparoscopy presents in wedge resections [40], such as intramural lesions. For the authors, the surgeon may find it difficult to demarcate the staple line and not remove the lesion completely [40]. Our group has no experience with this method, but we make the following considerations: In lesions close to the cardia or pylorus, SCL has superiority. The use of laparoscopic staplers in these topographies, even with intraoperative UDE, in addition to the technical difficulty, generates a high risk of stenosis. For the other locations we have used laparoscopic wedge resection plus intraoperative EDA with good results, all R0 resections. A downside for CSEC is the need to open the stomach, which increases the risk of infection.
Adjuvancy
Imatinib Mesylate has its adjuvant use standardized by a minimum of 3 in cases of completely resected GISTs but with a high risk of recurrence [41]. Tang et al. retrospectively evaluated 137 patients who received adjuvant Imatinib, 68 of which were gastric GISTs. In multivariate analysis they showed that the duration of adjuvant treatment was the only protective factor associated with disease-free survival [41]. A retrospective study at the Memorial Sloan Kettering Cancer Center, Cavnar et al. evaluated 1,000 patients who underwent surgical resection for GIST of the digestive tract, divided into non-metastatic primary tumor, synchronous metastatic and metachronic tumor. A total of 467 had primary tumor in the non-metastatic stomach. In the overall analysis of the groups, 162 received Imatinib, 86 of which were adjuvants, the majority non-gastric. For them, in the Imatinib era, tumors >10 cm presented themselves as an independent predictive factor for disease-free survival and overall survival [3]. Our patient received adjuvant therapy with Imatinib Mesylate and tumor size was the main factor considered to use the drug for more than 3 years, even without mitosis figures in the histopathological analysis.
Follow up
Our patient has been under outpatient follow-up for 7 years and during this period he used Imatinib for 18 months and stopped due to problems with the health care provider. Follow up without disease recurrence, with normal work activities and good quality of life.
Conclusion
Although the stomach is the most affected by stromal tumors, it is also generally the one with the best prognosis. Surgical treatment is the main predictor of cure, as long as surgery promotes complete resection of the tumor mass.
References
2. Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiology. 2016 Feb 1;40:39-46.
3. Cavnar MJ, Seier K, Curtin C, Balachandran VP, Coit DG, Yoon SS, et al. Outcome of 1,000 patients with gastrointestinal stromal tumor (GIST) treated by surgery in the pre-and post-imatinib eras. Annals of Surgery. 2021 Jan 1;273(1):128-38.
4. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Annals of Surgery. 2000 Jan;231(1):51-8.
5. Brainard JA, Goldblum JR. Stromal tumors of the jejunum and ileum: a clinicopathologic study of 39 cases. The American Journal of Surgical Pathology. 1997 Apr 1;21(4):407-16.
6. Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. The American Journal of Surgical Pathology. 2006 Apr 1;30(4):477-89.
7. Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. The American Journal of Surgical Pathology. 2000 Feb 1;24(2):211-22.
8. Tworek JA, Goldblum JR, Weiss SW, Greenson JK, Appelman HD. Stromal tumors of the abdominal colon: a clinicopathologic study of 20 cases. The American Journal of Surgical Pathology. 1999 Aug 1;23(8):937-45.
9. Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. The American Journal of Surgical Pathology. 2003 May 1;27(5):625-41.
10. Koyuncuer A, Gönlüşen L, Kutsal AV. A rare case of giant gastrointestinal stromal tumor of the stomach involving the serosal surface. International Journal of Surgery Case Reports. 2015 Jan 1;12:90-4.
11. Gastrointestinal Stromal Tumors. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Version 1.2021 – October 30, 2020. NCCN.org.
12. Nishida T, Goto O, Raut CP, Yahagi N. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer. 2016 Oct 15;122(20):3110-8.
13. Colović R, Micev M, Matić S, Colović N, Grubor N, Atkinson HD. Malignant stromal tumor of the stomach with giant cystic liver metastases prior to treatment with imatinib mesylate. Vojnosanitetski Pregled. 2013;70(2):225-8.
14. 14. Gheorghe G, Bacalbasa N, Ceobanu G, Ilie M, Enache V, Constantinescu G, et al. Gastrointestinal Stromal Tumors—A Mini Review. Journal of Personalized Medicine. 2021 Aug;11(8):694.
15. Zhang H, Liu Q. Prognostic indicators for gastrointestinal stromal tumors: a review. Translational Oncology. 2020 Oct 1;13(10):100812.
16. 16. P. Rubin, GIST, EGIST, Enzinger and Weiss’s Soft Tissue Tumors, 6th ed., Saunders, Philadelphia, PA, 2013, pp. 569–588.
17. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002 May;33(5):459-65
18. Demetri GD. NCCN task force report: optimal management of patient with gastrointestinal stromal tumor (GST)-expansion and update of NCCN clinical practice guidelines. JNCCN. 2004;2(1):S1-28.
19. Coe TM, Fero KE, Fanta PT, Mallory RJ, Tang CM, Murphy JD, et al. Population-based epidemiology and mortality of small malignant gastrointestinal stromal tumors in the USA. Journal of Gastrointestinal Surgery. 2016 Jun;20(6):1132-40.
20. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. InSeminars in Diagnostic Pathology 2006 May 1 (Vol. 23, No. 2, pp. 70-83). WB Saunders.
21. Supsamutchai C, Wilasrusmee C, Hiranyatheb P, Jirasiritham J, Rakchob T, Choikrua P. A cohort study of prognostic factors associated with recurrence or metastasis of gastrointestinal stromal tumor (GIST) of stomach. Annals of Medicine and Surgery. 2018 Nov 1;35:1-5.
22. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. The American Journal of Surgical Pathology. 2005 Jan 1;29(1):52-68.
23. Kitabayashi K, Seki T, Kishimoto K, Saitoh H, Ueno K, Kita I, et al. A spontaneously ruptured gastric stromal tumor presenting as generalized peritonitis: report of a case. Surgery Today. 2001 Mar;31(4):350-4.
24. Kimura H, Yoshida T, Kinoshita S, Takahashi I. Pedunculated giant gastrointestinal stromal tumor of the stomach showing extragastric growth: report of a case. Surgery Today. 2004 Feb;34(2):159-62.
25. Mehta RM, Sudheer VO, John AK, Nandakumar RR, Dhar PS, Sudhindran S, et al. Spontaneous rupture of giant gastric stromal tumor into gastric lumen. World Journal of Surgical Oncology. 2005 Dec;3(1):11.
26. Dal Corso HM, Solej M, Nano M. Giant gastrointestinal stromal tumor of the stomach in an elderly patient. Journal of Gastrointestinal Surgery. 2007 Jun 1;11(6):804-6.
27. Cruz RJ, Vincenzi R, Ketzer BM, Cecilio AL, Cepeda LA. Spontaneous intratumoral bleeding and rupture of giant gastric stromal tumor (> 30 cm) in a young patient. World Journal of Surgical Oncology. 2008 Dec;6(1):76.
28. Funahashi H, Okada Y, Sawai H, Wakasugi T, Akamo Y, Manabe T. Complete extragastric growth in a giant gastrointestinal stromal tumor: report of a case. International Surgery. 2008 Jan 1;93(1):45-9.
29. Cappellani A, Piccolo G, Cardì F, Cavallaro A, Menzo EL, Cavallaro V, et al. Giant gastrointestinal stromal tumor (GIST) of the stomach cause of high bowel obstruction: surgical management. World Journal of Surgical Oncology. 2013 Dec;11(1):172.
30. Notani H, Kawamura T, Sato T, Hoshino A, Sato Y, Nakajima A. A case of a giant gastrointestinal stromal tumor of the stomach with extramural growth. Gan to kagaku ryoho. Cancer & Chemotherapy. 2013 Nov 1;40(12):2179-81.
31. Skandalos IK, Hotzoglou NF, Matsi KC, Pitta XA, Kamas AI. Giant extra gastrointestinal stromal tumor of lesser omentum obscuring the diagnosis of a choloperitoneum. International Journal of Surgery Case Reports. 2013 Jan 1;4(10):818-21.
32. Schneider C, Hewin DF. Resection of giant gastric GIST with a new generation ultrasonic scalpel device. World Journal of Clinical Cases: WJCC. 2014 Jan 16;2(1):9-11.
33. Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali P, Choi H, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)—update of the NCCN clinical practice guidelines. Journal of the National Comprehensive Cancer Network. 2007 May 1;5(S2):S1-S26.
34. ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology. 2012 Oct;23:vii49-55.
35. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. The Lancet. 2006 Oct 14;368(9544):1329-38.
36. Reichardt P, Blay JY, Boukovinas I, Brodowicz T, Broto JM, Casali PG, et al. Adjuvant therapy in primary GIST: state-of-the-art. Annals of Oncology. 2012 Nov 1;23(11):2776-81.
37. Joseph CP, Abaricia SN, Angelis MA, Polson K, Jones RL, Kang YK, et al. Optimal avapritinib treatment strategies for patients with metastatic or unresectable gastrointestinal stromal tumors. The Oncologist. 2021 Apr;26(4):e622-31.
38. Koo DH, Ryu MH, Kim KM, Yang HK, Sawaki A, Hirota S, et al. Asian consensus guidelines for the diagnosis and management of gastrointestinal stromal tumor. Cancer research and treatment: Official Journal of Korean Cancer Association. 2016 Oct;48(4):1155-66.
39. Mohamed A, Al Qureshi T, Rakha SM. Giant Gastrointestinal Stromal Tumors of the Stomach Successfully Treated With Laparoscopic Resection: Case Report and Literature Review. Cureus. 2021 Feb;13(2):e13584.
40. Matsuda T, Nunobe S, Kosuga T, Kawahira H, Inaki N, Kitashiro S, et al. Laparoscopic and luminal endoscopic cooperative surgery can be a standard treatment for submucosal tumors of the stomach: a retrospective multicenter study. Endoscopy. 2017 May;49(05):476-83.
41. Tang J, Zhao R, Zheng X, Xu L, Wang Y, Feng L, et al. Using the recurrence risk score by Joensuu to assess patients with gastrointestinal stromal tumor treated with adjuvant imatinib: a retrospective cohort study. Medicine. 2018 Jul;97(29):e11400.