Introduction
We discuss the pathology perspective in the manuscript titled “The efficacy and safety of endoscopic ultrasoundguided liver biopsy versus percutaneous liver biopsy in patients with chronic liver disease: a retrospective singlecenter study” [1]. In this study, the safety and efficacy of liver biopsies performed by endoscopic ultrasound (EUS-LB) were compared with those performed via the traditional percutaneous route at our Medical Center between January 2018 and August 2019. The main findings of this study were EUS-LB was found to be safe and associated with less pain, shorter hospital stay, and high diagnostic yield (93%) compared to PC-LB. In this review paper, we would like to discuss our published paper and provide an in-depth discussion on the pathology perspective of EUSLB and its diagnostic yield.
Global Burden of Chronic Liver Disease
Global burden of liver disease is enormous with approximately 2 million deaths per year worldwide either secondary to complications of cirrhosis or due to viral hepatitis and hepatocellular carcinoma [2]. There are varying estimates for individuals with chronic liver diseases as many present with cirrhosis or hepatocellular carcinoma. Based on a United States epidemiological study in the year 2017, liver disease was the 11th leading cause of death, with a reported 41,000 deaths annually [3]. Nonalcoholic fatty liver disease (NAFLD) followed by chronic infection from hepatitis C and alcoholic liver diseases account for other major causes of chronic liver disease in the United States [2,4].
Histological assessment of the liver tissue remains the cornerstone for the determination of stage of liver fibrosis in patients with chronic liver diseases. Liver biopsy is commonly used in clinical settings to aid in the diagnosis, prognosis and management of chronic liver disease [5]. Although other modalities of assessing liver fibrosis using novel biomarkers have been developed, they have been found to be inferior compared to the gold standard histologic assessment via liver biopsy [6]. The shear wave elastography or Vibration-Controlled Transient Elastography (Fibroscan), a relatively newer modality to assess liver fibrosis, has been found to have some drawbacks such as difficultly to perform in obese individuals or people with ascites [7]. Fibroscan is yet to be validated for the assessment of liver disease in certain metabolic conditions such as Wilson’s disease and hemochromatosis. Furthermore, Fibroscan is operator dependent and has shown reduced sensitivity in patients with mild to moderate fibrosis [8]. Most leading societies and experts recommend liver biopsy as the gold standard diagnostic modality to diagnose and prognosticate liver diseases [9,10].
Liver biopsy has historically been performed percutaneously without image guidance, also known as “blind biopsy”. In the past several years, there has been more reliance on image guidance to direct the needle into the liver to limit the risk of complications. Surgical LB (either laparoscopic or open) is yet another way of obtaining liver tissue. A newer method of obtaining a LB is by EUS-guidance [11]. EUS provides a high resolution image of both lobes of the liver, and a biopsy needle can be safely directed into the liver for sampling under image guidance [12]. Doppler capability of the linear echoendoscope allows intrahepatic vessels to be avoided during fine needle aspiration (FNA). Intervening structures such as loops of bowel, gallbladder, and the pleural space can be easily observed and avoided, further lowering the risk of complications. Another potential advantage of EUS-LB is that it easily and safely allows biopsy of both left and right lobes of the liver, potentially addressing concerns about sampling error [13,14]. In addition, EUS-LB uses a 19-gauge needle, which is smaller than the 16-gauge needles that have traditionally been used for transcutaneous LB [11].
Indications for Liver Biopsy
There are several indications for liver biopsy obtained by clinicians and among these are the following: (a) evaluation of abnormal liver function tests; (b) grading and staging of chronic hepatitis; (c) documentation of steatosis and its possible complications; (d) diagnosis of neoplasms; (e) establishing a tissue diagnosis of metastatic disease; (f) determination of the effects of therapy; (g) investigating causes of jaundice or pyrexia of unclear etiology; (h) evaluation of liver with the clinical findings of ascites and portal hypertension; (i) evaluation of liver for adequacy of transplantation; and (j) evaluation of liver function dysfunction after kidney, liver or bone marrow transplantation [15-17].
In the era of personalized and precision medicine, liver biopsy for tumor diagnosis presents the opportunity of further genetic and molecular analysis for targeted tumoral therapy [18,19]. Further, with the advancement of contemporary techniques in immunohistochemical and special stains, as well as molecular and genomic methods, a wide range of basic and complex clinical questions can be answered using liver biopsy material of neoplastic and nonneoplastic conditions alike. Moreover, the risk of major complications is very low (1%), and only 1%-3% require hospitalization for a complication following liver biopsy [20,21]. This is even lower when the biopsy is performed for evaluation of non-neoplastic / medical liver biopsies [22].
Methods of Obtaining Liver Biopsy
Various modalities have been employed to perform liver biopsy procedure. The commonly used techniques include (a) percutaneous (CT-or US-guided); (b) transjugular route (fluoroscopy guided); (c) endoscopic ultrasoundguided liver biopsy (EUS-LB); (d) operative wedge biopsy; (e) laparoscopic; and (f) Fine-needle aspiration [23].
Percutaneous liver biopsy
Percutaneous liver biopsy (PC-LB) dates back to early 1900s when palpation/percussion technique was commonly employed to perform the biopsy procedure in the transthoracic region, but with the advent of imaging it is currently performed using an ultrasound-guided technique to avoid puncturing adjacent organs such as the lungs, gallbladder, kidney, viscera and to reduce other complications such as bleeding. The biopsy requires training and is performed either by the gastroenterologist, hepatologist or the interventional radiologist using the imaging modalities such as Ultrasound or Computed Tomography to increase the precision and reducing the risk of complications [24]. The PC-LB involves placing the patient in the supine position with right hand above the head on the table to extend the intercostal space. At this point, the patient is asked to inhale slightly and then maximally exhale and hold his/her breath. The biopsy needle is then inserted while simultaneously applying steady suction at this point and advancing the needle into the liver followed by withdrawal of the needle to obtain the specimen.
Transjugular liver biopsy
Transjugular liver biopsy is another technique of obtaining liver tissue. It was first described in the 1964 and was later brought into clinical practice in in 1967. This technique is commonly reserved for patients in whom PC-LB is contraindicated (such as those with ascites, morbid obesity, and coagulopathy). The procedure can also be utilized to evaluate for clinically significant portal hypertension through measurement of the hepatic venous pressure gradient (HVPG). This technique is thought to be associated with less bleeding compared to PC-LB in patients with thrombocytopenia and coagulopathy. In this technique the radiologists use fluoroscopic guidance to approach the liver via the internal jugular vein. The specimens are obtained via the same mentioned needles above either the Menghini or Tru-Cut needles. This technique has been found to have lesser success rates in obtaining adequate samples and usually requires multiple passes. A major disadvantage of the transjugular liver biopsy is that the obtained tissue specimen is usually smaller and associated with higher rates of specimen fragmentation compared to PC-LB.
Endoscopic ultrasound-guided liver biopsy
The more versatile and evolving techniques involve endoscopic ultrasound-guided liver biopsy (EUS-LB; see Figure 1) which involves the use of echoendoscope and color Doppler first described in 1991. In EUSLB, the biopsy needle is introduced via a linear array echoendoscope through the transgastric/transduodenal route and a real-time endoscopic ultrasound guidance is employed to obtain liver tissue. The color Doppler feature is used to evaluate for interposed vascular structures, avoiding damaging vessels as small as 1 mm in diameter in the path of the biopsy needle. Other major advantages of EUS-LB include less pain in addition to concomitant diagnostic or therapeutic upper endoscopic procedures to visualize the esophagus, stomach, and duodenum. Both the right lobe and the left lobe of the liver can be biopsied with this technique using a 19-gauge or 22-gauge fine needle aspiration (FNA; see Figures 2A and 2B). The EUS-FNA needles have a stiff needle stylet inside a steel or Nitinol needle while the Quick-Core employs Tru-cut mechanism to obtain liver tissue. The Quick-Core needles (Cook Medical, United States) are thought to be rigid; as a result, mechanical friction limited the use of these needles. The newer models include the ProCore fine needle biopsy (FNB) (Cook Medical, United States; see Figure 3A) designed with a reverse bevel side fenestration to suction the tissue into the bevel. The SharkCore FNB needle (Medtronic, United States) is the most recent modified tip design contacting 6 cutting edge surfaces and an opposing bevel to catch tissue as it is sheared off the liver [9,11,25,26]. The SharkCore FNB and Acquire FNB needle (Boston Scientific, United States) have been shown to yield the highest specimen adequacy and is most commonly used during the procedure (see Figures 3B and 3C).
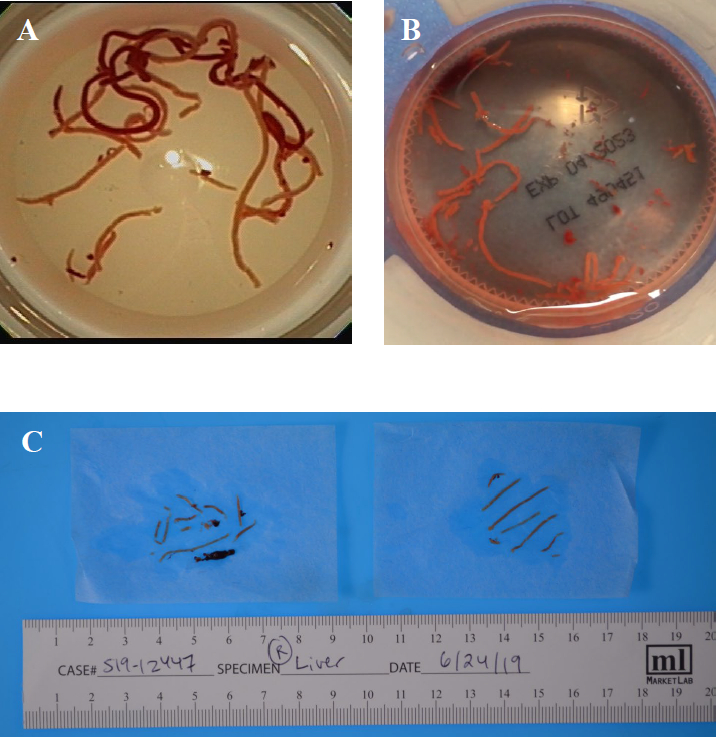
Figure 1. (A) and (B) show cores of liver tissue obtained by EUS-FNB placed in a formalin jar; and (C) shows multiple cores of liver tissue obtained by EUS-FNB separated from blood clots. Adapted from Ali et al. 2020 [1].
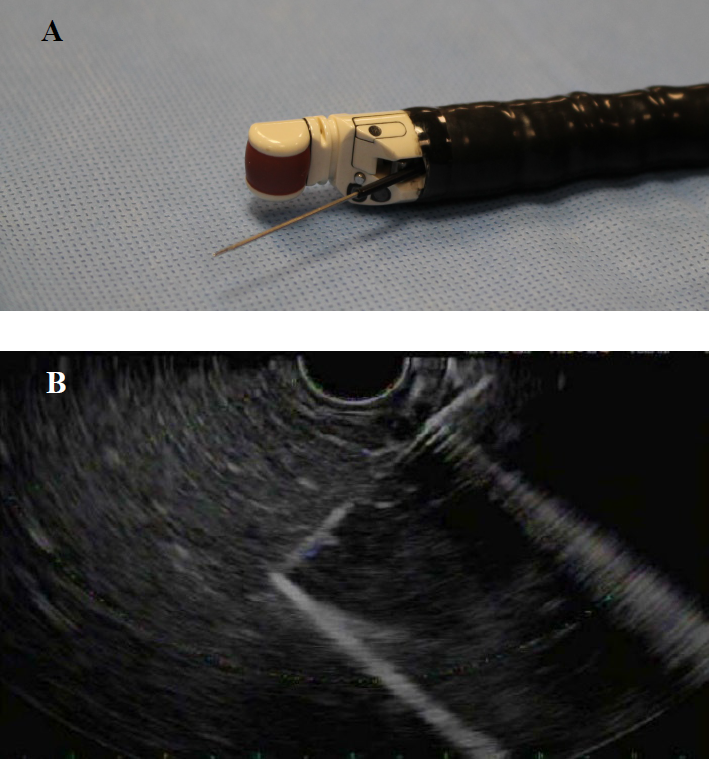
Figure 2. (A) GF-UCT180 Curvilinear Array Olympus Echoendoscope with find needle. (B) Linear echoendoscope showing fine needle biopsy of the right lobe of the liver using a SharkCore FNB needle.
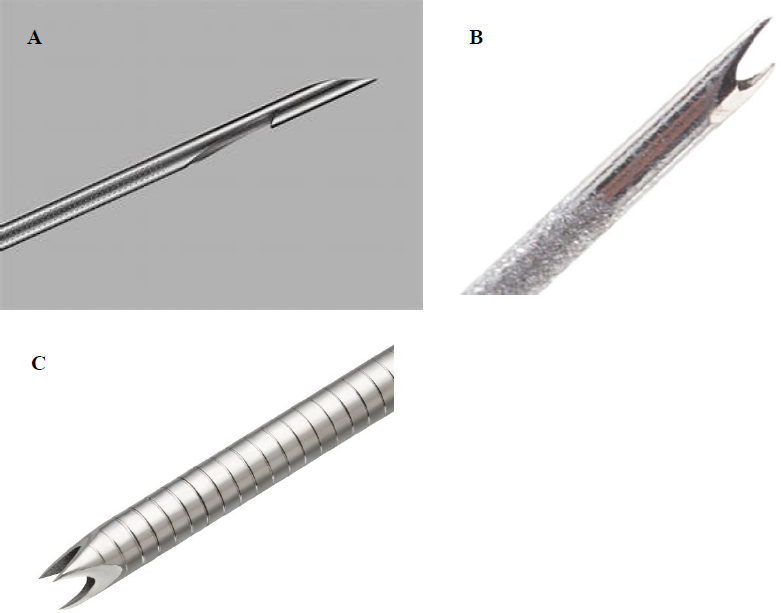
Figure 3. (A) ProCore needle (Cook Medical, United States); (B) Fork-tip SharkCore FNB needle (Medtronic, United States); and (C) Franseen-tip needle (Acquire, Boston Scientific, United States).
Types and Sizes of Needles Utilized to Obtain Liver Tissue
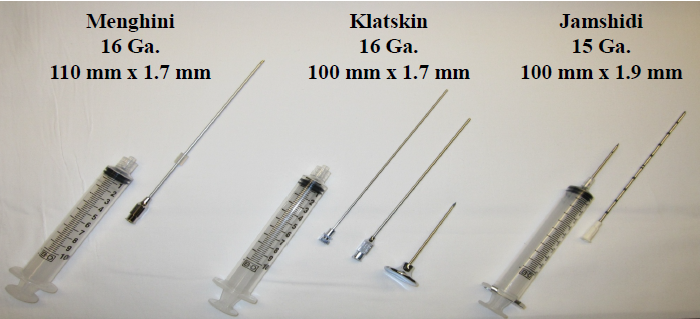
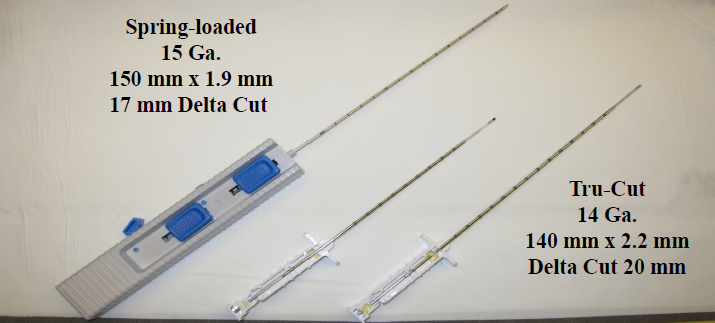
Two types of needles are commonly used in performing the PC-LB: (a) aspiration/suction type needle (Jamshidi, Klatskin and Menghini; see Figure 4), and (b) cutting-type needle (Tru-Cut, Vim-Silverman obturator and springloaded devices; see Figure 5). The aspiration/suction type needles such as the Menghini needle are mostly used by the interventional gastroenterologists. The Menghini needle is a semi-automatic device 6-8 cm in length with a retaining device in the proximal aspect to collect the liver tissue. The needle diameter is 1.4-1.6 mm in size which is used to obtain a 1.5 to 2.0 cm length samples with an average of 21 portal tracts and 7 terminal hepatic veins per specimen [27,28].
Figure 4. Aspiration/suction needles used for liver biopsy.
Figure 5. Cutting-type needles used for liver biopsy.
The Tru-Cut biopsy needle is a more automatic needle employed usually by the interventional radiologist and is a modification of the Vim-Silverman obturator and cannula. The automatic spring-loaded needles automatically trigger a rapid firing side-notch mechanism which is usually performed after the needle is advanced into the liver parenchyma. The device is able to obtain a specimen 1 mm in diameter and 1-2 cm in length [28]. Tru-Cut needles are superior to Menghini needles for diagnosis advanced fibrosis as the average length of the tissue specimen obtained is greater.
A widely quoted study using an open porcine model that assessed the impact of needle size on hemorrhage and specimen volume showed that large-caliber needles caused greater blood loss than smaller-caliber needles [29]. A recent ultrasound-guided liver biopsy study by Tublin et al. reported no difference in safety profile, rate of procedural complications and perception of pain as noted at 1-, 3-, and 24-hour post procedure using 18-gauge and 16-gauge core liver biopsies. The findings argue against the general perception of most practitioners that large-gauge needles are associated with greater risk of hemorrhage and are associated with increased pain. Some studies reported that the use of large-caliber needles were more efficient and potentially safer because fewer passes were needed to obtain equal volumes of tissue in open porcine model that assessed the impact of needle size on hemorrhage and specimen volume [30]. Further, the follow-up clinical studies attempting to address the impact of needle size on complication rates are rather difficult secondary to nonrandomized and retrospective study designs, widely varying operator experience, biopsy indications, techniques, needle types, and patient populations [31,32].
Adequate Interpretation of the Liver Biopsy
The biopsy obtained using any of the above techniques is considered adequate when the pathologist is able to make a detailed interpretation. The tissue obtained by the biopsy must be intact and should adequately demonstrate the architecture of the liver over several portal tracts. Pathologists prefer a liver tissue sample that is several centimeters in length with multiple portal tracts and hepatic veins embedded in a contiguous liver tissue; this allows the pathologist to grade and stage fibrosis in patients with chronic liver diseases with confidence [33,34]. Various scoring systems have been developed over the years to help grading (by assessing necroinflammation) and staging (by identifying the extent of fibrosis) of chronic liver disease. Recommendations on the size of the biopsy varies among different societies and scorning systems as the right amount of tissue varies among intra- and interobserver assessment of the tissue [35,36].
Hematoxylin and eosin (H&E) is the primary stain utilized in reviewing liver biopsy microscopy. However, minor structural changes are difficult to assess in sections stained with H&E alone, and thus, might indeed get missed altogether. Examination of a liver tissue with special stains are therefore often essential [37]. Although, the special stains routinely performed in addition to H&E vary according to the pathologists’ preference and individual laboratories, the minimum advised would include: (a) reticulin as reliable connective tissue stain such with its silver preparation to accurately assess the structural changes; (b) trichrome to reveal fibrosis and other structural changes not easily seen in a reticulin stained section; (c) periodic acid–Schiff with and without diastase digestion (PASD and PAS) to assess bile-duct basement membranes, activated macrophages and a rather crude but practical method to screen for α1-antitrypsin deficiency; and (d) iron for screening for iron storage diseases as well as to evaluate for bile, lipofuscin and other pigments [38-41].
Optimal biopsy length and width
It is noteworthy that several studies differ in their suggestion regarding biopsy specimen length ranging from 10 to 40 mm. Much of the supportive evidence is based on an exhaustive systematic review article pooling data from thirty-two relevant studies [42]. However, comparisons between and/or within these studies can be challenging given the widely varying study designs, patient populations, needle types and gauges, biopsy techniques, and criteria used for sample adequacy.
Colloredo et al. studied 161 liver biopsies from patients with chronic hepatitis. They recommended a sample larger than or equal to 20 mm was considered necessary for accurate evaluation [34]. In their study, they reported that the reduction in length led to an underestimation of grade and stage [34]. The shorter the specimen, the higher the rate of mild grades and mild fibrosis. Conversely, cases staged as severe both in terms of grading and staging decreased considerably among the shorter specimens [34]. Further, they also noted that reducing the diameter to 1 mm always resulted in a significant underestimation of both grade and stage, regardless of length [34]; so estimates of inflammatory activity and fibrosis based on liver biopsies of this size should be considered with great caution [23,34,43].
Another study by Bedossa et al. [44] was in agreement with the above study that liver disease staging differed on the same biopsy in two different sizes: 25 mm and 15 mm and thus, stating that larger fragments allowed for biopsy assessment with better accuracy. On the other hand, Schiano et al. analyzed 100 biopsies and did not find a significant difference in the stage of fibrosis when evaluating different sizes of the same liver specimen.
The guidelines regarding the width of the liver biopsy sample also differ. Colloredo et al. reported that thin needle biopsies (TNB) are rather inadequate and recommended biopsy sample size of ≥ 1.4 mm for accurate assessment [34]. Another study by Petz et al. came to the opposite conclusion, i.e., TNB were adequate for grading and staging chronic hepatitis [45]. On the other hand, an interventional radiology based study by Tublin et. al. noted no difference in adequacy or the mean number of portal tracts retrieved (14 vs 13) between core sizes [30].
Although more studies are needed to determine the liver biopsy specimen length and width, a perfect study to address this question is not feasible. Because, ideally, one would have to compare both TNB and long needle biopsies (LNB) in tandem biopsy set up. It is also noteworthy to mention that Colloredo et al., read a series of LNB, which were at least 3 cm long and 1.4 mm wide. Subsequently, these same biopsies were re-read after being made shorter and/or thinner by covering the slide with opaque paper to decrease the length or viewing the slide through a modified micrometer eyepiece to decrease the width. However, it is rather difficult to accurately simulate the TNB using LNB sample since the tissue from most LNB presents a curved profile on the slide. A reasonable possibility in this regard would be to employ deep learning-based algorithms on whole slide imaging of the LNB specimen to subtract the outer rim of a LNB until it reached the proportion of a TNB and compare the readings. Further, addressing the interobserver and intra-observer variabilities with a number of pathologists with and without expertise in liver disease diagnosis, and kappa statistics evaluation of results that were lacking in the above studies would be preferable.
Optimal number of portal tracts
The number of well-formed portal tracts (PT) in the liver biopsy specimen is important for adequate interpretation of liver biopsy. However, attention should be drawn to the fact that the vast majority of clinical trials in chronic hepatitis did not mention the number of portal tracts represented in the biopsy. Some studies have suggested a minimum of 4 to 6 PT per liver tissue biopsy are required to be considered acceptable [27,34,45], whereas others suggested at least 11 PT for making a meaningful evaluation of chronic hepatitis. Interestingly, studies also noted that number of complete PT beyond which no significant changes were observed in the severity of grade and stage was in the range of 11 to 15 [5,46-48]. In a study by Coral et al., a correlation between PT and size of fragment has been reported [48]. However, they suggested that less than 11 PT may still be suitable for assessment of adequate staging in chronic hepatitis and more than 5 PT did not adversely influence the staging [48]. Further, a systematic review by Cholongitas et al. evaluating the quality of liver biopsies demonstrated that the mean PT among the 32 included studies (total number of biopsies of 10,027) was 7.5. Thus, having a cut off of 11 PT as minimal for adequacy can be practically difficult to achieve [42].
Despite the disaccord regarding the number of PT, majority of the authors agree that the smallest number of portal tract had greatest impact on scoring for portal inflammation and fibrosis, presumably because the most inflamed or fibrotic portal tracts could be excluded from the sample. Another crucial remark noted by others is that the number of complete PT plays an important part in histological scoring in grading and staging chronic liver disease [49]. In the study by Ali et al. the Median (interquartile range) number of portal tracts obtained by EUS-guided liver biopsy were 5 (5-8) and histological diagnosis was established in 93% of cases [1]. Other recent studies showed variability of the Median complete PT ranging from 2 to 26 [50-56].
Limitations of Liver Biopsy
One of the limitations of liver biopsy interpretation by pathologist is sampling variability since a standard specimen represents only about 0.0002% of the whole liver [57]. In order to minimize sampling error, it is essential that the sample be representative of the whole liver for limiting the risk of inappropriate results biopsy. However, the optimal biopsy specimen size remains rather controversial.
The intuitive response regarding the liver biopsy sample size, by most pathologists, including hepatopathologists, is likely to be “the more the better”. However, pathologists are usually aware of clinical constraints. While several studies have focused on the relevance of intra- and interobserver variability in estimating the grade and stage of chronic hepatitis in liver biopsies [58,59], there has been a substantial lack of studies focusing on the impact of biopsy size variation on diagnostic assessment from pathologists’ perspective. The studies on this issue are mostly outdated and might not use the current and relevant semi-quantitative scoring systems utilized in grading and staging chronic liver diseases [60-64].
Conclusion Remarks
Liver biopsy is a diagnostic method widely used for staging of chronic hepatitis despite the emergence of novel noninvasive methods. EUS-guided liver biopsy has emerged as a new modality in evaluation of chronic liver disease/portal hypertension and obtaining a liver tissue for grading and staging of liver disease. However, liver biopsy is not devoid of some limitations. One of its limitations is sampling variability. In order to minimize sampling error, biopsy needs to be representative of the whole liver. The practical implications of these observations are important because the grading and staging of severity of liver disease is one of the parameters used for making therapeutic decision and thus, a number of patients whose biopsies were small may have been precluded from treatment because they were considered at low risk of progression [5,23].
EUS-guided liver biopsy is capable of providing the pathologist with adequate liver sample to reach a histopathologic diagnosis. In order to make the clinician aware of the limitations of histological interpretation, specimen size and the number of complete portal tracts should be clearly indicated in the histological report which in turn, can facilitate future research studies to address the questions of optimal biopsy sampling. Although, histopathologic diagnosis, grading, and staging of chronic liver disease are an inexact science and are sensitive to sampling issues, liver biopsy can still stand out as a crucial way of obtaining a significant amount of reliable information about the liver despite the availability of noninvasive methods. With increasing incidence of fatty liver disease and other disorders that lead to chronic liver disease and its consequent complications, the need for liver histology as part of the patients’ care is expected to rise in the future.
Funding
This work was partly supported by R01 DK113701 for Jamal A. Ibdah.
References
2. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. Journal of hepatology. 2019;70(1):151-71.
3. Heron M. Deaths: Leading Causes for 2017. Natl Vital Stat Rep. 2019;68(6):1-77.
4. Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L, Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: The multiethnic cohort. Hepatology. 2016;64(6):1969-77.
5. Neuberger J, Patel J, Caldwell H, Davies S, Hebditch V, Hollywood C, et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut. 2020;69(8):1382- 403.
6. Lai M, Afdhal NH. Liver Fibrosis Determination. Gastroenterol Clin North Am. 2019;48(2):281-9.
7. Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics. 2017;7(5):1303-29.
8. El Saadany S, Soliman H, Ziada DH, Hamisa M, Hefeda M, Selim A, et al. Fibroscan versus liver biopsy in the evaluation of response among the Egyptian HCV infected patients to treatment. The Egyptian Journal of Radiology and Nuclear Medicine. 2016;47(1):1-7.
9. Johnson KD, Laoveeravat P, Yee EU, Perisetti A, Thandassery RB, Tharian B. Endoscopic ultrasound guided liver biopsy: Recent evidence. World J Gastrointest Endosc. 2020;12(3):83-97.
10. Hollerbach S, Willert J, Topalidis T, Reiser M, Schmiegel W. Endoscopic ultrasound-guided fineneedle aspiration biopsy of liver lesions: histological and cytological assessment. Endoscopy. 2003;35(9):743-9.
11. Diehl DL, Johal AS, Khara HS, Stavropoulos SN, Al-Haddad M, Ramesh J, et al. Endoscopic ultrasoundguided liver biopsy: a multicenter experience. Endosc Int Open. 2015;3(3):E210-5.
12. Chon HK, Yang HC, Choi KH, Kim TH. Endoscopic Ultrasound-Guided Liver Biopsy Using a Core Needle for Hepatic Solid Mass. Clin Endosc. 2019;52(4):340-6.
13. Lew M, Hissong EM, Westerhoff MA, Lamps LW. Optimizing small liver biopsy specimens: a combined cytopathology and surgical pathology perspective. J Am Soc Cytopathol. 2020;9(5):405-21.
14. Jhala NC, Jhala DN, Chhieng DC, Eloubeidi MA, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration. A cytopathologist’s perspective. American journal of clinical pathology. 2003;120(3):351-67.
15. Tannapfel A, Dienes HP, Lohse AW. The indications for liver biopsy. Dtsch Arztebl Int. 2012;109(27-28):477- 83.
16. Bianchi L. [Significance of liver biopsy in the diagnostic evaluation of liver diseases (author’s transl)]. Ther Umsch. 1978;35(9):707-15.
17. Scoazec JY. [Liver biopsy: Which role in patient management?]. Ann Pathol. 2010;30(6):464-9.
18. Krzyszczyk P, Acevedo A, Davidoff EJ, Timmins LM, Marrero-Berrios I, Patel M, et al. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci). 2018;6(3-4):79-100.
19. Makki JS. Diagnostic Implication and Clinical Relevance of Ancillary Techniques in Clinical Pathology Practice. Clin Med Insights Pathol. 2016;9:5-11.
20. Takyar V, Etzion O, Heller T, Kleiner DE, Rotman Y, Ghany MG, et al. Complications of percutaneous liver biopsy with Klatskin needles: a 36-year single-centre experience. Alimentary pharmacology & therapeutics. 2017;45(5):744-53.
21. Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344(7):495-500.
22. Palmirotta R, Lovero D, Cafforio P, Felici C, Mannavola F, Pellè E, et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol. 2018;10:1758835918794630.
23. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49(3):1017-44.
24. Tapper EB, Lok AS. Use of Liver Imaging and Biopsy in Clinical Practice. N Engl J Med. 2017;377(8):756-68.
25. Eskandari A, Koo P, Bang H, Gui D, Urayama S. Comparison of Endoscopic Ultrasound Biopsy Needles for Endoscopic Ultrasound-Guided Liver Biopsy. Clin Endosc. 2019;52(4):347-52.
26. Shuja A, Alkhasawneh A, Fialho A, Fialho A, Shukri A, Harris C, et al. Comparison of EUS-guided versus percutaneous and transjugular approaches for the performance of liver biopsies. Dig Liver Dis. 2019;51(6):826-30.
27. Crawford AR, Lin XZ, Crawford JM. The normal adult human liver biopsy: a quantitative reference standard. Hepatology. 1998;28(2):323-31.
28. Al Knawy B, Shiffman M. Percutaneous liver biopsy in clinical practice. Liver Int. 2007;27(9):1166-73.
29. Plecha DM, Goodwin DW, Rowland DY, Varnes ME, Haaga JR. Liver biopsy: effects of biopsy needle caliber on bleeding and tissue recovery. Radiology. 1997;204(1):101-4.
30. Tublin ME, Blair R, Martin J, Malik S, Ruppert K, Demetris A. Prospective Study of the Impact of Liver Biopsy Core Size on Specimen Adequacy and Procedural Complications. AJR Am J Roentgenol. 2018;210(1):183-8.
31. Hegarty JE, Williams R. Liver biopsy: techniques, clinical applications, and complications. Br Med J (Clin Res Ed). 1984;288(6426):1254-6.
32. Thampanitchawong P, Piratvisuth T. Liver biopsy:complications and risk factors. World J Gastroenterol. 1999;5(4):301-4.
33. Cholongitas E, Quaglia A, Dhillon AP, Patch D, Burroughs AK. Length versus width in liver biopsies. Journal of hepatology. 2006;44(4):822-3; author reply 3-4.
34. Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. Journal of hepatology. 2003;39(2):239-44.
35. Lindh G, Weiland O, Glaumann H. The application of a numerical scoring system for evaluating the histological outcome in patients with chronic hepatitis B followed in long-term. Hepatology. 1988;8(1):98-103.
36. Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1(5):431-5.
37. Standish RA, Cholongitas E, Dhillon A, Burroughs AK, Dhillon AP. An appraisal of the histopathological assessment of liver fibrosis. Gut. 2006;55(4):569-78.
38. Alturkistani HA, Tashkandi FM, Mohammedsaleh ZM. Histological Stains: A Literature Review and Case Study. Glob J Health Sci. 2015;8(3):72-9.
39. Krishna M. Role of special stains in diagnostic liver pathology. Clin Liver Dis (Hoboken). 2013;2(Suppl 1):S8- s10.
40. Clark I, Torbenson MS. Immunohistochemistry and Special Stains in Medical Liver Pathology. Adv Anat Pathol. 2017;24(2):99-109.
41. Lefkowitch JH. Special stains in diagnostic liver pathology. Semin Diagn Pathol. 2006;23(3-4):190-8.
42. Cholongitas E, Senzolo M, Standish R, Marelli L, Quaglia A, Patch D, et al. A systematic review of the quality of liver biopsy specimens. American journal of clinical pathology. 2006;125(5):710-21.
43. Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. British Society of Gastroenterology. Gut. 1999;45 Suppl 4:Iv1-iv11.
44. Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38(6):1449-57.
45. Petz D, Klauck S, Rohl FW, Malfertheiner P, Roessner A, Rocken C. Feasibility of histological grading and staging of chronic viral hepatitis using specimens obtained by thin-needle biopsy. Virchows Arch. 2003;442(3):238-44.
46. Chan J, Alwahab Y, Tilley C, Carr N. Percutaneous medical liver core biopsies: correlation between tissue length and the number of portal tracts. J Clin Pathol. 2010;63(7):655-6.
47. Fryer E, Wang LM, Verrill C, Fleming K. How often do our liver core biopsies reach current definitions of adequacy? J Clin Pathol. 2013;66(12):1087-9.
48. Coral GP, Antunes AD, Serafini AP, Araujo FB, Mattos AA. Liver Biopsy: Importance of Specimen Size in the Diagnosis and Staging of Chronic Viral Hepatitis. Rev Inst Med Trop Sao Paulo. 2016;58:10.
49. Moreira RK, Chopp W, Washington MK. The concept of hepatic artery-bile duct parallelism in the diagnosis of ductopenia in liver biopsy samples. Am J Surg Pathol. 2011;35(3):392-403.
50. Sey MS, Al-Haddad M, Imperiale TF, McGreevy K, Lin J, DeWitt JM. EUS-guided liver biopsy for parenchymal disease: a comparison of diagnostic yield between two core biopsy needles. Gastrointest Endosc. 2016;83(2):347-52.
51. Nieto J, Khaleel H, Challita Y, Jimenez M, Baron TH, Walters L, et al. EUS-guided fine-needle core liver biopsy sampling using a novel 19-gauge needle with modified 1-pass, 1 actuation wet suction technique. Gastrointest Endosc. 2018;87(2):469-75.
52. Saab S, Phan J, Jimenez MA, Grotts JF, Walters L, Hathaway KA, et al. Endoscopic Ultrasound Liver Biopsies Accurately Predict the Presence of Fibrosis in Patients With Fatty liver. Clin Gastroenterol Hepatol. 2017;15(9):1477-8.
53. Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898-906.
54. Schulman AR, Thompson CC, Odze R, Chan WW, Ryou M. Optimizing EUS-guided liver biopsy sampling: comprehensive assessment of needle types and tissue acquisition techniques. Gastrointest Endosc. 2017;85(2):419-26.
55. Mok SRS, Diehl DL, Johal AS, Khara HS, Confer BD, Mudireddy PR, et al. Endoscopic ultrasound-guided biopsy in chronic liver disease: a randomized comparison of 19-G FNA and 22-G FNB needles. Endosc Int Open. 2019;7(1):E62-E71.
56. Bazerbachi F, Vargas EJ, Matar R, Storm AC, Mounajjed TM, Topazian MD, et al. EUS-guided core liver biopsy sampling using a 22-gauge fork-tip needle: a prospective blinded trial for histologic and lipidomic evaluation in nonalcoholic fatty liver disease. Gastrointest Endosc. 2019;90(6):926-32.
57. Skripenova S, Trainer TD, Krawitt EL, Blaszyk H. Variability of grade and stage in simultaneous paired liver biopsies in patients with hepatitis C. Journal of clinical pathology. 2007;60(3):321-4.
58. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20(1 Pt 1):15-20.
59. Younossi ZM, Gramlich T, Liu YC, Matteoni C, Petrelli M, Goldblum J, et al. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod Pathol. 1998;11(6):560-5.
60. Robert M, Sofair AN, Thomas A, Bell B, Bialek S, Corless C, et al. A comparison of hepatopathologists’ and community pathologists’ review of liver biopsy specimens from patients with hepatitis C. Clin Gastroenterol Hepatol. 2009;7(3):335-8.
61. Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97(10):2614-8.
62. Guido M, Mangia A, Faa G. Chronic viral hepatitis: the histology report. Dig Liver Dis. 2011;43 Suppl 4:S331- 43.
63. Lefkowitch JH. Liver biopsy assessment in chronic hepatitis. Arch Med Res. 2007;38(6):634-43.
64. Krishna M. Patterns of necrosis in liver disease. Clin Liver Dis (Hoboken). 2017;10(2):53-6.