Commentary
Malaria, caused by various strains of malaria parasites such as Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi, is a major threat to human health worldwide. It is estimated that around 3.3 billion people are at risk of developing this disease [1]. Recent research on the human microbiome has revealed a link between resident microbial communities and the risk of blood parasites, offering potential for microbial-based disease treatments such as probiotics [2]. The immune response plays a crucial role in the pathophysiology of malaria. While chloroquine was once the drug of choice for treating P. falciparum malaria, the emergence of resistance among this species has made it less effective [1]. Probiotics are microorganisms that provide health benefits to consumers. They are typically gram-positive bacteria, primarily isolated from gut microflora, and are known to enhance the immune response in the host. Probiotics have strain-specific effectiveness against various pathogens and can modulate intestinal microorganisms, thereby influencing immune cells and Peyer's patches cells [1]. By interacting with these cells, probiotics can stimulate the production of antibodies, such as IgA and IgM, leading to an overall boost in the immune response. Given these properties, fermented foods that contain beneficial microbes and potential probiotics may be a promising avenue for enhancing the immune response and reducing the severity of malaria.
Gut Microbiome Composition Associated with Plasmodium Infection
Recent studies have demonstrated a connection between the host microbiome and susceptibility to malaria, particularly in mammalian laboratory settings. For instance, genetically similar mice that are resistant to Plasmodium yoelii exhibit differential bacterial gene expression compared to susceptible mice [3]. Additionally, the gut microbiome of resistant mice is enriched with Lactobacillus and Bifidobacterium [4]. As example, longitudinal human stool samples collected during a high Plasmodium falciparum transmission season reveal bacterial community profiles that correlate with malaria infection risk. Individuals who are resistant to malaria exhibit a higher proportion of Bifidobacterium, Streptococcus, and Enterobacteriaceae Escherichia/Shigella [5]. Conversely, infection by Plasmodium parasites may cause dysbiosis in the microbiome, as Plasmodium berghei infection in mice is accompanied by physical changes in the intestine, including shortening and increased permeability, as well as alterations in bacterial communities [6].
In certain instances, resistance to malaria can be induced in animals that were previously susceptible through the microbiome. For instance, injecting mice with Lactobacillus casei can confer resistance to Plasmodium chabaudi, resulting in reduced parasite load and shorter infection period. Similarly, transplanting the caecal microbiome from resistant or susceptible mice to new germ-free mice can transfer resistance or risk, respectively [4]. Treating susceptible mice with antibiotics followed by yogurt probiotics containing Lactobacillus and Bifidobacterium can also lead to lower parasite infection intensity [4]. While the precise mechanism of resistance in these examples is unclear, increased levels of host immune response are associated with reduced infection severity in mice [4], indicating an interactive relationship between the microbiome, host immune system, and pathogen infection.
While the majority of microbiome research in the context of Plasmodium infection has been conducted using model organisms and humans, the extent to which microbial communities may impact parasite infection in wild populations and non-mammals remains poorly understood. To investigate the correlation between gut microbiome composition and Plasmodium infection in wild birds, Rohrer et al. [2] conducted a study using faecal and blood samples from wild Eurasian tree sparrows (Passer montanus) in the United States. The results indicated differential abundances in the number of significantly varying bacteria, with the greatest representation within the phyla Proteobacteria and Firmicutes in Plasmodium-infected birds. These differentially abundant taxa may serve as a starting point for experimental investigations aimed at establishing the relationship between microbial abundance and Plasmodium infection [2]. In addition, recent research by Aželyt? et al. [7] has utilized the computational tool PICRUSt2 to predict functional profiles in bacterial communities in infected and healthy birds. The study found that infection with P. homocircumflexum was linked to the presence of specific degradation and biosynthesis metabolic pathways that were not present in healthy birds. Interestingly, some of the metabolic pathways that decreased in abundance in the infected group showed a significant increase in the later stages of infection. These results suggest that avian malaria parasites can have a significant impact on the assembly of bacterial communities within the host gut microbiome. Moreover, modulation of the microbiome by malaria parasites could have negative consequences for the health of the host bird. Thus, understanding the intricate interactions between birds, malaria parasites, and the microbiota may prove beneficial in identifying key microbial players and developing interventions aimed at promoting animal health.
Alpha-Gal Related Immunity: A Mechanism to Explore
In addition to its production by certain bacteria, alpha-Gal (Galα1-3Galβ1-4GlcNAc-R) is a carbohydrate moiety that is of interest in the context of malaria prevention. Alpha-Gal is a glycan epitope that is produced by many pathogens, including Plasmodium, and provokes an immune response in humans, apes, Old World monkeys, and other non-mammalian vertebrates, including birds and fishes [8-11]. Galili [12] reported that all non-immunocompromised individuals can produce antibodies against alpha-Gal. In the zebrafish model for tuberculosis, probiotic bacterial with high alpha-Gal content protect against mycobacterial infection through activation of different immune mechanisms [13].
Studies have found that individuals with high levels of alpha-Gal antibodies in their blood are less susceptible to malaria, possibly due to the recognition and termination of Plasmodium sporozoites by these antibodies [8]. These studies have confirmed that Plasmodium species produce alpha-Gal, which is present on the surface of sporozoites [8]. Higher levels of antibodies specific to alpha-Gal have been observed in humans with greater resistance to Plasmodium infection, but these antibody levels do not predict febrile malaria outcomes [8]. Notably, in humanized mice lacking the ability to produce alpha-Gal, treatment with antibiotics and inoculation with Escherichia coli strain O86:B7, which exhibits high alpha-Gal levels, led to increased antibody production against this glycan and resistance to Plasmodium colonization through antibody-mediated sporozoite blockage [8]. However, further research is needed to identify specific mechanisms linking the microbiome to host immune response and Plasmodium risk.
One possible approach for malaria prevention involves the development of a vaccine that targets alpha-Gal and stimulates the immune system, or the use of microbes that produce alpha-Gal to stimulate an immunological response in humans [14] (Figure 1). It is also possible that the association between the microbiome and Plasmodium infection is related to bacterial priming of the immune system before parasite invasion. Although the potential of alpha-Gal as a strategy for malaria prevention is promising, more research is required to fully understand its effectiveness and safety in humans.
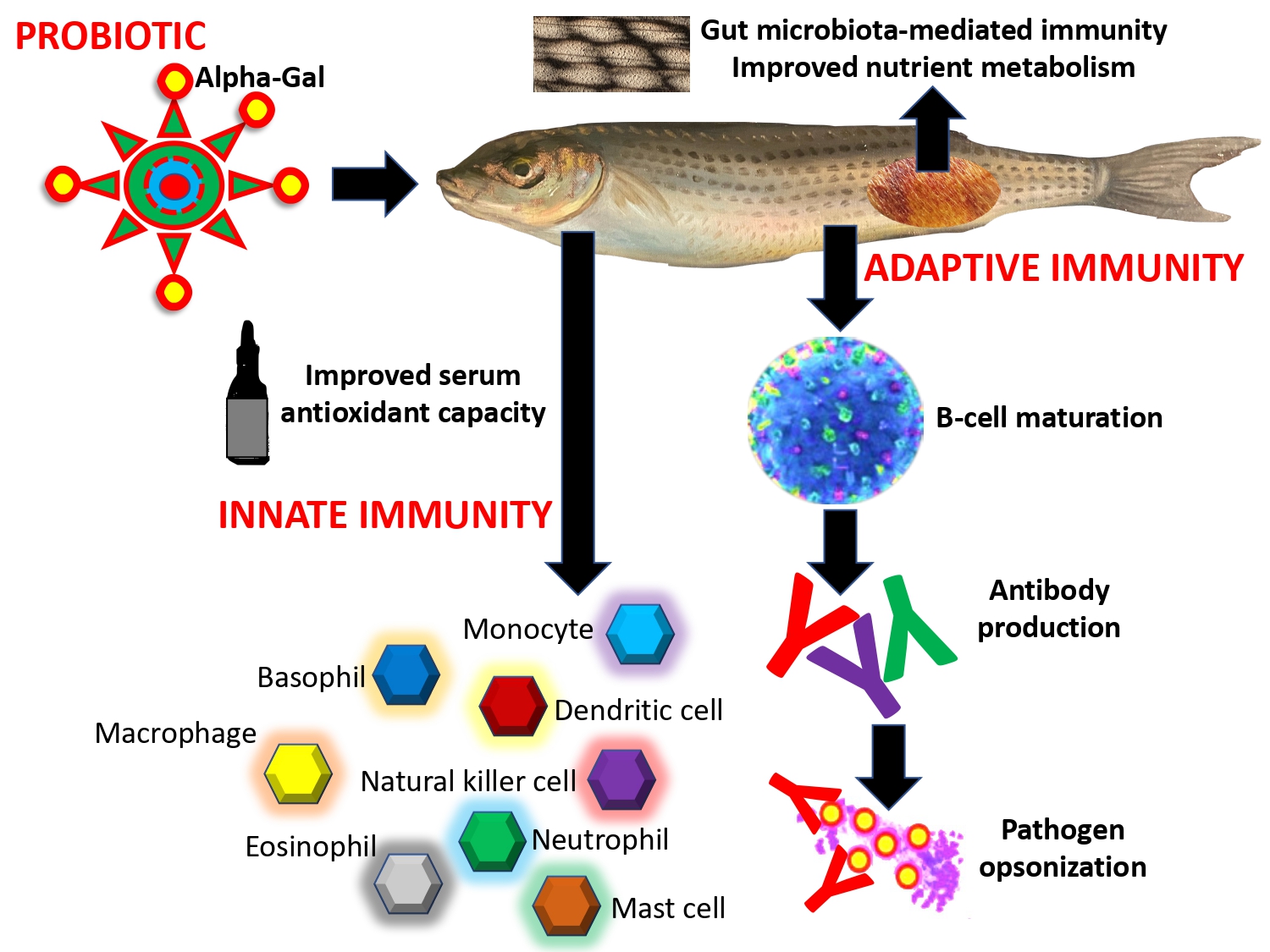
Figure 1. Example of the protective mechanisms regulating immunity and metabolism activated in response to probiotics with high α-Gal content. Results were obtained in the zebrafish animal model [13].
Functional Food to Change Microbiota Composition for Malaria Prevention
In order to modulate the gut microbiome and potentially protect against malaria transmission or reduce disease severity, the development of a functional food containing probiotic organisms is worth considering [15-17]. The use of probiotics has been shown to enhance both specific and nonspecific immune responses, as evidenced by studies using in vitro systems, animal models, and human subjects. These immune-stimulating effects are thought to be achieved through mechanisms such as activation of macrophages, elevation of cytokine levels, enhancement of natural killer cell activity, and/or increases in immunoglobulin levels. Further research is needed to determine the specific probiotic strains and doses that could effectively modulate the immune response against malaria. Evidence support that probiotics can bolster immunity and improve resistance against infections, as highlighted by recent studies [18]. Various research outputs have reported success in using fermented foods to modulate gut flora, although such changes are generally observed as broad shifts in microbial populations and may not always correspond to the microorganisms present in the fermented foods. Lactic acid bacteria (LAB) are particularly appealing for probiotic applications due to their ability to modulate both innate and adaptive immune responses. They produce antimicrobial agents and can interact with intestinal epithelial cells (IECs) and dendritic cells (DCs) to inhibit the growth of pathogenic organisms, thus providing chemical and physical barriers to boost innate immunity. Moreover, they have been shown to activate antigen-specific responses, thereby improving both innate and adaptive immune functions [15].
Effect of Probiotic as Add-on Therapy with Conventional Therapy
The co-administration of L. casei, a probiotic with chloroquine in mice infected with malaria has opened a new potential treatment avenue for malaria. In a three-day treatment, administering L. casei at a dosage of 0.1 ml resulted in lower serum alanine aminotransferase activities compared to the control group, indicating an improvement in liver function which was further confirmed by rat serum toxicological data. The study showed that the combination of probiotic and conventional drug therapy significantly reduced the parasitaemia rate, as well as the histopathological damage caused by the Plasmodium parasite in organs such as the liver and spleen. The study also found that administering L. casei with chloroquine resulted in a greater decrease in parasitaemia count and maximum suppression of parasite growth. Prior to treatment, blood film microscopic examination revealed a parasitaemia rate of less than 2%, which was cleared by the third day of treatment with chloroquine and L. casei. However, further investigation is needed through well-designed clinical trials to confirm the potential benefits of probiotics as an add-on therapy for malaria treatment [1].
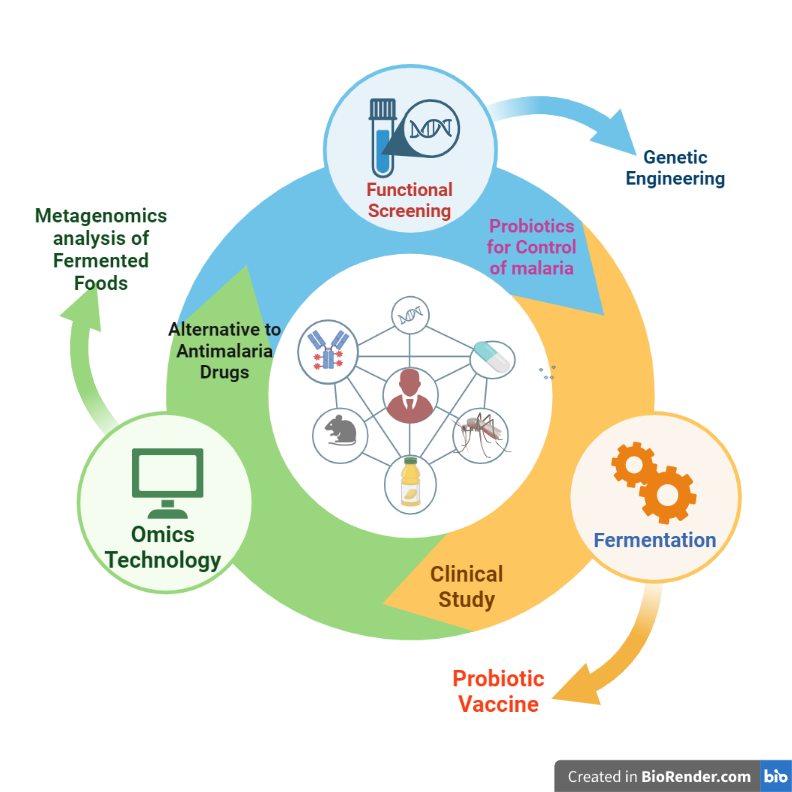
Graphical Abstract depicting the workflow for the possible selection of lactic acid bacteria (LAB) from fermented foods with desired probiotic properties for malaria prevention and treatment and vaccine discovery for infectious diseases. The approach will involve the use of omics technologies, including metagenomics and molecular identification based on 16S rRNA, to isolate and identify specific genera of LAB. The isolated LAB should be evaluated for their probiotic properties in vitro and in vivo, including the detection of probiotic marker genes and their ability to modify the gut microbiota composition and stimulate immune responses. The study can also investigate the expression of antimalarial peptides in genetically engineered LAB for the development of a probiotic-based vaccine after clinical studies as a means to develop alternatives to antimalarial drugs.
Conclusions
In recent years, the human gut microbiome has become a popular area of research due to increasing evidence suggesting its impact on physical health and its connection to various metabolic disorders. Lifestyle factors, such as nutrition, can influence the composition of gut microbiota, and there is increasing interest in using food to modify the gut microbiota in a beneficial way. Although the mechanisms behind the action of probiotics in enhancing the immune system's response to malaria is yet to be fully elucidated, their use holds great promise. Therefore, it is crucial to conduct further clinical studies with specific protocols to better understand the molecular mechanisms of probiotics in malaria prevention and their clinical application.
References
2. Bamgbose T, Alberdi P, Abdullahi IO, Inabo HI, Bello M, Sinha S, et al. Functional characterization of α-Gal producing lactic acid bacteria with potential probiotic properties. Scientific Reports. 2022 May 6;12(1):7484.
3. Bamgbose T, Anvikar AR, Alberdi P, Abdullahi IO, Inabo HI, Bello M, et al. Functional food for the stimulation of the immune system against malaria. Probiotics and Antimicrobial Proteins. 2021 Oct 1:13(5):1254-66.
4. Contreras M, Pacheco I, Alberdi P, Díaz-Sánchez S, Artigas-Jerónimo S, Mateos-Hernández L, et al. Allergic reactions and immunity in response to tick salivary biogenic substances and red meat consumption in the zebrafish model. Frontiers in Cellular and Infection Microbiology. 2020 Mar 10;10:78.
5. Galili U. Human natural antibodies to mammalian carbohydrate antigens as unsung heroes protecting against past, present, and future viral infections. Antibodies. 2020 Jun 22;9(2):25.
6. Gupta M, Raut R, Manandhar S, Chaudhary A, Shrestha U, Dangol S, et al. Identification and characterization of probiotics isolated from indigenous chicken (Gallus domesticus) of Nepal. PloS one. 2023 Jan 19;18(1):e0280412.
7. Hodžić A, Mateos-Hernández L, de La Fuente J, Cabezas-Cruz A. α-Gal-based vaccines: advances, opportunities, and perspectives. Trends in parasitology. 2020 Dec 1;36(12):992-1001.
8. Mahajan E, Sinha S, Bhatia A, Sehgal R, Medhi B. Evaluation of the effect of probiotic as add-on therapy with conventional therapy and alone in malaria induced mice. BMC Research Notes. 2021 Dec;14(1):1-5.
9. Pacheco I, Díaz-Sánchez S, Contreras M, Villar M, Cabezas-Cruz A, Gortázar C, et al. Probiotic bacteria with high alpha-Gal content protect zebrafish against mycobacteriosis. Pharmaceuticals. 2021 Jun 30;14(7):635.
10. Rohrer SD, Robertson BQ, Chubiz LM, Parker PG. Gut microbiome composition associated with Plasmodium infection in the Eurasian tree sparrow. Journal of Avian Biology. 2023 Jan:e03027.
11. Shah AM, Tarfeen N, Mohamed H, Song Y. Fermented Foods: Their Health-Promoting Components and Potential Effects on Gut Microbiota. Fermentation. 2023 Jan 26;9(2):118.
12. Stough JM, Dearth SP, Denny JE, LeCleir GR, Schmidt NW, Campagna SR, et al. Functional characteristics of the gut microbiome in C57BL/6 mice differentially susceptible to Plasmodium yoelii. Frontiers in Microbiology. 2016 Sep 27;7:1520.
13. Taniguchi T, Miyauchi E, Nakamura S, Hirai M, Suzue K, Imai T, et al. Plasmodium berghei ANKA causes intestinal malaria associated with dysbiosis. Scientific Reports. 2015 Oct 27;5(1):15699.
14. Thorel M, Mateos-Hernandez L, Mulot B, Azzouni MN, Hodžić A, Gaillot H, et al. Assessment of the Safety and Efficacy of an Oral Probiotic-Based Vaccine Against Aspergillus Infection in Captive-Bred Humboldt Penguins (Spheniscus humboldti). Frontiers in Immunology. 2022:1975.
15. Villarino NF, LeCleir GR, Denny JE, Dearth SP, Harding CL, Sloan SS, et al. Composition of the gut microbiota modulates the severity of malaria. Proceedings of the National Academy of Sciences. 2016 Feb 23;113(8):2235-40.
16. Yilmaz B, Portugal S, Tran TM, Gozzelino R, Ramos S, Gomes J, et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell. 2014 Dec 4;159(6):1277-89.
17. Yooseph S, Kirkness EF, Tran TM, Harkins DM, Jones MB, Torralba MG, et al. Stool microbiota composition is associated with the prospective risk of Plasmodium falciparum infection. BMC Genomics. 2015 Dec;16(1):1-15.
18. Vaz-Rodrigues R, Mazuecos L, Villar M, Urra JM, Gortázar C, de la Fuente J. Serum biomarkers for nutritional status as predictors in COVID-19 patients before and after vaccination. Journal of Functional Foods. 2023 Jan 10:105412.
