Abstract
Biological cells are sensitive to both pathological and non-pathological stress, which can activate the innate immune response. This activation often involves the release of heat shock proteins (HSPs), such as HSPA1A and HSPB1. However, the implications and relationships between HSP release and immune system activation remain unclear. While evidence suggests that HSPs play a role in immune system activation and regulation, their immune regulatory characteristics are still debated.
Antigen-presenting cells (APCs) are a vital component of the immune system, utilizing recognition receptors such as toll-like receptors (TLRs). There is evidence that HSPs interact with CD91 and CD40 to activate the immune system. However, the potential promiscuity of HSP interactions with recognition receptors raises questions about their biological function.
To investigate additional receptors involved in HSP-induced cytokine secretion, either HSPA1A or HSPB1 was exposed to differentiated U937 cells. U937 differentiated cells, along with HSPA1A or HSPB1, were preincubated with TLR2, TLR4, TLR5, and TLR7 blocking peptides. Subsequently, HSP or HSP-blocking peptide mixtures were applied to the cells, followed by the measurement of secreted IL-1β, TNF-α, and IL-10 via ELISA. The results showed a partial decrease in the HSPA1A and HSPB1-induced cytokine secretion with the blocking peptides. Indicating, some level of HSPA1A and HSPB1 interaction with the TLR2, TLR4, TLR5, and TLR7 in differentiated monocytic U937 cells.
Keywords
Immune response, Cytokine, Differentiated U937 cells, HSPA1A, HSPB1, TLR2, TLR4, TLR5, TLR7
Introduction
Exogenous molecules such as lipopolysaccharide (LPS) and lipoteichoic acid (LTA) are well-known for their ability to elicit an immune response [1]. Evidence has shown that certain natural endogenous molecules such as heat shock proteins (HSPs) can exhibit similar biological properties following cell stress [2]. Among these endogenous HSP molecules are HSPA1A (HSP72) and HSPB1 (HSP27), which are pivotal members of the heat shock protein family known to activate the immune system [3]. When cells experience stress, endogenous molecules can escape into the exogenous environment, interacting with cell receptors such as toll-like receptors (TLRs), leading to an immune response [4]. This observation is suggestive of the possible role of extracellular HSPs including HSPA1A and HSPB1 to modulate immune response [5]. This suggestion of HSPs function as an immune modulating protein agrees with several studies [6,7]. HSPs have been reported to activate immune system by acting as a danger signal or alarm proteins [2]. This has been shown to be possible by their interaction with receptor proteins such as TLRs [2]. While several receptors such as CD91, CD40, TLR2, and TLR4 have been demonstrated in the HSPs-induced cytokine secretion [8,9]. What is unclear however, is whether those are the only receptors employed by HSPs to activate cytokine secretion. HSPs such as HSPA1A and HSPB1 have been reported as promiscuous in their interaction with receptors [5]. This can be attributed to the type of cells involved, which may have an impact on the type of receptor protein available for HSPs interaction [5]. For example, evidence has shown that the absence of the CD14 receptor protein in naïve U937 cells is due to the lack of CD14 gene transcription and translation [10]. However, activation of U937 cells by PMA has been shown to induce CD14 gene activation, resulting in its transcription and translation [5,10]. This could explain why, in addition to TLRs, CD14 is expressed in differentiated U937 cells [5]. Therefore, the complex interactions between HSPs and TLRs have been proposed to form the primary immunological basis for molecular communication in both the activation and regulation of immune responses [11,12]. These HSPs have emerged as key regulators, orchestrating cellular responses to stress, and their interaction with TLRs adds layer of complexity to the finely tuned immune system [13,14]. It is well-established that microorganisms, such as bacteria and their products, can trigger immune responses [1]. However, a long-standing issue has been the potential contamination of human HSPs expressed in E. coli, which has raised concerns about whether cytokine secretion is induced by the HSPs themselves or by bacterial components like LPS [15]. This debate has affected our understanding of HSPs biological functions in immune activation. However, our previous research and other studies have shown that HSPs, such as HSPA1A and HSPB1, can induce cytokine secretion independently of LPS contamination [5]. To gain better understanding into the HSPs role as danger signal molecules, it is important to explore receptor proteins not exclusive to E. coli. This study specifically investigated TLRs that recognize antigens from various microbial sources. For instance, TLR2 detects LTA, lipoproteins, and peptidoglycan [16,17], while TLR4 responds to LPS [18,19], TLR5 to bacterial flagellin [20], and TLR7 to single-stranded RNA [21].
Exploring the role of HSPs such as HSPA1A and HSPB1 biological activities in immune regulation can help manipulate the immune system therapeutically.
Interestingly, HSPs were initially reported to function as an intracellular protein that facilitates protein folding [22]. Later, it became clear that these stress proteins can be secreted to the extracellular environment in cell stress to act as a danger signaling biomarker [2,22]. Explaining why extracellular HSPs activate immune response against adverse cellular conditions [22]. Evidence showed that HSPs function as an immunomodulatory protein due to their role in the activation of both pro-inflammatory and anti-inflammatory immune responses [5,22].
HSPA1A is a vital inducible member of the HSP70 family, and HSPB1, which belongs to the small HSP family, are known for their multifaceted roles in cellular homeostasis and stress adaptation [23,24]. Recent studies have shown their interactions with TLRs such as TLR2, TLR4, TLR5, and TLR7 [25,26,27]. TLRs are the integral components of the innate immune system, which serve as alarm proteins for detecting microbial pathogens and endogenous danger signals [5,27]. The convergence of HSPA1A and HSPB1 with these TLRs signifies a crucial connection where stress response pathways intersect with immune signaling, influencing the outcome of host defense and inflammatory processes [5,8]. Extracellular HSPA1A and HSPB1 activates host immune and inflammatory responses by interacting with these TLRs such as TLR2 and TLR4, through signaling pathways dependent on myeloid differentiation factor 88 (MyD88), this in turn activates nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK), leading to the release of pro-inflammatory cytokines [28,29]. Conversely, HSPA1A and HSPB1 bind to endocytic receptors such as CD91 and TLRs like TLR4, which facilitates their endocytosis and grants them access to antigen- presenting cell (APC) pathways. Once internalized, these proteins can modulate the APC phenotype towards a more tolerogenic state, promoting the production of the anti-inflammatory cytokine IL-10. This change will then lead to immunosuppression by dampening excessive immune responses [30,31]. Therefore, blocking peptides can bind specific receptors following incubation with cells [32].
The U937 cell line is a human monocyte cell line capable of differentiating into macrophage-like cells due to their monoblastic properties [33]. Phorbol 12-myristate 13-acetate (PMA) is used to differentiate U937 cells because it induces changes in gene expression by activating protein kinase C (PKC). This activation promotes the differentiation of U937 cells into macrophage-like cells, which function as antigen- presenting cells (APCs) [34-36]. These differentiated U937 cells have been shown to express TLRs strongly, making them an ideal model for studying the interaction between HSPs and TLRs. Several studies have supported the use of U937 cells in vitro for investigating cytokine secretion and the expression of receptor proteins such as TLRs [37-39]. This study will further explore the role of TLRs, including TLR2, TLR4, TLR5, and TLR7, in HSPA1A- and HSPB1-induced cytokine secretion [5,38]. Studies have shown that macrophages are critical components of the immune response and express these Toll-like receptors to detect and respond to various pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) [40,41]. The interplay between HSPA1A, HSPB1, and these TLRs in U937-derived macrophages offers a detailed perspective on how stress response proteins modulate the immune response, influencing the expression and functionality of TLRs [40,42].
In this study, we examined the interactions between HSPA1A and HSPB1 with TLR2, TLR4, TLR5, and TLR7, and their role in modulating the immune response. These TLRs are known to recognize various exogenous molecules, such as lipoteichoic acid (LTA) and lipopolysaccharide (LPS) via TLR2 and TLR4, respectively, as well as viral components [43-46], flagellin via TLR5 [47], and viral or parasitic components via TLR7 [45]. HSPs, including HSPA1A and HSPB1, have been shown to induce cytokine secretion through interactions with various receptors, such as TLRs, which this study aims to further investigate [25,26,48]. A study by Ogbodo et al. [5], reported the potential interaction of HSPA1A and HSPB1 with several receptor proteins, including TLRs. Understanding these interactions provides valuable insights into the modulation of immune responses, offering potential avenues for therapeutic interventions in conditions characterized by dysregulated stress and inflammation. This study aims to investigate HSPA1A and HBPB1 interaction with TLR2, TLR4, TLR5, and TLR7 in activation of cytokine secretion (TNF-α, IL-1β, IL-10) in macrophage-like differentiated U937 cells.
Material and Methods
Cell cultures
U937 macrophages (differentiated U937 cells) from ATCC (CRL-1593.2) were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) at 37oC in a humidified atmosphere of 5% CO2. Cells were regularly tested for viability using the trypan blue exclusion method.
U937 cells differentiation
Naïve U937 cells, actively growing in the log phase and with >95% cells viability were centrifuged at 500g for 5 minutes at 25oC, and the supernatant was discarded. Cells were then re-suspended in fresh RPMI 1640 with 10% heat-inactivated FBS (10% HI- RPMI). Cells were washed three times in 10% HI-RPMI and then re-suspended in HI- RPMI before treatment with 10 ng/ml of phorbol 12-myristate 13-acetate (PMA) (Sigma-Adrich Merck United Kingdom) for 24 h. Cell differentiation was monitored through morphological changes using light microscopy. The differentiated cells were adherent, formed clusters, and were larger in size than the undifferentiated cells.
Preparation of cells for treatment
Viable cells cultured in 10% FBS and RPMI 1640 were transferred into a centrifuge tube and centrifuged at 500g for 5 minutes at 25oC. The supernatant was removed and discarded. The cell pellet was gently resuspended in 10% HI-RPMI at a density of 5 x 105 cells/ml for U937 cells washed three times. Before resuspending the cell pellet in 10% HI-RPMI ready for experimental treatment.
Cell viability was determined using trypan blue exclusion dye. There was >90% viable cells in both naïve and differentiated U937 cells upon either HSPA1A or HSPB1 incubation and after HSPA1A or HSPB1 incubation.
The treatment of differentiated U937 cells with TLR blocking peptides
Treatments included the addition of 20 µg/ml of TLR2, TLR4, TLR5, and TLR7 blocking peptides (Sigma-Adrich United Kingdom) diluted in 10% HI-RPMI and preincubated for 2 h with differentiated U937 cells before applying either HSPA1A or HSPB1. Also, HSPA1A or HSPB1 were preincubated for 2 h with blocking peptides before applied to the differentiated U937 monocytic cells and incubated for 6 h. There were no observable morphological changes in differentiated U937 cells following co-treatment with HSPs and blocking peptides, indicating that the treatments did not affect cell structure or viability under these conditions. Incubation of 10 µg/ml exogenous HSPs with cell line including U397 cells for 15 – 30 minutes showed activation of cytokine secretion following their interaction with these extracellular receptors [49]. Similar responses were seen in other studies [48,50,51]. Blocking peptides are used in this study because they competitively block protein-protein interaction, by mimicking protein-specific binding sites [52]. Therefore, blocking peptides can bind specific receptors following incubation with cells. This can limit molecular interaction with the exogenous receptors and further indicate molecular specificity to the extracellular receptors [53]. To determine the effects of direct HSPA1A and HSPB1 binding to specific receptors, this study measured cytokine secretion across several treatment conditions: untreated cells, cells treated with HSPs alone (without blocking peptides), and cells treated with blocking peptides alone (without HSPs). This approach ensures that any observed changes in cytokine secretion can be specifically attributed to HSPA1A and HSPB1 interactions with TLRs, rather than to nonspecific effects.
Supernatant collection and cytokine secretion by ELISA
Following cell treatment with 1000 ng/ml HSPA1A and HSPB1, the 5 x 105 cells/ml cell suspension was transferred from the tissue culture wells into a microcentrifuge tube and centrifuged at 500g for 5 minutes. The concentration of IL-1β, TNF-α and IL-10 in the cell culture supernatants were measured by ELISA kit (eBioscience) following the manufacture’s procedures. Optical density (OD) at 450 nm was determined using a Varioskanlux (Thermo-scientific) microplate plate reader. 1000 ng/ml is used in this study as our previous study showed maximum cytokine secretion at 1000 ng/ml concentration [5].
Determination of cell surface receptor proteins expression by flow cytometry
Flow cytometry was used to measure TLR2, TLR4, TLR5, TLR7 on differentiated U937 cells. 5 x 105 cells/ml differentiated U937 cells were either treated in the presence or absence of 1000 ng/ml of HSPA1A or HSPB1 and incubated for 6 h. Cells were either immune stained with or without 5 µg Anti-TLR2 (0.1 mg/ml), Anti-TLR4 (0.1 mg/ml), Anti-TLR5 (0.1 mg/ml), Anti-TLR7 (0.2 mg/ml): PE (BD BioSciences). Cells were also stained with 10 µg of 50 µg/ml of propidium iodide staining solution (BD BioSciences) for 1 hour at 4oC in the dark. Analyzed cells were gated to include only viable cells using BD AccuriTM C6 flow cytometry [54].
Statistical analysis
Data are presented as the mean ± SD, n=3. Each set of the experiments were done at least three times with three replicates undertaken for each of the independent experiments tested. Analysis was performed with GraphPad PrismTM version 7.0 (GraphPad Software, Inc, San Diego, USA). The one-way ANOVA was carried out to analyze the significance in the differences between cells treatments and control cells (10% FBS/HI-RPMI). The statistical significance level was set as *p<0.05; **p<0.01; and ***p<0.001.
Results
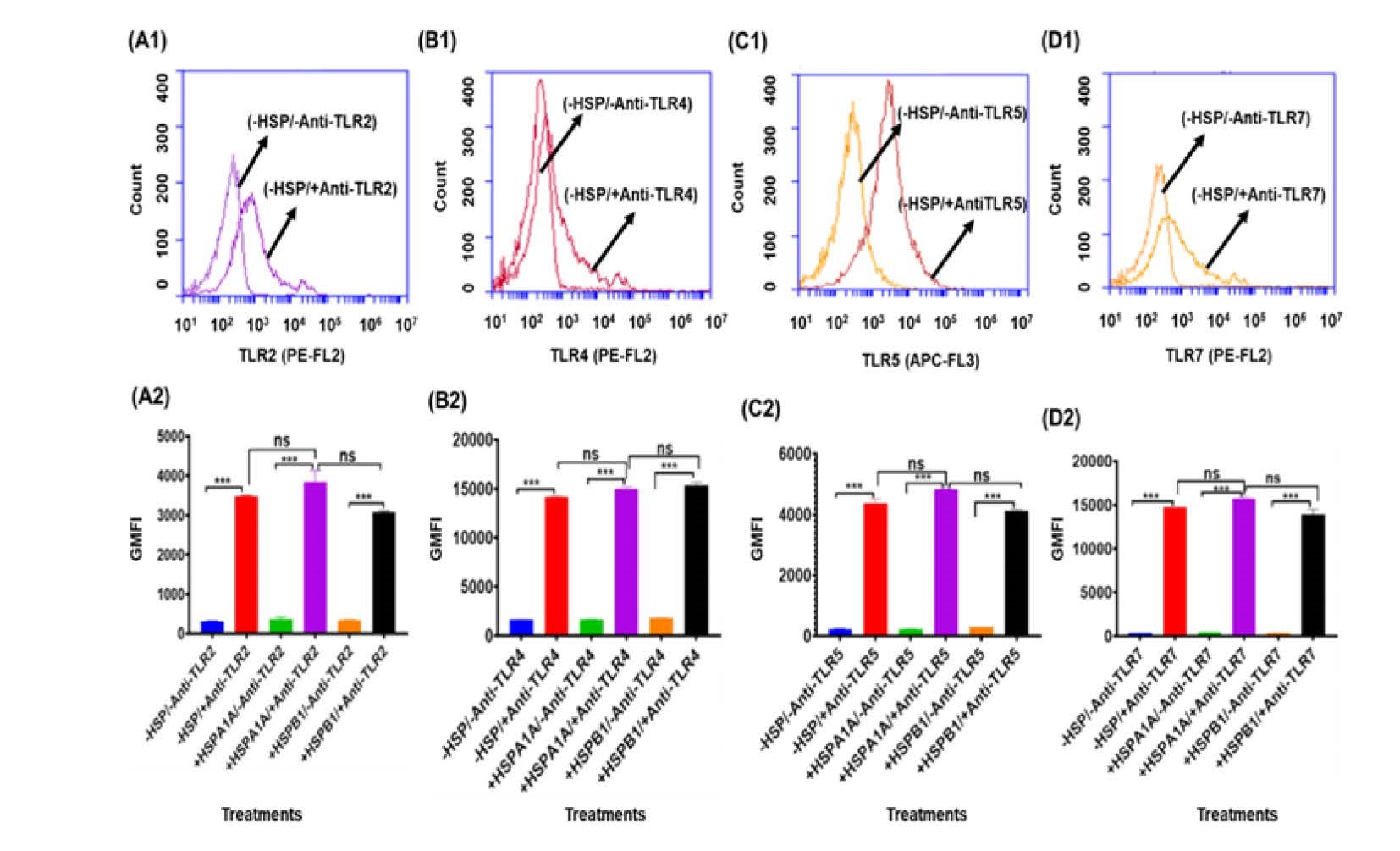
The activation of differentiated U937 cells to secrete cytokines such as TNF-α, IL-1β, and IL-10 requires the interaction of extracellular antigen receptor proteins. To determine the expression of extracellular antigens TLR2, TLR4, TLR5, and TLR7, flow cytometry was performed. Differentiated U937 cells were immuno-stained with Anti- TLR2, Anti-TLR4, Anti-TLR5, and Anti-TLR7 antibodies, and analyzed against control cells that were not immuno-stained (Figure 1: A1, B1, C1, and D1). The results showed that the antibodies effectively distinguished TLR expression compared to the control, indicating that the differentiated U937 cells were positive for TLR2, TLR4, TLR5, and TLR7 (Figure 1: A1, B1, C1, and D1). The fold difference in expression between immuno- stained and non-immuno-stained cells varied among the TLRs. Specifically, TLR2 expression showed a >10-fold difference (-HSP/-Anti-TLR2 & -HSP/+Anti-TLR2) (Figure 1: A1), TLR4 showed an 8.7-fold difference (-HSP/-Anti-TLR4 & -HSP/+Anti-TLR4) (Figure 1: B1), TLR5 showed a >10-fold difference (-HSP/-Anti-TLR5 & -HSP/+Anti- TLR5) (Figure 1: C1), and TLR7 showed a >10-fold difference (-HSP/-Anti-TLR7 & - HSP/+Anti-TLR7) (Figure 1: D1).
Figure 1. Expression of cell surface TLR2, TLR4, TLR5, and TLR7 from differentiated U937 cells. Differentiated U937 cells (1x10^6/ml) were suspended in 10% HI-RPMI and either treated or not treated with 1000 ng/ml HSPA1A or HSPB1, then incubated for 6 hours. The cell suspension was then centrifuged, and the supernatant was discarded. The cell pellet was immuno-stained with Anti-TLR2, Anti-TLR4, Anti-TLR5, and Anti-TLR7 antibodies, followed by flow cytometry analysis. The panels show the proportion of highly labelled differentiated U937 cells: Panel A1: (-HSP/-Anti-TLR2 & -HSP/+Anti- TLR2). Panel B1: (-HSP/-Anti-TLR4 & -HSP/+Anti-TLR4). Panel C1: (-HSP/-Anti-TLR5 & -HSP/+Anti- TLR5). Panel D1: (-HSP/-Anti-TLR7 & -HSP/+Anti-TLR7). These are compared to cells without immuno-staining with the respective antibodies. A similar response was observed in differentiated U937 cell pellets treated with either HSPA1A or HSPB1. Data (Panels A1, B1, C1, and D1) are representative of one experiment. Overall, the experiment was performed and analysed in three replicates. The geometric mean fluorescence intensity (GMFI) was derived from the traces in the panels (Panels A1, B1, C1, and D1) using software from the BD Accuri™ C6 Plus analyzer. Data are presented as mean ± SD, n=3, and statistical analysis was performed using one-way ANOVA with Bonferroni’s multiple comparison post hoc test. Significant differences were observed in the following comparisons: Panel A2: (-/+HSP/+Anti-TLR2 and -/+HSP/-Anti-TLR4). Panel B2: (-/+HSP/+Anti-TLR4 and -/+HSP/-Anti- TLR4). Panel C2: (-/+HSP/+Anti-TLR5 and -/+HSP/-Anti-TLR5). Panel D2: (-/+HSP/+Anti-TLR7 and -/+HSP/-Anti-TLR7). Significant differences are indicated by *** (P<0.001). No significant differences (P>0.05) were found between -HSP/+TLRs and +HSP/+TLRs.
The geometric mean fluorescence intensity (GMFI) (Figure 1: A2, B2, C2, and D2), derived from these traces (Figure 1: A1, B1, C1, and D1), shows that there was a proportion of differentiated U937 cells that were more highly labelled when compared to cells that were not immuno-stained with the Anti-TLR2, Anti-TLR4, Anti-TLR5, and Anti-TLR7 antibodies (Figure 1). These antibodies were able to distinguish the expression of TLR2, TLR4, TLR5, and TLR7 in differentiated U937 cells compared to the non immuno- stained (P<0.001) (Figure 1). The addition of HSPA1A or HSPB1 had no effect on the binding of any of the anti-TLR antibodies to cells (Figure 1). Figure 1 confirms previous studies that demonstrated the presence of TLRs, including TLR2, TLR4, TLR5, and TLR7, in monocytic cells such as U937 cells [87,88].
Following the expression analysis of TLR2, TLR4, TLR5, and TLR7 in differentiated U937 cells, the effect of TLR2, TLR4, TLR5, and TLR7 blocking peptides on HSPA1A- and HSPB1-induced TNF-α, IL-1β, and IL-10 production was investigated.
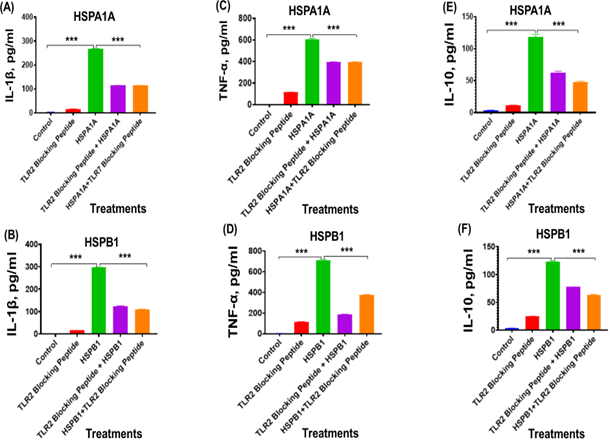
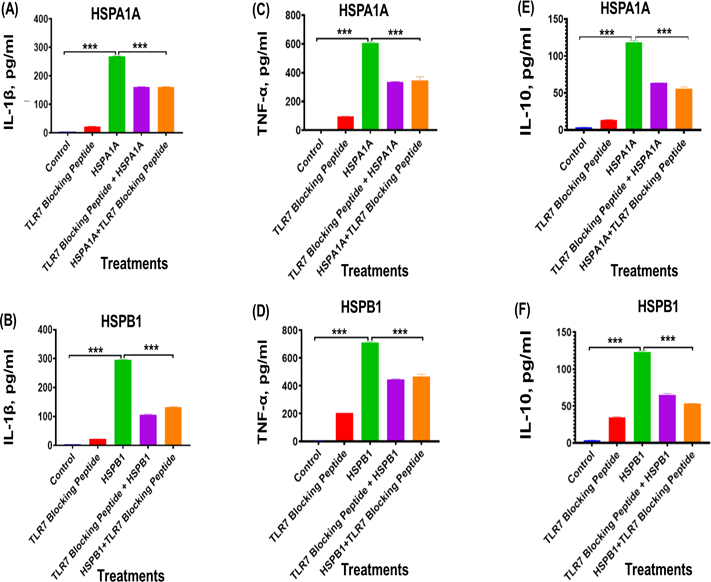
In this experiment, we investigated the effect of inhibiting surface TLRs using specific blocking peptides on HSPA1A- or HSPB1-induced cytokine production in differentiated U937 cells. Preincubation of differentiated U937 cells with TLR2 blocking peptides for 2 hours before the application of either HSPA1A (Figures 2A, 2C, and 2E) or HSPB1 (Figures 2B, 2D, and 2F) significantly decreased (P<0.001) the production of IL-1β (Figures 2A and 2B), TNF-α (Figures 2C and 2D), and IL-10 (Figures 2E and 2F), compared to cells treated with HSPA1A or HSPB1 alone (Figure 2). Specifically, the TLR2 blocking peptide significantly reduced (P<0.001) IL-1β secretion from 268.6 pg/ml with HSPA1A treatment alone to 115.0 pg/ml when co- treated with the TLR2 blocking peptide, representing approximately a 43% decrease in IL-1β secretion (Figure 2A), IL-1β secretion from 296.1 pg/ml with HSPB1 treatment alone to 124.6 pg/ml when co-treated with TLR2 blocking peptide, representing approximately a 37% decrease (Figure 2B). The treatment of TLR2 blocking peptide showed reduction (P<0.001) TNF-α secretion from 606.7 pg/ml with HSPA1A alone to 395.1 pg/ml when HSPA1A is preincubated with the TLR2 blocking peptide, indicating approximately a 65% decrease in TNF-α secretion (Figure 2C), the same treatment with HSPB1 alone showed reduction (P<0.001) in the TNF-α secretion from 710.6 pg/ml to 187.7 pg/ml when HSPB1 is co-treated with TLR2 blocking peptide, reflecting approximately 53% in TNF-α secretion (Figure 2D). The same treatment showed reduction in the IL-10 secretion from 118.8 pg/ml of HSPA1A treatment alone to 47.51 pg/ml when HSPA1A is co-treated with TLR2 blocking peptide, representing approximately 40% decrease in IL-10 secretion (Figure 2E), and similar reponse is shown in the HSPB1 treatment alone (122.8 pg/ml) to 62.95 pg/ml when HSPB1 is co-treated with TLR2 blocking peptide, which showed approximately 51% reduction in IL-10 secretion (Figure 2F).
Figure 2. The effect of TLR2 blocking peptide on HSPA1A- or HSPB1-induced IL-1β, TNF-α, and IL-10 secretion from differentiated U937 cells. Panels show results obtained from differentiated U937 cells treated with 20 µg/ml of TLR2 blocking peptide alone, incubated for 2 hours, followed by the addition of either 1000 ng/ml of HSPA1A or HSPB1. Additional results are presented for 1000 ng/ml of either HSPA1A (A, C, and E) or HSPB1 (B, D, and F) preincubated with 20 µg/ml of TLR2 blocking peptide for 2 hours before being applied to the differentiated U937 cells and incubated for 6 hours. The cell suspension was then centrifuged, and the supernatant was collected to measure IL-1β, TNF-α, and IL- 10 secretion into the media by ELISA. Data are presented as mean ± SD, n=3, and analysed by one- way ANOVA with Bonferroni’s multiple comparison post hoc test. Significant differences between either HSPA1A or HSPB1 and (HSPA1A+TLR2 or HSPB1+TLR2), (TLR2 blocking peptide only), and control (HI-RPMI) treatments are indicated by ** (P<0.01) and *** (P<0.001).
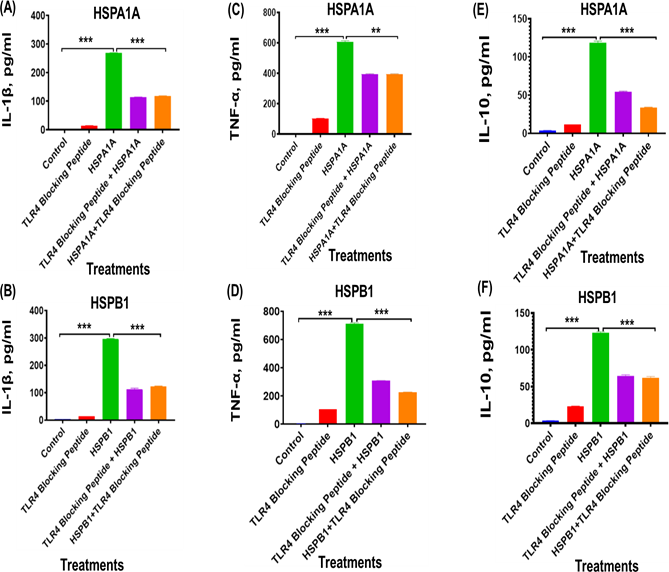
Similarly, treatment with TLR4 blocking peptides resulted in a significant (P<0.001 to P<0.01) reduction in IL-1β (Figures 3A, 3B), TNF-α (Figures 3C and 3D), and IL-10 (Figures 3E and 3F) secretion, compared to cells treated with HSPA1A or HSPB1 alone (Figure 3). The treatment of TLR4 blocking peptide showed reduction (P<0.001) IL-1β secretion from 268.6 pg/ml with HSPA1A treatment alone to 114.1 pg/ml when co-treated with the TLR2 blocking peptide, representing approximately a 42% decrease in IL-1β secretion (Figure 3A). The same treatment showed IL-1β secretion from 296.1 pg/ml with HSPB1 treatment alone to 112.2 pg/ml (P<0.001) when co-treated with TLR2 blocking peptide, representing approximately a 42% decrease in IL-1β secretion (Figure 3B). The treatment of TLR2 blocking peptide showed reduction (P<0.001) TNF-α secretion from 606.7 pg/ml with HSPA1A alone to 395.9 pg/ml when HSPA1A is preincubated with the TLR2 blocking peptide, indicating approximately a 65% decrease in TNF-α secretion (Figure 3C), the same treatment with HSPB1 alone showed reduction in the TNF-α secretion from 710.6 pg/ml to 225.0 pg/ml when HSPB1 is co- treated with TLR2 blocking peptide, reflecting approximately 43% in TNF-α secretion (Figure 3D). Similarly, treatment of HSPA1A alone showed reduction (P<0.001) in the IL- 10 secretion from 118.8 pg/ml to 47.51 pg/ml when HSPA1A is co-treated with TLR2 blocking peptide, representing approximately 46% decrease in IL-10 secretion (Figure 3E), and the same treatment showed 122.8 pg/ml of IL-1β secretion following cell treatment with HSPB1 alone to 61.91 pg/ml (P<0.001) when HSPB1 is co-treated with TLR2 blocking peptide, which showed approximately 52% reduction in IL-10 secretion (Figure 3F).
Figure 3. The effect of TLR4 blocking peptide on HSPA1A- or HSPB1-induced IL-1β, TNF-α, and IL-10 secretion from differentiated U937 cells. Panels show results obtained from differentiated U937 cells treated with 20 µg/ml of TLR4 blocking peptide alone, incubated for 2 hours, followed by the addition of either 1000 ng/ml of HSPA1A or HSPB1. Additional results are presented for 1000 ng/ml of either HSPA1A (A, C, and E) or HSPB1 (B, D, and F) preincubated with 20 µg/ml of TLR4 blocking peptide for 2 hours before being applied to the differentiated U937 cells and incubated for 6 hours. The cell suspension was then centrifuged, and the supernatant was collected to measure IL-1β, TNF-α, and IL- 10 secretion into the media by ELISA. Data are presented as mean ± SD, n=3, and analysed by one- way ANOVA with Bonferroni’s multiple comparison post hoc test. Significant differences between either HSPA1A or HSPB1 and (HSPA1A+TLR4 or HSPB1+TLR4), (TLR4 blocking peptide only), and control (HI-RPMI) treatments are indicated by ** (P<0.01) and *** (P<0.001).
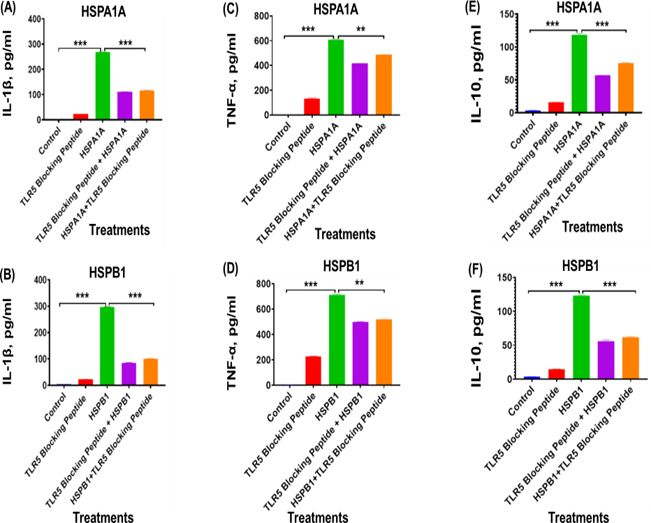
The treatment with TLR5 blocking peptide significantly (P<0.001 to P<0.01) reduced cytokines secretion in differentiated U937 IL-1β (Figures 4A and 4B), TNF-α (Figures 4C and 4D), and IL-10 (Figures 4E and 4F), compared to cells treated with HSPA1A or HSPB1 alone (Figure 4). Specifically, IL-1β levels decreased from 268.6 pg/ml (HSPA1A alone) to 110.4 pg/ml (HSPA1A with TLR4 blocking peptide), marking an approximate 41% reduction (P<0.001) (Figure 4A). Similarly, co-treatment with TLR4 blocking peptide and HSPB1 decreased IL-1β secretion from 296.1 pg/ml to 84.03 pg/ml, also aproximately 28% reduction (P<0.001) (Figure 4B). Treatment with TLR2 blocking peptide also significantly impacted TNF-α secretion. With HSPA1A, TNF-α levels dropped from 606.7 pg/ml to 415.8 pg/ml, an approximate 68% reduction (P<0.001) (Figure 4C). The co-treatment with TLR2 blocking peptide and HSPB1 similarly reduced TNF-α from 710.6 pg/ml to 498.8 pg/ml, indicating an approximately 70% decrease (P<0.001) (Figure 4D). For IL- 10, co-treatment with TLR2 blocking peptide and HSPA1A reduced secretion from 118.8 pg/ml to 56.98 pg/ml, approximately a 48% decrease (P<0.001) (Figure 4E). When HSPB1 was co-treated with TLR2 blocking peptide, IL-10 levels dropped from 122.8 pg/ml to 55.47 pg/ml, representing approximately 45% reduction (P<0.001) (Figure 4F).
Figure 4. The effect of TLR5 blocking peptide on HSPA1A- or HSPB1-induced IL-1β, TNF-α, and IL-10 secretion from differentiated U937 cells. Panels show results obtained from differentiated U937 cells treated with 20 µg/ml of TLR5 blocking peptide alone, incubated for 2 hours, followed by the addition of either 1000 ng/ml of HSPA1A or HSPB1. Additional results are presented for 1000 ng/ml of either HSPA1A (A, C, and E) or HSPB1 (B, D, and F) preincubated with 20 µg/ml of TLR5 blocking peptide for 2 hours before being applied to the differentiated U937 cells and incubated for 6 hours. The cell suspension was then centrifuged, and the supernatant was collected to measure IL-1β, TNF-α, and IL- 10 secretion into the media by ELISA. Data are presented as mean ± SD, n=3, and analysed by one- way ANOVA with Bonferroni’s multiple comparison post hoc test. Significant differences between either HSPA1A or HSPB1 and (HSPA1A+TLR5 or HSPB1+TLR5), (TLR5 blocking peptide only), and control (HI-RPMI) treatments are indicated by ** (P<0.01) and *** (P<0.001).
Treatment with TLR7 blocking peptides significantly reduced cytokine secretion, specifically IL-1β (Figures 5A and 5B), TNF-α (Figures 5C and 5D), and IL-10 (Figures 5E and 5F), in comparison to cells treated with HSPA1A or HSPB1 alone (P<0.001 to P<0.01). For IL-1β, levels decreased from 268.6 pg/ml with HSPA1A alone to 160.6 pg/ml when co-treated with TLR7 blocking peptide, representing an approximate 60% reduction (P<0.001) (Figure 5A). Similarly, IL-1β secretion decreased from 296.1 pg/ml to 106.4 pg/ml with TLR7 blocking in HSPB1-treated cells, also approximately 36% reduction (P<0.001) (Figure 5B). Regarding TNF-α secretion, HSPA1A co-treatment with TLR7 blocking peptide led to a decrease from 606.7 pg/ml to 395.9 pg/ml, indicating approximately 55% reduction (P<0.001) (Figure 5C). Co-treatment with TLR7 blocking peptide and HSPB1 resulted in a drop from 710.6 pg/ml to 468.4 pg/ml, approximately a 66% reduction (P<0.001) (Figure 5D). For IL-10, co-treatment with TLR7 blocking peptide reduced secretion from 118.8 pg/ml with HSPA1A alone to 55.61 pg/ml, representing approximately 53% reduction (P<0.001) (Figure 5E). Similarly, co-treatment of HSPB1 with TLR7 blocking peptide lowered IL-10 secretion from 122.8 pg/ml to 53.0 pg/ml, marking an approximate 51% reduction (P<0.001) (Figure 5F).
Figure 5. The effect of TLR7 blocking peptide on HSPA1A- or HSPB1-induced IL-1β, TNF-α, and IL-10 secretion from differentiated U937 cells. Panels show results obtained from differentiated U937 cells treated with 20 µg/ml of TLR7 blocking peptide alone, incubated for 2 hours, followed by the addition of either 1000 ng/ml of HSPA1A or HSPB1. Additional results are presented for 1000 ng/ml of either HSPA1A (A, C, and E) or HSPB1 (B, D, and F) preincubated with 20 µg/ml of TLR7 blocking peptide for 2 hours before being applied to the differentiated U937 cells and incubated for 6 hours. The cell suspension was then centrifuged, and the supernatant was collected to measure IL-1β, TNF-α, and IL- 10 secretion into the media by ELISA. Data are presented as mean ± SD, n=3, and analysed by one- way ANOVA with Bonferroni’s multiple comparison post hoc test. Significant differences between either HSPA1A or HSPB1 and (HSPA1A+TLR7 or HSPB1+TLR7), (TLR7 blocking peptide only), and control (HI-RPMI) treatments are indicated by ** (P<0.01) and *** (P<0.001).
Similarly, preincubation of either HSPA1A or HSPB1 with TLR2 (Figure 2), TLR4 (Figure 3), TLR5 (Figure 4), or TLR7 (Figure 5) blocking peptide before introduction to the differentiated U937 cells showed a significant decrease in cytokine secretion, when compared to differentiated U937 cells treated with either HSPA1A or HSPB1 alone (Figures 3–6). The results demonstrated variable levels of TLR binding, as indicated by the reduction in IL-1β, TNF-α, and IL-10 secretion. The differing levels of cytokine secretion observed with TLR2, TLR4, TLR5, and TLR7 blocking peptides may be attributed to variations in either the concentration of the peptides or the specific binding mechanisms involved [55,56]. The reduction in IL-1β, TNF-α, and IL-10 secretion in response to HSPA1A or HSPB1 following TLR inhibition suggests a receptor-specific interaction with the blocking peptides (Figure 3–6). Additionally, the 20 µg/ml concentration used in this study aligns with Kwon et al. [57], who reported cytokine inhibition by peptides at concentrations ranging from 12.5 to 50 µg.
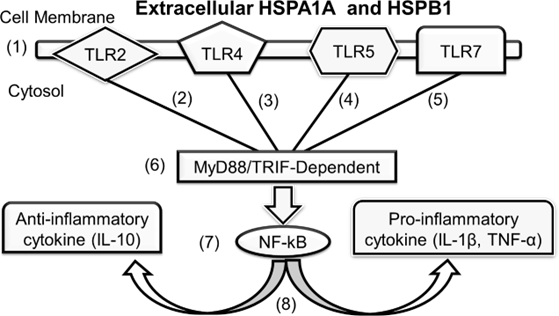
Figure 6. Possible HSPA1A and HSPB1 cytokine activation via MyD88/TRIFF. Cell stress triggers the release of HSPA1A and HSPB1 into the extracellular environment (1). These extracellular HSPA1A and HSPB1 proteins bind to (2, 3, 4, and 5) TLR proteins on the cell membrane, activating MyD88/TRIF-dependent mechanisms (6). This activation leads to NF-κB signaling (7), resulting in the release of cytokines (8) [Adapted from 85,86].
Discussion
Our study aimed to elucidate the role of heat shock proteins (HSPs), specifically HSPA1A and HSPB1, in the activation of the innate immune response via their interaction with toll-like receptors (TLRs) on differentiated U937 cells. The findings from this investigation provide significant insights into the complex mechanisms through which HSPs such as HSPA1A and HSPB1 influence immune system activation and regulation. HSPs including HSPA1A and HSPB1 have been demonstrated to activate cytokine secretion in many cells [14,40,58,59]. For example, HSPA1A has been shown to activate cytokine secretion in human bronchial epithelial cells [14], fibroblast cell [58]. Similarly, HSPB1 has been shown to activate cytokine secretion in coronary vascular endothelial cell and U937 cells [14,40]. HSPA1A and HSPB1 have been further demonstrated in ovarian cancer cells, neutrophils, monocytic cell lines such as U937 cells and human monocytes to induce cytokine secretion via TLR2 and TLR4 [8,14,40,58]. This study further investigates HSPA1A and HSPB1 in both TLR5 and TLR7.
Cytokines are widely recognized as biomarkers for inflammatory immune responses, with cells responding to immune stimuli in either a pro- or anti-inflammatory manner [60,61]. TNF-α and IL-1β are predominantly associated with pathological conditions, acting as pro-inflammatory markers, whereas IL-10 is typically involved in cellular repair and serves an anti-inflammatory role [61]. In an acute immune response, cells initially release pro-inflammatory cytokines such as TNF-α and IL-1β, initiating the primary immune response. This response subsequently triggers the release of IL-10. Thereby, facilitating inflammation resolution and tissue repair [62]. The roles of TNF-α, IL-1β, and IL-10 in modulating immune responses underscore their relevance as reliable biomarkers for inflammation [60-62]. Our previous study demonstrated that HSPA1A and HSPB1 treatments stimulate the secretion of TNF-α, IL-1β, and IL- 10, highlighting their role in immune activation [5].
Our results suggest that HSPA1A and HSPB1 interaction with TLR2, TLR4, TLR5, and TLR7 contributes to the both HSPA1A and HSPB1-induced cytokine secretion IL-1β, TNF-α, and IL-10 in differentiated U937 cells. This interaction underscores the HSPs role as an immunomodulatory protein. Previous studies have highlighted HSPs involvement in immune activation [63,64]. For example, Luo et al. [64] demonstrated anti- inflammatory effect of HSPA1A on rheumatoid arthritis (RA) by downregulating production of both IL-6, IL-8, and monocytes chemoattractant protein (MCP-1) in fibroblast-like synoviocytes, but our study extends this knowledge by investigating more interactions between HSPA1 and HSPB1 with several TLRs rather than focusing on individual receptors [8,14,59,65]. TLR-blocking peptides have been widely used in several studies to explore the role of TLRs in cytokine secretion activation. For instance, TLR2, TLR4, TLR5, and TLR7 have been shown to reduce cytokine secretion levels in monocytic cells and macrophages [66-69]. These studies provide strong evidence supporting the effectiveness of TLR-blocking peptides, which are therefore utilised in this study. By incubating TLR-blocking peptides with either U937 macrophages or HSPs, interactions can be assessed, and a reduction in cytokine secretion suggest specific binding between the TLR-blocking peptides, the cells, and HSPs. Thereby, confirm the presence of these TLRs on the cell membrane and their role in activating signalling pathways that lead to cytokine production [40,70].
Interestingly, there are little or no differences between the HSPA1A and HSPB1 interaction with TLR2, TLR4, TLR5, and TLR7 blocking peptides (Figures 2-5). Also, each of these TLRs showed a similar reduction in cytokine secretion for both HSPA1A- and HSPB1-induced cytokine responses. This suggests that these HSPs may interact with multiple receptor proteins such as CD91 and CD40 or may be working in conjunction with TLRs in the activation of cytokines via MyD88 [71], which could explain why blocking a single TLR did not completely inhibit the cytokine effects of HSPA1A and HSPB1. For example, while blocking a specific receptor might limit HSPA1A and HSPB1 interaction at that site, these HSPs could simultaneously engage other receptors, which would account for the partial cytokine activity observed even with TLR blockade. This potential for multi-receptor interaction may underline the inability of TLR-blocking peptides to fully abrogate HSPA1A and HSPB1-induced cytokine secretion (Figures 2-5). HSPs have been shown to interact with various receptor proteins, including TLR2, TLR4, CD14, CD40, CD91, and CD36 [8,9]. This observation aligns with findings from our previous study [5], further supporting the notion of receptor- specific but overlapping pathways in HSPA1A and HSPB1-mediated cytokine production.
The elevated levels of IL-1β, TNF-α, and IL-10 following HSPA1A and HSPB1 incubation with differentiated U937 cells indicate that these HSPs can effectively trigger both pro-inflammatory and anti-inflammatory regulatory cytokine responses. This observation suggests dual role of HSPs in activation of both pro and anti- inflammatory immune responses, which demonstrates their functions as immune modulation proteins [5,13,72]. This explains why HSPs can promote inflammation in some scenarios while aiding in immune regulation in others [5,13,72].
Preincubation with TLR2, TLR4, TLR5, and TLR7 blocking peptides significantly reduced cytokine secretion, indicating that these receptors are critical for HSP- mediated immune responses. Also, this observation may be suggestive of HSPs interaction with other receptors or mechanisms other than the TLRs demonstrated in this study. However, this study observed that the blocking peptides in some cases inhibited the level of IL-1β and IL-10 secretion than that seen in the TNF-α secretion. While it is unclear why that should be the case. However, evidence has shown variation in the cytokine secretion from immune cells to be attributed to the cell pathways involved, structure, and cellular response pattern [73,74]. For example, it has been documented that cytokines can be released from secretary granules, constitutive secretary pathways with more dynamic vesicular carriers, and compartment recycling endosomes [74,75]. This finding addresses the promiscuity concerns regarding HSP interactions with recognition receptors [5], demonstrating specificity in the HSP-TLR interactions that drive cytokine production [25,59]. Our previous study showed HSPA1A and HSPB1-induced cytokine secretion via interactions with CD14, CD36, and CD11b [5]. While there has been overwhelming evidence of HSP-induced immune response via interaction with CD91 and CD40 [76-78]. Studies have shown HSPs interaction with LOX-1, scavenger receptor (SR) in the activation of immune responses [77,79].
Our findings align with previous research demonstrating the role of HSPs in immune activation through interactions with receptors such as CD91 and CD40 [11,78,80]. However, the identification of specific TLRs involved in HSP-induced cytokine secretion provides a more nuanced understanding of the underlying mechanisms. While previous studies have suggested a broad immunoregulatory function for HSPs [13,72], While the specific signalling pathways behind HSP-induced cytokine secretion remain unclear, our data suggest that the reduced levels of TNF-α, IL-1β, and IL-10 observed when using TLR2, TLR4, TLR5, and TLR7 blocking peptides with HSPA1A and HSPB1 indicate the specificity of these TLRs in mediating HSPA1A- and HSPB1-induced cytokine production. This specificity suggests that HSPA1A and HSPB1 may activate MAPKs and NF-κB pathways through MyD88 and TRIF- dependent mechanisms (Figure 6). MyD88 has been widely shown to mediate cytokine release in several TLR pathways, while TRIF is known to play a role in TLR4-mediated cytokine activation [28,29]. Our findings underscore the importance of receptor- specific interactions in shaping immune responses. It is important to mention that HSP- induced cytokine secretion through TLR5 and TLR7 pathways has been less commonly studied. However, evidence from this study indicates that in addition to traditional receptors like TLR2 and TLR4 in cytokine activation, TLR5 and TLR7 also support both HSPA1A- and HSPB1-induced cytokine secretion. This finding is consistent with studies suggesting that TLR5 and TLR7 signalling, similar to TLR2 and TLR4, occurs via MyD88-dependent pathways and the activation of NF-κB (Figure 6).
The ability of HSPA1A and HSPB1 to induce cytokine secretion through TLRs positions these HSPs as potential modulators of immune responses in pathological and non-pathological contexts. This insight opens avenues for therapeutic interventions targeting HSP-TLR pathways to modulate immune responses in diseases characterized by chronic inflammation or immune dysregulation such as inflammatory diseases and cancer [5]. The TLRs studied in this manuscript are present in monocytic U937 cells and that has been demonstrated in other studies [81,82]. Understanding how modulation of HSP levels or blocking specific receptors, as demonstrated in this study and our previous work [5], could offer a viable strategy for reducing unwanted immune activation in conditions such as autoimmune disorders and hypersensitivity reactions [83,84].
While our study provides critical insights, it is essential to acknowledge the limitations. Given the promiscuity of both HSPA1A and HSPB1 in their interaction with receptors shown previously in [5] and in the current study, it is important to investigate how the different interactions demonstrated in [5] and the current study operate in vivo. For example, how does the context influence receptor usage? Future studies should explore these interactions in primary immune cells and animal models to validate the findings. Additionally, investigating the structural basis of HSP-TLR interactions, along with using Western blot analysis to identify downstream signalling pathways associated with the HSP-TLR mechanism, could further clarify the specificity and affinity of these interactions. The level of IL-1β, TNF-α, and IL-10 inhibition can be attributed to the concentration of TLR2, TLR4, TLR5, and TLR7 used. Future study can explore higher concentrations to further elucidate the study of HSPs interaction with TLRs.
Conclusion
Our study showed that HSPA1A and HSPB1-induced cytokine secretion may occur via interaction with TLR2, TLR4, TLR5, and TLR7. The promiscuity identified here and in [5] needs further investigation into the effect of context on receptor usage. These findings contribute to a deeper understanding of HSP-mediated immune modulation and highlight potential therapeutic targets for modulating immune responses in various disease contexts. Further research is warranted to explore the clinical implications of these interactions and their potential in therapeutic applications.
Authorship Contribution Statement
EO and JW conceived of the study and designed the experiments. JW and FM supervised the study. EO executed the experiments and analysed the data. EO, JW and FM interpreted the data and planned the publication. EO wrote the first draft of the manuscript, and all authors edited and approved of the manuscript.
Declaration of Competing Interest
None.
Acknowledgments
Chester Medical School, University of Chester, UK.
References
2. Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009 Jun;85(6):905-10.
3. Periard JD, Ruell P, Caillaud C, Thompson MW. Plasma Hsp72 (HSPA1A) and Hsp27 (HSPB1) expression under heat stress: influence of exercise intensity. Cell Stress Chaperones. 2012 May;17(3):375-83.
4. Hu C, Yang J, Qi Z, Wu H, Wang B, Zou F, et al. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm (2020). 2022 Aug 2;3(3):e161.
5. Ogbodo E, Michelangeli F, Williams JHH. Exogenous heat shock proteins HSPA1A and HSPB1 regulate TNF-α, IL-1β and IL-10 secretion from monocytic cells. FEBS Open Bio. 2023 Oct;13(10):1922-40.
6. Zininga T, Ramatsui L, Shonhai A. Heat Shock Proteins as Immunomodulants. Molecules. 2018 Nov 1;23(11):2846.
7. Multhoff G, Pockley AG, Schmid TE, Schilling D. The role of heat shock protein 70 (Hsp70) in radiation-induced immunomodulation. Cancer Lett. 2015 Nov 28;368(2):179-84.
8. Klink M, Nowak M, Kielbik M, Bednarska K, Blus E, Szpakowski M, et al. The interaction of HspA1A with TLR2 and TLR4 in the response of neutrophils induced by ovarian cancer cells in vitro. Cell Stress Chaperones. 2012 Nov;17(6):661-74.
9. Hauet-Broere F, Wieten L, Guichelaar T, Berlo S, van der Zee R, Van Eden W. Heat shock proteins induce T cell regulation of chronic inflammation. Ann Rheum Dis. 2006 Nov;65 Suppl 3(Suppl 3):iii65-8.
10. Baek YS, Haas S, Hackstein H, Bein G, Hernandez-Santana M, Lehrach H, et al. Identification of novel transcriptional regulators involved in macrophage differentiation and activation in U937 cells. BMC Immunol. 2009 Apr 2;10:18.
11. Grotegut P, Kuehn S, Dick HB, Joachim SC. Destructive Effect of Intravitreal Heat Shock Protein 27 Application on Retinal Ganglion Cells and Neurofilament. Int J Mol Sci. 2020 Jan 15;21(2):549.
12. Winter J, Hammer E, Heger J, Schultheiss HP, Rauch U, Landmesser U, et al. Adenine Nucleotide Translocase 1 Expression is Coupled to the HSP27- Mediated TLR4 Signaling in Cardiomyocytes. Cells. 2019 Dec 6;8(12):1588.
13. Milani A, Akbari E, Pordanjani PM, Jamshidi F, Ghayoumi S, Sadeghi SA, et al. Immunostimulatory effects of Hsp70 fragments and Hsp27 in design of novel HIV-1 vaccine formulations. HIV Med. 2024 Feb;25(2):276-90.
14. Chase MA, Wheeler DS, Lierl KM, Hughes VS, Wong HR, Page K. Hsp72 induces inflammation and regulates cytokine production in airway epithelium through a TLR4- and NF-kappaB-dependent mechanism. J Immunol. 2007 Nov 1;179(9):6318-24.
15. Tsan MF, Gao B. Cytokine function of heat shock proteins. Am J Physiol Cell Physiol. 2004 Apr;286(4):C739-44.
16. Shin HS, Xu F, Bagchi A, Herrup E, Prakash A, Valentine C, et al. Bacterial lipoprotein TLR2 agonists broadly modulate endothelial function and coagulation pathways in vitro and in vivo. J Immunol. 2011 Jan 15;186(2):1119-30.
17. Travassos LH, Girardin SE, Philpott DJ, Blanot D, Nahori MA, Werts C, et al. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 2004 Oct;5(10):1000-6.
18. Mazgaeen L, Gurung P. Recent Advances in Lipopolysaccharide Recognition Systems. Int J Mol Sci. 2020 Jan 7;21(2):379.
19. Zamyatina A, Heine H. Lipopolysaccharide Recognition in the Crossroads of TLR4 and Caspase-4/11 Mediated Inflammatory Pathways. Front Immunol. 2020 Nov 27;11:585146.
20. Yang J, Yan H. TLR5: beyond the recognition of flagellin. Cell Mol Immunol. 2017 Dec;14(12):1017-9.
21. Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004 Apr 13;101(15):5598-603.
22. Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007 Jan;81(1):15-27.
23. Choi SK, Kam H, Kim KY, Park SI, Lee YS. Targeting Heat Shock Protein 27 in Cancer: A Druggable Target for Cancer Treatment? Cancers (Basel). 2019 Aug 16;11(8):1195.
24. Qi W, White MC, Choi W, Guo C, Dinney C, McConkey DJ, et al. Inhibition of inducible heat shock protein-70 (hsp72) enhances bortezomib- induced cell death in human bladder cancer cells. PLoS One. 2013 Jul 18;8(7):e69509.
25. Ferat-Osorio E, Sanchez-Anaya A, Gutierrez-Mendoza M, Bosco-Garate I, Wong-Baeza I, Pastelin-Palacios R, et al. Heat shock protein 70 down-regulates the production of toll-like receptor-induced pro-inflammatory cytokines by a heat shock factor- 1/constitutive heat shock element-binding factor-dependent mechanism. J Inflamm (Lond). 2014 Jul 12;11:19.
26. Gupta A, Cooper ZA, Tulapurkar ME, Potla R, Maity T, Hasday JD, et al. Toll-like receptor agonists and febrile range hyperthermia synergize to induce heat shock protein 70 expression and extracellular release. J Biol Chem. 2013 Jan 25;288(4):2756-66.
27. Chen F, Zou L, Williams B, Chao W. Targeting Toll-Like Receptors in Sepsis: From Bench to Clinical Trials. Antioxid Redox Signal. 2021 Nov 20;35(15):1324-39.
28. Hulina A, Grdic Rajkovic M, Jaksic Despot D, Jelic D, Dojder A, cepelak I, et al. Extracellular Hsp70 induces inflammation and modulates LPS/LTA- stimulated inflammatory response in THP-1 cells. Cell Stress Chaperones. 2018 May;23(3):373-84.
29. Sur R, Lyte PA, Southall MD. Hsp27 regulates pro-inflammatory mediator release in keratinocytes by modulating NF-kappaB signaling. J Invest Dermatol. 2008 May;128(5):1116-22.
30. Borges TJ, Wieten L, van Herwijnen MJ, Broere F, van der Zee R, Bonorino C, et al.The anti-inflammatory mechanisms of Hsp70. Front Immunol. 2012 May 4; 3:95.
31. Miller-Graziano CL, De A, Laudanski K, Herrmann T, Bandyopadhyay S. HSP27: an anti-inflammatory and immunomodulatory stress protein acting to dampen immune function. Novartis Found Symp. 2008; 291:196-208.
32. Singh AV, Katz A, Maharjan RS, Gadicherla AK, Richter MH, Heyda J, et al. Coronavirus-mimicking nanoparticles (CorNPs) in artificial saliva droplets and nanoaerosols: Influence of shape and environmental factors on particokinetics/particle aerodynamics. Sci Total Environ. 2023 Feb 20;860:160503.
33. Ragg SJ, Kaga S, Berg KA, Ochi A. The mitogen-activated protein kinase pathway inhibits ceramide-induced terminal differentiation of a human monoblastic leukemia cell line, U937. J Immunol. 1998 Aug 1;161(3):1390-8.
34. Biggs JR, Ahn NG, Kraft AS. (1998). Activation of the mitogen-activated protein kinase pathway in U937 leukemic cells induces phosphorylation of the amino terminus of the TATA-binding protein. Cell Growth Differ. 1998 Aug;9(8):667-76.
35. Jalagadugula G, Dhanasekaran DN, Rao AK. Phorbol 12-myristate 13-acetate (PMA) responsive sequence in Galphaq promoter during megakaryocytic differentiation. Regulation by EGR-1 and MAP kinase pathway. Thromb Haemost. 2008 Nov;100(5):821-8.
36. Song MG, Ryoo IG, Choi HY, Choi BH, Kim ST, Heo TH, et al. (2015). NRF2 Signaling Negatively Regulates Phorbol-12-Myristate-13- Acetate (PMA)-Induced Differentiation of Human Monocytic U937 Cells into ProInflammatory Macrophages. PLoS One. 2015 Jul 29;10(7):e0134235.
37. Liu G, Chen S, Hu A, Zhang L, Sun W, Chen J, et al. The Establishment and Validation of the Human U937 Cell Line as a Cellular Model to Screen Immunomodulatory Agents Regulating Cytokine Release Induced by Influenza Virus Infection. Virol Sin. 2019 Dec;34(6):648- 61.
38. Nascimento CR, Rodrigues Fernandes NA, Gonzalez Maldonado LA, Rossa Junior C. Comparison of monocytic cell lines U937 and THP-1 as macrophage models for in vitro studies. Biochem Biophys Rep. 2022 Nov 18; 32:101383.
39. Sanchez-Reyes K, Bravo-Cuellar A, Hernandez-Flores G, Lerma-Diaz JM, Jave-Suarez LF, Gomez-Lomeli P, et al. Cervical cancer cell supernatants induce a phenotypic switch from U937-derived macrophage-activated M1 state into M2- like suppressor phenotype with change in Toll-like receptor profile. Biomed Res Int. 2014;2014:683068.
40. Jin J, Samuvel DJ, Zhang X, Li Y, Lu Z, Lopes-Virella MF, et al. Coactivation of TLR4 and TLR2/6 coordinates an additive augmentation on IL-6 gene transcription via p38MAPK pathway in U937 mononuclear cells. Mol Immunol. 2011 Dec;49(3):423-32.
41. Greene CM, McElvaney NG, O'Neill SJ, Taggart CC. Secretory leucoprotease inhibitor impairs Toll-like receptor 2- and 4-mediated responses in monocytic cells. Infect Immun. 2004 Jun;72(6):3684-7.
42. Xiong F, Wang XB, Zhang JH, Liu W, Sun S, Liu LQ, et al. [Expression and role of toll-like receptors in U937 cells]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007 Jun;15(3):449-53.
43. Francisco S, Billod JM, Merino J, Punzon C, Gallego A, Arranz A, et al. Induction of TLR4/TLR2 Interaction and Heterodimer Formation by Low Endotoxic Atypical LPS. Front Immunol. 2022 Jan 24;12:748303.
44. Szentirmai E, Massie AR, Kapas L. Lipoteichoic acid, a cell wall component of Gram-positive bacteria, induces sleep and fever and suppresses feeding. Brain Behav Immun. 2021 Feb;92:184-92.
45. Chen D, Zhao Y, Feng Y, Jin C, Yang Q, Qiu H, et al. Expression of TLR2, TLR3, TLR4, and TLR7 on pulmonary lymphocytes of Schistosoma japonicum-infected C57BL/6 mice. Innate Immun. 2019 May;25(4):224-34.
46. Jacinto R, Hartung T, McCall C, Li L. Lipopolysaccharide- and lipoteichoic acid- induced tolerance and cross-tolerance: distinct alterations in IL-1 receptor- associated kinase. J Immunol. 2002 Jun 15;168(12):6136-41.
47. Song WS, Jeon YJ, Namgung B, Hong M, Yoon SI. A conserved TLR5 binding and activation hot spot on flagellin. Sci Rep. 2017 Jan 20;7:40878.
48. Zheng G, Zhang Z, Liu H, Xiong Y, Luo L, Jia X, et al. HSP27-Mediated Extracellular and Intracellular Signaling Pathways Synergistically Confer Chemoresistance in Squamous Cell Carcinoma of Tongue. Clin Cancer Res. 2018 Mar 1;24(5):1163-75.
49. Somensi N, Brum PO, de Miranda Ramos V, Gasparotto J, Zanotto-Filho A, Rostirolla DC, et al. Extracellular HSP70 Activates ERK1/2, NF-kB and Pro-Inflammatory Gene Transcription Through Binding with RAGE in A549 Human Lung Cancer Cells. Cell Physiol Biochem. 2017;42(6):2507-22.
50. Murshid A, Theriault J, Gong J, Calderwood SK. Molecular Chaperone Receptors. Methods Mol Biol. 2018;1709:331-44.
51. Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002 Apr 26;277(17):15028-34.
52. Havasi A, Lu W, Cohen HT, Beck L, Wang Z, Igwebuike C, et al. (2017) Blocking peptides and molecular mimicry as treatment for kidney disease. American journal of physiology. Renal physiology 312(6): F1016-25.
53. Chavez-Sanchez L, Chavez-Rueda K, Legorreta-Haquet MV, Zenteno E, Ledesma-Soto Y, Montoya-Diaz E, et al. (2010) The activation of CD14, TLR4, and TLR2 by mmLDL induces IL-1β, IL-6, and IL-10 secretion in human monocytes and macrophages. Lipids in health and disease 9:117.
54. Ajay Vikram Singh, Romi Singh Maharjan, Harald Jungnickel, Heike Romanowski, Yves Uwe Hachenberger, Philipp Reichardt, et al., (2021). Evaluating Particle Emissions and Toxicity of 3D Pen PrintedFilaments with Metal Nanoparticles As Additives: In Vitro and inSilico Discriminant Function Analysis. ACS Sustainable Chemistry & Engineering 2021 9 (35), 11724-37.
55. Brasino M, Wagnell E, Hamilton S, Ranganathan S, Gomes MM, Branchaud B, et al. Turning antibodies off and on again using a covalently tethered blocking peptide. Commun Biol. 2022 Dec 10;5(1):1357.
56. Jiang SJ, Tsai PI, Peng SY, Chang CC, Chung Y, Tsao HH, et al. A potential peptide derived from cytokine receptors can bind proinflammatory cytokines as a therapeutic strategy for anti-inflammation. Sci Rep. 2019 Feb 19;9(1):2317.
57. Kwon HK, Patra MC, Shin HJ, Gui X, Achek A, Panneerselvam S, et al. A cell- penetrating peptide blocks Toll-like receptor-mediated downstream signaling and ameliorates autoimmune and inflammatory diseases in mice. Exp Mol Med. 2019 Apr 26;51(4):1-19.
58. Arai C, Nomura Y, Matsuzawa M, Hanada N, Nakamura Y. Extracellular HSP72 induces proinflammatory cytokines in human periodontal ligament fibroblast cells through the TLR4/NFκB pathway in vitro. Arch Oral Biol. 2017 Nov;83:181-6.
59. Jin C, Cleveland JC, Ao L, Li J, Zeng Q, Fullerton DA, et al. Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: the proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)- 2 and TLR4. Mol Med. 2014 Jun 9;20(1):280-9.
60. Ng PC, Li K, Wong RP, Chui K, Wong E, Li G, et al. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch Dis Child Fetal Neonatal Ed. 2003 May;88(3):F209-13.
61. Kessler B, Rinchai D, Kewcharoenwong C, Nithichanon A, Biggart R, Hawrylowicz CM, et al. Interleukin 10 inhibits pro-inflammatory cytokine responses and killing of Burkholderia pseudomallei. Sci Rep. 2017 Feb 20;7:42791.
62. Teodorczyk-Injeyan JA, Triano JJ, Injeyan HS. Nonspecific Low Back Pain: Inflammatory Profiles of Patients With Acute and Chronic Pain. Clin J Pain. 2019 Oct;35(10):818-25.
63. Wan Q, Song D, Li H, He ML. Stress proteins: the biological functions in virus infection, present and challenges for target-based antiviral drug development. Signal Transduct Target Ther. 2020 Jul 13;5(1):125.
64. Luo X, Zuo X, Zhou Y, Zhang B, Shi Y, Liu M, et al. Extracellular heat shock protein 70 inhibits tumour necrosis factor-alpha induced proinflammatory mediator production in fibroblast-like synoviocytes. Arthritis Res Ther. 2008;10(2):R41.
65. Thuringer D, Jego G, Wettstein G, Terrier O, Cronier L, Yousfi N, et al. Extracellular HSP27 mediates angiogenesis through Toll-like receptor 3. FASEB J. 2013 Oct;27(10):4169-83.
66. Javmen A, Zou J, Nallar SC, Szmacinski H, Lakowicz JR, Gewirtz AT, et al. TLR5-Derived, TIR-Interacting Decoy Peptides to Inhibit TLR Signaling. J Immunol. 2023 May 1;210(9):1419-27.
67. Achek A, Kwon HK, Patra MC, Shah M, Hong R, Lee WH, et al. A peptide derived from the core β-sheet region of TIRAP decoys TLR4 and reduces inflammatory and autoimmune symptoms in murine models. EBioMedicine. 2020 Feb;52:102645.
68. Veloso Junior PHH, Simon KS, de Castro RJA, Coelho LC, Erazo FAH, de Souza ACB, et al. Peptides ToAP3 and ToAP4 decrease release of inflammatory cytokines through TLR-4 blocking. Biomed Pharmacother. 2019 Oct;118:109152.
69. Kanno A, Tanimura N, Ishizaki M, Ohko K, Motoi Y, Onji M, et al. Targeting cell surface TLR7 for therapeutic intervention in autoimmune diseases. Nat Commun. 2015 Feb 4;6:6119.
70. Ruiperez V, Astudillo AM, Balboa MA, Balsinde J. Coordinate regulation of TLR- mediated arachidonic acid mobilization in macrophages by group IVA and group V phospholipase A2s. J Immunol. 2009 Mar 15;182(6):3877-83.
71. Cohen-Sfady M, Nussbaum G, Pevsner-Fischer M, Mor F, Carmi P, Zanin- Zhorov A, et al. Heat shock protein 60 activates B cells via the TLR4-MyD88 pathway. J Immunol. 2005 Sep 15;175(6):3594-602.
72. Kaiser F, Steptoe A, Thompson S, Henderson B. Monocyte cytokine synthesis in response to extracellular cell stress proteins suggests these proteins exhibit network behaviour. Cell Stress Chaperones. 2014 Jan;19(1):135-44.
73. Bonaventura P, Lamboux A, Albarede F, Miossec P. Differential effects of TNF- α and IL-1β on the control of metal metabolism and cadmium-induced cell death in chronic inflammation. PLoS One. 2018 May 16;13(5):e0196285.
74. Stow JL, Low PC, Offenhauser C, Sangermani D. Cytokine secretion in macrophages and other cells: pathways and mediators. Immunobiology. 2009;214(7):601-12.
75. Stanley AC, Lacy P. Pathways for cytokine secretion. Physiology (Bethesda). 2010 Aug;25(4):218-29.
76. Pawaria S, Binder RJ. CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat Commun. 2011 Nov 1;2:521.
77. Fischer N, Haug M, Kwok WW, Kalbacher H, Wernet D, Dannecker GE, et al. Involvement of CD91 and scavenger receptors in Hsp70-facilitated activation of human antigen-specific CD4+ memory T cells. Eur J Immunol. 2010 Apr;40(4):986-97.
78. Becker T, Hartl FU, Wieland F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol. 2002 Sep 30;158(7):1277-85.
79. Borges T. J., Lima K., Murshid A., Lape I. T., Zhao Y., Rigo M. M, et al. (2023). Innate extracellular Hsp70 inflammatory properties are mediated by the interaction of Siglec-E and LOX-1 receptors. BioRxiv, 2023-12.
80. Zhou YJ, Messmer MN, Binder RJ. Establishment of tumor-associated immunity requires interaction of heat shock proteins with CD91. Cancer Immunol Res. 2014 Mar;2(3):217-28.
81. Grigoryeva LS, Cianciotto NP. Human macrophages utilize a wide range of pathogen recognition receptors to recognize Legionella pneumophila, including Toll-Like Receptor 4 engaging Legionella lipopolysaccharide and the Toll-like Receptor 3 nucleic-acid sensor. PLoS Pathog. 2021 Jul 19;17(7):e1009781.
82. Okamoto M, Hirai H, Taniguchi K, Shimura K, Inaba T, Shimazaki C, et al. Toll-like receptors (TLRs) are expressed by myeloid leukaemia cell lines, but fail to trigger differentiation in response to the respective TLR ligands. Br J Haematol. 2009 Nov;147(4):585-7.
83. Pan SY, Chia YC, Yee HR, Fang Cheng AY, Anjum CE, Kenisi Y, et al. Immunomodulatory potential of anti-idiotypic antibodies for the treatment of autoimmune diseases. Future Sci OA. 2020 Oct 29;7(2):FSO648.
84. Warrington R, Watson W, Kim HL, Antonetti FR. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 2011 Nov 10;7 Suppl 1(Suppl 1):S1.
85. Chen H, Wang F, Wu X, Yuan S, Dong H, Zhou C, et al. Chronic Heat Stress Induces Oxidative Stress and Induces Inflammatory Injury in Broiler Spleen via TLRs/MyD88/NF-κB Signaling Pathway in Broilers. Vet Sci. 2024 Jul 1;11(7):293.
86. Rai V, Mathews G, Agrawal DK. Translational and Clinical Significance of DAMPs, PAMPs, and PRRs in Trauma-induced Inflammation. Arch Clin Biomed Res. 2022;6(5):673-85.
87. Cho HY, Choi EK, Lee SW, Kim KH, Park SJ, Lee CK, et al. All-trans retinoic acid induces TLR-5 expression and cell differentiation and promotes flagellin- mediated cell functions in human THP-1 cells. Immunol Lett. 2011 Apr 30;136(1):97-107.
88. Zhou J, An H, Xu H, Liu S, Cao X. Heat shock up-regulates expression of Toll- like receptor-2 and Toll-like receptor-4 in human monocytes via p38 kinase signal pathway. Immunology. 2005 Apr;114(4):522-30.