Abstract
The pathological evaluation of ovaries is a critical endpoint in reproductive toxicology assessments. Currently, there is no standardized method for the quantification of oocytes and follicles in preclinical drug safety evaluations. This study developed a simplified quantitative method based on stereological principles to efficiently assess the number of oocytes in experimental rabbits. The quantitative results obtained with this method are consistent with those from traditional counting methods. This innovative approach reduces the time of slide preparation and cell quantification, making it efficient for evaluating oocyte quality in large scale of toxicity studies.
Keywords
Oocyte, Reproductive toxicity, Safety evaluation, Experimental animals, New drug approval
Introduction
Reproductive toxicity studies are a crucial component of non-clinical safety evaluations for drugs and are essential for the clinical trial entry and market approval of new drugs [1]. A new candidate drug molecule should be tested in preclinical model, often lab animals, to identify potential toxic effects in different organs [2]. Any toxic effect on the testis and ovaries is seriously concerned [3]. Quantitative examination of oocytes may need to investigate potential risk of reproductive toxicity by innovative drug molecules [4].
However, the assessment of the female reproductive system has long been a focal point as well as a challenging aspect in the non-clinical safety evaluation of drugs. This is largely due to the influence of the reproductive cycle. The ovarian reserve serves as a crucial indicator of ovarian function [5]. The endocrine functions of the ovaries are not only crucial for fertility but also play a vital role in regulating skeletal, cardiovascular, and brain health [6]. The follicular maturation process in experimental animals is mostly the same and is regulated by the hypothalamic-pituitary-gonadal axis, with primordial follicles gradually developing into primary follicles, secondary follicles, and mature follicles, leading to ovulation. In preclinical trials, pathological evaluation of the ovaries serves as a valuable early warning indicator for potential toxicity to the female reproductive system that may manifest in subsequent clinical trials [7]. Concurrently, paying attention to the morphological developmental characteristics of follicles at various stages can assist pathologists in assessing the reproductive function of the ovary. Subtle variations in cell or particle density frequently elude the sensitivity of the human eye. Typically, differences must reach a threshold of at least 25% to 40%, contingent upon the specific tissue, in order to become perceptible [8]. Under normal physiological conditions, the ovarian reserve of primordial follicles undergoes natural depletion and eventual exhaustion [9]. Qualitative assessment of tissue sections by pathologists remains the gold standard for routine diagnosis, experimental, and toxicological research.
Exact ovarian cell quantification is time consuming. The often-used method needs pathologists to count follicles on hundreds of sections from entire ovary [10]. This method is practically possible for small ovaries from small lab animals such as rodents, while for larger lab animals it becomes time-consuming and inefficient. An alternative approach is to apply stereological principle to quantify the number of cells in sections through designed tissue sampling [10-12]. The stereological method can save much time though, it requires specialized microscope quipped with an electrically driven stage and operated by stereological software [13]. This requirement restricted its wide application in reproductive toxicity evaluations.
There is a need to develop a practical method to quality of oocytes in large experimental animals in safety assessment of new drug molecules [14]. To address this issue, we developed a method that applied the sampling principles of stereology to quantify various oocytes and verified the method in rabbit ovary. This new method provides a low cost and highly efficient approach to evaluate oocytes health in safety assessment of female reproductive system of larger lab animals.
Materials and Methods
Experimental animals
Six female New Zealand rabbits (5-6 months old, weighing ≥ 2.5 kg) were obtained from Qingdao BioSino Biotechnology Co., Ltd. All animal experiments in this study were conducted in accordance with the relevant regulations of the Animal Management and Ethics Committee of TriApex Preclinical Research Laboratories Co., Ltd.
Materials and equipment
The main materials used for tissue sectioning included Hematoxylin and Eosin (H.E.) staining solution, 10% formalin, anhydrous ethanol, gradient alcohol, xylene, and neutral resin. The primary instruments used included a paraffin tissue microtome (RM2015, Leica, Germany), a scanner (AT2, Leica, Germany), an optical microscope (DP73, Olympus, Japan), and HALO software.
Ovarian tissue collection and sectioning
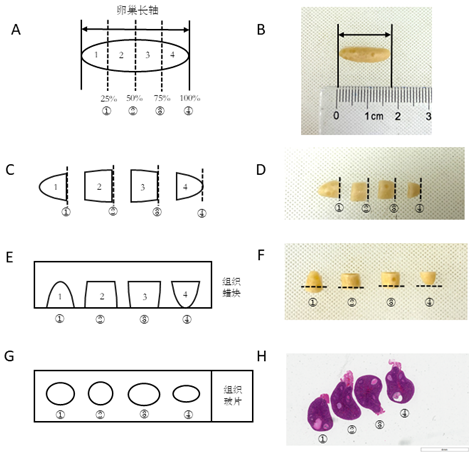
The new method: Three rabbits were euthanized using carbon dioxide asphyxiation. After shaving, the abdominal skin was disinfected with povidone-iodine and 75% ethanol. The abdomen was then opened, and the connective tissue attached to the surface of the ovaries and the connected fallopian tubes were removed. The left and right ovaries were collected and placed in physiological saline. After washing the surface, the ovaries were transferred to 10% formalin for fixation for 24 hours. The long axis diameter of the ovary was measured. The ovaries of each rabbit were cut transversely along the long axis diameter into four equal parts using a self-made device (as shown in Figure 1). The ovarian slices were then rotated 90° clockwise to expose four different cut surfaces, representing section faces at 25%, 50%, 75%, and 100% of the long axis of the ovary. The four ovarian slices were arranged in order and embedded in one paraffin block. During sectioning, rough cutting was performed until the four ovarian tissues were fully exposed (the cortex and medulla of the ovarian tissue could be seen under a microscope). A single section (6 μm) was collected for microscopic examination and counting.
Figure 1. Schematic diagram of the innovative method for oocyte quality assessment in New Zealand rabbits. A-H illustrate the process where the ovarian tissue of the experimental animals is divided equally into 4n parts along the long axis diameter. Each of the 4n ovarian parts is then rotated 90° clockwise along the cutting direction and sequentially fixed and arranged in an embedding cassette for dehydration. They are then embedded and fixed in the same wax block, followed by sectioning, staining, and mounting. Finally, the numbers of follicles at various stages in the 4 n sections are counted under a microscope and summed to obtain the total number of follicles at each stage, where n = 1 or a positive integer greater than 1.
Classic method: The bilateral ovaries of 3 rabbits were collected using the same method and fixed in 10% formalin for 24 hours. The bilateral ovaries were embedded routinely as they were placed side by side. After rough trimming to completely expose both ovaries, the first section exposing both ovaries were taken as the starting point. The tissue was cut into sections with a thickness of 6 μm. Every tenth section was retained for microscopic examination and counting.
Pathological examination and quantification of ovarian follicles
The primordial follicles, primary follicles, secondary follicles, and mature follicles are examined by pathologists using images from whole-slide scanning. The different follicles are counted, and the total numbers were calculated.
Follicles quantification of the innovative counting method
The total number of each stage of follicles on a single section of the left or right ovary for each animal (Q1) was multiplied by the reciprocal of the sampling interval (f1) to obtain the total number of each stage of follicles in the entire ovarian tissue (N1).
N1 = Q1 × (1/ f1)
N1: Total number of follicles in the ovarian tissue.
Q1: Total number of each stage of follicles in the sampled sections (each type of follicle is counted separately).
f1: Theoretical sampling interval. In this study, the theoretical number of sections obtainable from the ovarian diameter is used to calculate the interval when extracting 4 sections. In this study, the ovaries of the animals were 1.8 cm, 1.5 cm, and 1.6 cm, and the corresponding Theoretical sampling intervals were 750, 625, and 666, respectively.
Follicles quantitative formula of the direct counting method
The total number of each stage of follicles on a single section of the left or right ovary for each animal (Q2) was multiplied by the reciprocal of the sampling interval (f2) to obtain the total number of each stage of follicles in the entire ovarian tissue (N2).
N2 = Q2 × (1/ f2)?
N2: Total number of follicles in the ovarian tissue.
Q2: Total number of each stage of follicles in the sampled sections (each type of follicle is counted separately).
f2: Sampling interval. In this study, one section is sampled every 10 sections for counting, so the sampling interval is 10.
The density calculation and statistical analysis
Using HALO software, the area size of the ovary on sections is measured on whole-slide scanning images. The density of follicles at each stage is obtained by dividing the number of follicles by the ovarian area.
Statistical analysis
The mean and standard deviation values of the number of each stage of follicles, obtained from the direct counting method and the innovative counting method, are calculated. The results are expressed as mean ± standard error of the mean (SEM). Statistical analysis is performed using GraphPad Prism 7, and a t-test is used for comparing differences. P <0.05 is considered statistically significant, while "ns" indicates no significant difference.
Results
Observation of ovarian structure and follicle structure
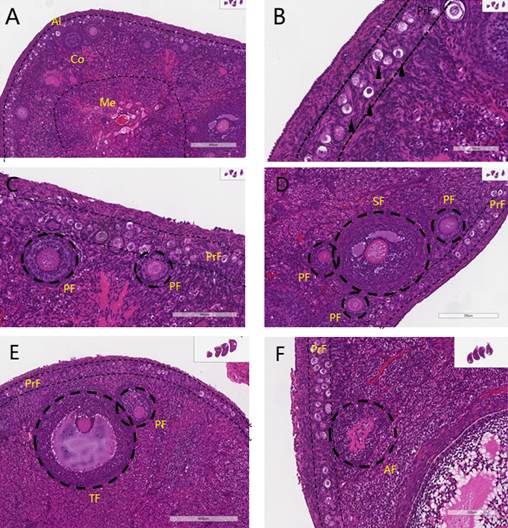
The ovarian structure of New Zealand rabbits as previous described (REF) has 4 regions, from the surface to the center there are germinal epithelium, tunica albuginea (Al), cortex (Co), and medulla (Me) as shown in (Figure 2A). The surface germinal epithelial cells are flat or cuboidal, covered by the tunica albuginea composed of dense connective tissue. The cortex is located at the periphery of the ovary and has follicles at various developmental stages, atretic follicles, stroma, and blood vessels. Primordial follicles (PrF) and primary follicles (PF) are distributed in the superficial layer. Secondary follicles (SF) and mature follicles (TF) are predominantly located in the deeper layers of the cortex, where they are less numerous and exhibit one or more irregular, antrum-like structures within the follicular cells, all surrounded by a distinct zona pellucida. Most follicles undergo degeneration during development, forming atretic follicles (AF). The medulla is located beneath the cortex and had more elastic fibers, larger blood vessels, lymphatic vessels, and nerves.
Quantification of follicles
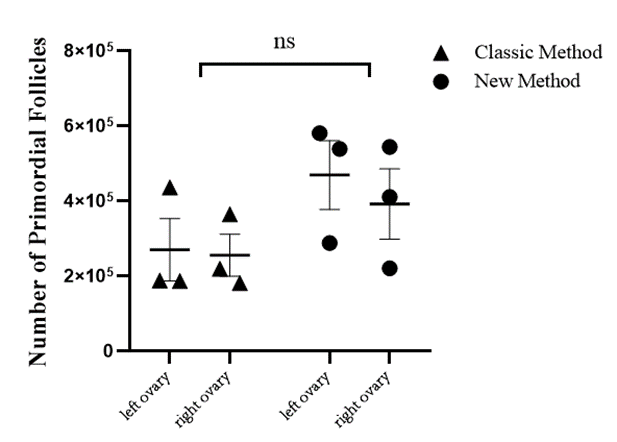
The total number of primordial follicles obtained from the two methods has been analyzed statistically and summarized in (Figure 3). The average numbers of primordial follicles from two different methods were slightly variable between unilateral ovary and between the methods, but the variations were not statistically different (P >0.05).
Figure 2. Tissue Structure of Ovaries and Various Stages of Follicles in New Zealand Rabbits. (A) Al indicates the tunica albuginea; Co indicates the cortex; Me indicates the medulla. (B) Primordial follicles (black arrows). (C) Primordial follicles (PrF) and primary follicles (PF). (D) Primordial follicles (PrF), primary follicles (PF), and secondary follicles (SF). (E) Mature follicle (TF) and primary follicles (PF). (F) Atretic follicles (AF).
Figure 3. Quantification of primordial follicles in the ovaries. To eliminate the influence of individual animal variability on the comparison between the two methods, we further analyzed the density of different follicles at proportions of 25%, 50%, 75%, and 100% of the diameter of the major axis of ovary and compared the follicle counts per unit area (number/mm2).
The results from further analysis of different follicles showed a consistent density of the follicles between the two methods (Table 1).
|
Group |
Follicle Counts per Unit Area (number/mm2) |
|||
|
Primordial Follicles |
Primary Follicles |
Secondary Follicles |
Mature Follicles |
|
|
Classic Method |
37.9 ± 15.9 |
2.16 ± 0.86 |
0.36 ± 0.18 |
0.29 ± 0.12 |
|
New Method |
35.9 ± 13.5 |
2.19 ± 0.53 |
0.45 ± 0 .20 |
0.31 ± 0.17 |
|
Note: The values represent the mean ± standard deviation obtained from three identical animals using both methods. |
||||
Discussion
The assessment of female reproductive toxicity is critical in environmental safety and drug R&D [16]. It needs a combination of various methods, including the evaluation of estrous cycle duration, oocyte fertility, corpora lutea counting, organ weights and histopathology of ovary [17]. Loss of oogonia, oocytes, or ovarian stromal cells due to the test substance may cause dysfunction of female reproductive system [18]. Accurate follicle quantification is important to identify potential ovarian toxicants and understand the mode of action and the severity of the effect [19]. The EPA, FDA, and OECD guidelines mandate the evaluation of primordial follicles in ovaries, focusing on F1 generation for EPA and OECD, and both F0 and F1 for FDA [20]. The FDA also suggests assessing follicle presence. Preclinical study data must include statistical analysis of animal numbers, ovarian sections, and section selection [21]. Female reproductive toxicity assessment is essential and necessitates quantification.
Although regulatory agencies have not recommended any specific method for follicle counting, they did require scientific rationale and counting accuracy. Given the fact that diverse species and large number of experimental animals used in general toxicity studies, ovarian histopathological assessment of oocyte quality is a workload challenge for toxicologic pathologists. Currently, common methods for follicle counting are direct counting and stereological counting [22]. Direct counting of small ovaries can be shown through studies evaluating the effects of the PARP inhibitor Olaparib on mouse ovarian reserve and fertility [23]. This approach of processing entire ovary is possible for small ovaries but becomes very tedious for big ovaries from large experimental animals such as rabbits and non-human primates [24]. The large ovaries are often divided into segments, sectioned, and examined, the follicles are counted either at regular intervals or randomly sampled [25].
Stereological counting is beneficial but technically a challenge. It requires specialized equipment that makes it difficult to widely implement in toxicological pathology studies [26]. Some studies have compared primordial follicle counts from the two methods and found consistent results [27]. For stereological method, the key is its random sampling, using the principle of systematic uniform random sampling. The purpose is to ensure the equal chance of being sampled of all cells of the region of interest. It is done typically by capturing about 8 to 10 sections at different intervals through the tissue (or region of interest). In this study, we used the ovaries of adult healthy New Zealand rabbits to innovate a quantitative method that is much efficient in practice but results are comparable with traditional direct counting method. We followed the stereology principle and divided the ovary into 4 parts equidistantly along the long axis. This approach allowed us to examine ovarian structure and quantify the follicles 4 particular sections on one slide, covering the area at 1/4, 2/4, 3/4, and 4/4 of the total length. As there were no reports of application of traditional counting methods in medium to large experimental animals, we adopted a correction factor of 10 from rodent studies. Based on the thickness of sections and total long-axis diameter of the rabbit ovaries, selection of four sections from 1.5 cm long ovary was equivalent to collecting one section out of every 625 sections, making the correction factor 625. As shown in (Figure 3), in the direct counting method, since the total number is obtained by multiplying the counting result by the correction factor, the correction factor has a significant impact on the final result, and it can lead to differences in follicle numbers within the same species by several-fold or even dozens of times. Reports indicate that the smaller the correction factor, i.e., the fewer the intervals between sections, the smaller the impact on the final counting result. Statistical analysis of the results demonstrated no significant difference (P > 0.05) of the follicle counts from the direct counting method and the innovative counting method. To circumvent the issue of inconsistent pathologist statistical criteria and taking into account the commonalities in follicle development across all species, we have formulated classification criteria for follicles at every stage that are applicable to each species. These criteria are based on reports and preclinical experiences from various institutions. For instance, secondary follicles and mature follicles, which are often challenging to differentiate in sections, can be effectively graded by utilizing their diameter size [28].
Obtaining pathological data of drugs in development directly from humans is challenging. Animal models that possess strong predictive capabilities can act as effective preclinical tools, on the condition that the functional endpoints are homologous or sufficiently similar [29]. The study of female reproduction in preclinical research has great implications for clinical research. Preclinical investigations of the female reproductive system in large animals, particularly non-human primates, are capable of furnishing safe dosage levels and anticipated pharmacological effects for subsequent clinical trials [30, 31]. The initial size of the primordial follicle pool serves as a representation of ovarian reserve and plays a pivotal role in determining the reproductive lifespan in humans [32]. Owing to the limited availability of non-human primate tissue, our approach has thus far been validated solely on the New Zealand rabbit species. We anticipate expanding this validation to non-human primates in the near future, with the ultimate goal of progressing to clinical trials. This progression is essential for substantiating the statistical integrity of ovarian storage methods.
Up to now qualitative assessment of tissue sections by pathologists remains the gold standard for routine diagnosis, experimental, and toxicological research. Some studies have shown that the number of granulosa cell layers, the thickness of the zona pellucida, and the diameter of the oocyte in developing follicles are also correlated with oocyte quality [33]. Researchers have currently established 3D quantitative analysis methods to show the dynamic changes in oocyte loss during the ovarian development of C57BL/6J mice, which can be used to quantitatively analyze the loss of oocytes during the three waves of oocyte clearance [34].
As future directions in this field may include the expansion and adoption of new molecular pathology techniques, the quantification of follicles is still the critical criteria for evaluation of oocyte quality [35]. The major advantage of our new method is to significantly improve the efficiency of the sectioning and counting process for this type of time-consuming work. In the daily practice the direct counting follicle in a general rabbit reproductive toxicity study requires at least 100 sections for each animal in a total of 30-100 animals. The innovative counting method requires only one cut of a block with 4 regions, which reduces the number of slides by 90% and thereby cutting down workload massively and speedup the evaluation process.
Conclusion
This work established a quantitative method to evaluate follicles at various stages throughout the entire ovarian tissue of New Zealand rabbit. This method providing efficient safety assessment of female reproductive system in preclinical phase for innovative drug molecules.
Conflicts of Interest
All authors disclosed no relevant relationships.
Acknowledgement
The authors gratefully acknowledge the support of TriApex Laboratories Co., LTD.
Author Contributions Statement
Yafei Zhang: Conceptualization, Data curation, manuscript writing. Haoyun Li, Mengjun Dai: Formal analysis, Methodology. Minghui Feng, Yi Lou : Formal analysis, Methodology, Software. Dandan Li, Ji Yang, Juan Lv: Methodology, Resources, Validation. Lingna Li: Project administration, Resources, Supervision. Hui Zhang: Conceptualization, Funding acquisition, manuscript revising.
References
2. Li W, Picard F. Toxicokinetics in preclinical drug development of small-molecule new chemical entities. Biomed Chromatogr. 2023;37(7):e5553.
3. Scudiero R. Molecular Research on Reproductive Toxicity. Int J Mol Sci. 2023;24(4):3538.
4. Heiligentag M, Eichenlaub-Ritter U. Preantral follicle culture and oocyte quality. Reprod Fertil Dev. 2017;30(1):18-43.
5. Zhu Q, Li Y, Ma J, Ma H, Liang X. Potential factors result in diminished ovarian reserve: a comprehensive review. J Ovarian Res. 2023 Oct 25;16(1):208.
6. Ge W, Li L, Dyce PW, De Felici M, Shen W. Establishment and depletion of the ovarian reserve: physiology and impact of environmental chemicals. Cell Mol Life Sci. 2019;76(9):1729-46.
7. Ciucci A, Buttarelli M, Fagotti A, Scambia G, Gallo D. Preclinical models of epithelial ovarian cancer: practical considerations and challenges for a meaningful application. Cell Mol Life Sci. 2022;79(7):364.
8. Kagami K, Shinmyo Y, Ono M, Kawasaki H, Fujiwara H. Three-dimensional evaluation of murine ovarian follicles using a modified CUBIC tissue clearing method. Reprod Biol Endocrinol. 2018 Aug 2;16(1):72.
9. Forabosco A, Sforza C. Establishment of ovarian reserve: a quantitative morphometric study of the developing human ovary. Fertil Steril. 2007;88(3):675-83.
10. Tschanz SA, Burri PH, Weibel ER. A simple tool for stereological assessment of digital images: the STEPanizer. J Microscopy. 2010;243(1):47-59.
11. Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127(5):569-80.
12. Foote RH, Carney EW. The rabbit as a model for reproductive and developmental toxicity studies. Reprod Toxicol. 2000;14(6):477-93.
13. Altunkaynak BZ, Akgül N, Yahyazadeh A, Altunkaynak ME, Turkmen AP, Akgül HM, et al. Effect of mercury vapor inhalation on rat ovary: Stereology and histopathology. J Obstet Gynaecol Res. 2016 Apr;42(4):410-16.
14. Taketa Y, Horie K, Goto T, Ohta E, Nakano-Ito K, Hayakawa K, et al. Histopathologic Characterization of Mifepristone-induced Ovarian Toxicity in Cynomolgus Monkeys. Toxicol Pathol. 2018 Apr;46(3):283-9.
15. Laffan SB, Posobiec LM, Uhl JE, Vidal JD. Species Comparison of Postnatal Development of the Female Reproductive System. Birth Defects Res. 2018;110(3):163-89.
16. Rickard BP, Rizvi I, Fenton SE. Per- and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination, endocrine-mediated effects, and disease. Toxicology. 2022 Jan 15;465:153031.
17. Regan KS, Cline JM, Creasy D, Davis B, Foley GL, Lanning L, et al. STP position paper: ovarian follicular counting in the assessment of rodent reproductive toxicity. Toxicol Pathol. 2005;33(3):409-12.
18. Johansson HKL, Damdimopoulou P, van Duursen MBM, Boberg J, Franssen D, de Cock M, et al. Putative adverse outcome pathways for female reproductive disorders to improve testing and regulation of chemicals. Arch Toxicol. 2020 Oct;94(10):3359-79.
19. Bucci TJ, Bolon B, Warbritton AR, Chen JJ, Heindel JJ. Influence of sampling on the reproducibility of ovarian follicle counts in mouse toxicity studies. Reprod Toxicol. 1997;11(5):689-96.
20. U.S. Environmental Protection Agency. Health Effects Test Guideline. OPPTS 870.3550. Reproduction/Developmental Toxicity Screening Test. EPA 712-C-00-367. U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Washington, DC. 2000.
21. U.S. Food and Drug Administration. Redbook 2000. Toxicologial Principles for the Safety Assessment of Food Ingredients. IV.C.9.a. Guidelines for Reproductive Studies. U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition, Washington, DC. 2000.
22. Tilly JL. Ovarian follicle counts--not as simple as 1, 2, 3. Reprod Biol Endocrinol. 2003 Feb 6;1:11.
23. Winship AL, Griffiths M, Lliberos Requesens C, Sarma U, Phillips KA, Hutt KJ. The PARP inhibitor, olaparib, depletes the ovarian reserve in mice: implications for fertility preservation. Hum Reprod. 2020;35(8):1864-74.
24. Miller PL, Meyer TW. Methods in laboratory investigation: effects of tissue preparation on glomerular volume and capillary structure in the rat. Lab Invest. 1990;63(6):862-6.
25. Tu W, Ni D, Yang H, Zhao F, Yang C, Zhao X, et al. Deciphering the dynamics of the ovarian reserve in cynomolgus monkey through a quantitative morphometric study. Sci Bull (Beijing). 2022 Sep 30;67(18):1854-9.
26. Gundersen HJG, Mirabile R, Brown D, Boyce RW. Stereological principles and sampling procedures for toxicologic pathologists. In: Waschek WM, Rousseaux CG, Wallig MA, eds. Haschek and Rousseaux’s Handbook of Toxicologic Pathology. 3rd ed. London, UK: Academic Press; 2013:215-86.
27. Sarma UC, Winship AL, Hutt KJ. Comparison of methods for quantifying primordial follicles in the mouse ovary. J Ovarian Res. 2020 Oct 14;13(1):121.
28. Léveillé P, Tarrade A, Dupont C, et al. Maternal high-fat diet induces follicular atresia but does not affect fertility in adult rabbit offspring. J Dev Orig Health Dis. 2014;5(2):88-97.
29. Giuliano F, Pfaus J, Balasubramanian S, Hedlund P, Hisasue SI, Marson L, et al. Experimental models for the study of female and male sexual function. J of Sexual Medicine. 2010 Sep;7(9):2970-95.
30. Cai Y, Chen Z, Zhang Z, Zhang L, Li M, Liu C. A 30-day preclinical safety evaluation study of recombinant human follicle-stimulating hormone in female rhesus monkeys. Int J Toxicol. 2011;30(2):153-61.
31. Francés-Herrero E, Bueno-Fernandez C, Rodríguez-Eguren A, Gómez-Álvarez M, Faus A, Soto-Prado A, et al. Growth factor-loaded ovarian extracellular matrix hydrogels promote in vivo ovarian niche regeneration and enhance fertility in premature ovarian insufficiency preclinical models. Acta Biomater. 2024 Sep 15;186:125-40.
32. Wang C, Zhou B, Xia G. Mechanisms controlling germline cyst breakdown and primordial follicle formation [published correction appears in Cell Mol Life Sci. 2017 Jul;74(14):2567.
33. BAKER TG. A QUANTITATIVE AND CYTOLOGICAL STUDY OF GERM CELLS IN HUMAN OVARIES. Proc R Soc Lond B Biol Sci. 1963 Oct 22;158:417-33.
34. Boateng R, Boechat N, Henrich PP, Bolcun-Filas E. Whole Ovary Immunofluorescence, Clearing, and Multiphoton Microscopy for Quantitative 3D Analysis of the Developing Ovarian Reserve in Mouse. J Vis Exp. 2021 Sep 3;(175):10.3791/62972.
35. Winship AL, Sarma UC, Alesi LR, Hutt KJ. Accurate Follicle Enumeration in Adult Mouse Ovaries. J Vis Exp. 2020 Oct 16;(164).