Abstract
Aims: To assess the impact of different intravenous (IV) irons on healthcare resource utilization (HCRU), cost of care (CoC), and post-treatment iron deficiency/iron deficiency anemia (ID/IDA) in elderly patients with heart failure (HF) and ID/IDA.
Methods: This retrospective cohort study used a US healthcare claims database. Eligible patients were ≥ 65 years old with ≥ 1 medical claim for HF and ID/IDA. Patients were indexed on their first IV iron claim and had ≥ 12 months continuous enrollment before and after the index date. Inverse probability of treatment weighting (IPTW) was used to compare HF-related HCRU, CoC, and ID/IDA rates in the 12 months post-index in patients receiving different IV iron products.
Results: After IPTW, 6,471 patients were included with a mean age of approximately 82 years. Nearly 60% of patients were female and around 80% of patients were white. The mean CCI scores ranged from 3.70 to 3.84 across different IV iron cohorts. Above 90% of patients had hypertension, which was the most prevalent comorbidity. Patients receiving ferric carboxymaltose (FCM) had fewer HF-related medical visits than patients receiving sodium ferric gluconate complex in sucrose (FG) (ratio=0.74, P=0.03), ferumoxytol (FM) (ratio=0.97, P=0.75) and iron sucrose (IS) (ratio=0.87, P=0.11). Patients taking FCM had the shortest HF-related hospital inpatient stay (vs. FG ratio=0.20, P=0.02; vs. low-molecular-weight iron dextran [LMWID] ratio=0.27, P=0.04; vs. IS ratio=0.32, P=0.01; vs. FM ratio=0.40, P=0.08) and lowest HF-related CoC (vs. FG ratio=0.48, P<0.0001; vs. LMWID ratio=0.67, P<0.0001; vs. IS ratio=0.69, P<0.0001; vs. FM ratio=0.93, P=0.32). FCM recipients were least likely to have ID/IDA versus patients taking other IV irons in the follow-up period.
Limitations: Findings may not be generalizable to non-Medicare patients or those with HF as a secondary or other diagnosis. Patients with ID/IDA may not be fully captured due to lack of transferrin values.
Conclusions: Patients receiving FCM had the shortest HF-related hospital inpatient stay, lowest HF-related medical CoC, and lowest proportions of ID/IDA in the follow-up period compared to other IV irons. FCM may confer potential benefits in reducing HCRU and CoC that decreases the economic burden of ID/IDA on HF patients and healthcare plans.
Keywords
Cost of care, Ferric carboxymaltose, Healthcare resource utilization, Health outcomes, Heart failure, Intravenous iron, Iron deficiency, Iron deficiency anemia
Introduction
Iron deficiency (ID) and iron deficiency anemia (IDA) are highly prevalent in patients with heart failure (HF), with up to 50% of ambulatory patients with HF having ID [1]. However, the actual proportion of patients with HF having ID potentially could be higher, as iron status is not always measured [2-4]. ID, regardless of anemia, is an independent predictor of poor exercise capacity and reduced quality of life, resulting in worse survival among patients with HF [1,5,6]. Likewise, anemia is associated with poor prognosis in patients with HF [7], with increased risk of all-cause mortality and HF-related events as well as reduced New York Heart Association (NYHA) functional capacity [8,9]. Anemia is also an independent predictor of 1-year mortality among patients with HF, with the mortality rate increasing with the severity of the anemia [10].
In studies investigating health care costs associated with HF and anemia in US patients, patients with anemia have been found to have higher health care costs, longer length of stay (LOS), and higher hospital mortality rate than those without anemia [11-15]. Average annual anemia-attributed expenses were estimated to be $563 per patient in 2005 [13].
Treatment with intravenous (IV) iron has been shown to improve outcomes in patients with HF and ID/IDA, including reduced HF-related hospitalization and improved iron status and functional status [16-21]. The 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure recommends obtaining iron studies for all patients with HF, and IV iron replacement for patients with HF and ID regardless of anemia [22]. IV iron products (i.e., ferric carboxymaltose, low-molecular-weight iron dextran, iron sucrose, sodium ferric gluconate complex in sucrose, ferric derisomaltose, and ferumoxytol [23]) are preferred over oral iron treatments to manage ID/IDA in patients with HF [24,25]. IV iron products differ by the type of carbohydrate used to encase the iron salt and consequently have different physicochemical properties, dosages, and administration times [23]. The FDA approved the ferric carboxymaltose injection for the treatment of ID in adult patients with HF in June 2023, making it the first and only IV iron replacement therapy indicated for this patient population. However, health care resource utilization (HCRU) and cost of care (CoC) related to different IV iron products have not been well studied. Therefore, in this study, we aimed to investigate HF-related HCRU, medical CoC, and ID/IDA rates associated with different IV iron products in patients with HF and ID/IDA.
Methods
Study design
This was a retrospective, observational study using a claims database of the US Medicare population. The database included de-identified information linked to claims for medical, laboratory, and pharmacy costs for a large representative sample of individuals enrolled in Medicare Advantage in the US [26]. There are limitations to using a claims database, such that the data is collected for billing purposes. Therefore, there are constraints on lab data such as lack of transferrin values, which limits the capability of identifying patients with ID/IDA. As data were fully anonymized, institutional review board approval was not necessary.
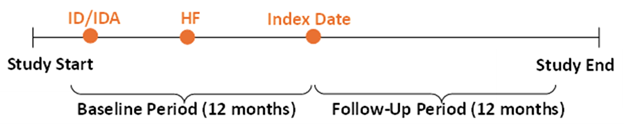
The study period was between January 2007 and September 2022. The index period was between January 2008 and September 2021, allowing for a 12-month baseline period and a 12-month follow-up period (Figure 1). The index date was the date of the first claim for an IV iron product during the index period. Patients were classified into five study cohorts (i.e., ferric carboxymaltose, ferumoxytol, sodium ferric gluconate complex in sucrose, low-molecular-weight iron dextran, or iron sucrose) based on their index treatment.
Figure 1. Study design. HF: Heart Failure; ID/IDA: Iron Deficiency/Iron Deficiency Anemia.
Patients were included in the analysis if they had one of the IV iron products during the index period, had at least one medical claim with an International Classification of Diseases code of HF as the primary diagnosis during the baseline period, had a diagnosis of ID or IDA during the study period, were aged ≥ 65 years on the index date, and were continuously enrolled during the baseline and follow-up periods.
Patients were excluded from the analysis if they had received any IV iron products in the 12 months prior to the index date, received more than one IV iron product during the follow-up period, or had one of the following conditions in the study period: stage 5 chronic kidney disease, end-stage renal disease, dialysis, a left ventricular assist device implantation, amyloidosis, cardiac transplantation, or congenital heart disease.
Outcomes and patient characteristics
This study evaluated HF-related HCRU, medical CoC, and proportions of patients with ID/IDA in each of the 5 study cohorts, which were generated from claims with HF being the primary diagnosis during the 12-month follow-up period. For each claim, there was a cost and place of service associated with the specific service rendered such as implantation of hemodynamic monitor in hospital inpatient and transthoracic echocardiography in an outpatient setting, which allowed for calculating HCRU and CoC by different service locations, including hospital inpatient, skilled nursing facility, emergency department (ED), outpatient, and physician office visits. The place of service was determined based on the Place of Service Codes for Professional Claims published by Centers for Medicare and Medicaid Services [27]. Hospital inpatient was defined as a portion of a hospital that primarily provided diagnostic, therapeutic and rehabilitation services under the supervision of physicians to patients who were admitted. Skilled nursing facilities were defined as facilities that primarily provided inpatient skilled nursing care and related services, but did not provide the level of care or treatment available in a hospital. ED was defined as a portion of a hospital where emergency diagnosis and treatment of illness or injury were provided. Outpatient was defined as a portion of a hospital that provided diagnostic, therapeutic and rehabilitation services to patients who did not require hospitalization. Office was defined as a location where the health professionals routinely provided services to patients on an ambulatory basis.
HF-related HCRU was assessed as the number of medical visits (1000 patients per month [1000PPM]), which were further categorized into hospital inpatient, skilled nursing facility, ED, outpatient, and physician office visits. LOS was calculated as the number of days per 1000 PPM, which included stays at hospital inpatient and skilled nursing facilities. HF-related direct medical CoC (per patient per month [PPPM]) was evaluated, which was further categorized into costs related to inpatient, skilled nursing facility, ED, outpatient, and physician office visits. Costs were inflated to 2022 US dollars. Proportions of patients with ID (serum ferritin <100 μg/L) or IDA (Hb <13 g/dL in males or <12 g/dL in females) were evaluated during the follow-up period.
Baseline demographic characteristics were assessed, including age as of the index date, sex (female/male), race (white, black, other), and US geographic region (Midwest, Northeast, South, West). Clinical characteristics were also assessed during the baseline period, including the Charlson Comorbidity Index (CCI) calculated using the Deyo adaptation [28] with a weighted approach, and chronic comorbidities (coronary artery disease, atrial fibrillation, hypertension, diabetes, hyperlipidemia, renal disorders, cancers, and inflammatory bowel diseases). Baseline HF-related HCRU and medical CoC were also evaluated for each cohort.
Statistical analysis
Descriptive statistics were reported for demographic and clinical characteristics. Frequencies and percentages were used to describe categorical variables, and comparisons were made using Chi-square tests. Means with standard deviations (SDs) or 95% confidence intervals (CIs) were used to describe continuous variables, and comparisons were made using analysis of variance (ANOVA).
Inverse probability of treatment weighting (IPTW) was used to balance baseline demographics, clinical characteristics, HCRU, and CoC. IPTW is a method to adjust for confounding and reduce selection biases in observational studies [29]. It calculates the probability (i.e., propensity score) of receiving the treatment accounting for patients’ baseline characteristics. Then each patient is weighted by the inverse probability of receiving the treatment. Balance was checked using the standardized mean difference (SMD), with SMD <0.1 indicating balance was achieved between cohorts [29].
Generalized linear models with negative binomial distribution and log-link function were used to assess number of visits and LOS. Generalized linear models with gamma distribution and log-link function were used to assess medical CoC. Presence of ID/IDA in the follow-up period was compared using logistic regression.
All statistical analyses were conducted using SAS® 9.4 (SAS Institute Inc., Cary, NC, US), with the significance level set at P<0.05.
Results
With IPTW, 6,471 patients were identified and included in the analysis and were divided into 5 cohorts, including the ferric carboxymaltose (n=1600; 24.7%), ferumoxytol (n=1341; 20.7%), sodium ferric gluconate complex in sucrose (n=522; 8.1%), low-molecular-weight iron dextran (n=717; 11.1%), and iron sucrose cohorts (n=2291; 35.5%) (Supplementary Figure S1).
Patient demographics are summarized in Table 1. Across the 5 cohorts, patients had a mean age of approximately 82 years and nearly 60% of patients were female. Around 80% of patients were white, and two-thirds of patients were from the Southern US. The mean (SD) CCI scores were similar among cohorts and ranged from 3.70 (2.47) for the iron sucrose cohort to 3.84 (2.59) for the low-molecular-weight iron dextran cohort. Among the comorbidities, hypertension was the most prevalent, affecting more than 95% of patients in each cohort. In terms of baseline HCRU (Table 2), total medical HF-related visits were lowest in the low-molecular-weight iron dextran cohort (mean [95% CI]: 196 [176, 219] 1000PPM) and highest in the sodium ferric gluconate complex in sucrose cohort (231 [203, 263] 1000PPM). Baseline HF-related total medical CoC was lowest in the ferric carboxymaltose cohort (mean [95% CI ]: $70 [65, 77] PPPM) and highest in the sodium ferric gluconate complex in sucrose cohort ($112 [96, 130] PPPM; Table 2).
|
|
Overall (n=6471) |
Ferric carboxymaltose |
Ferumoxytol |
Ferric gluconate |
Low-molecular-weight iron dextran |
Iron sucrose |
P value |
|
Age, years, mean (SD) |
81.6 (13.06) |
81.3 (13.08) |
81.7 (13.14) |
81.53 (13.00) |
81.18 (12.59) |
81.78 (13.17) |
0.74 |
|
Age groups, years, n (%) |
|
|
|
|
|
|
0.94 |
|
65–74 |
2365 (36.55) |
590 (36.85) |
484 (36.09) |
194 (37.25) |
272 (37.88) |
826 (36.04) |
|
|
75–84 |
2479 (38.31) |
629 (39.29) |
514 (38.31) |
196 (37.51) |
267 (37.26) |
874 (38.15) |
|
|
≥ 85 |
1626 (25.13) |
382 (23.86) |
343 (25.59) |
132 (25.25) |
178 (24.86) |
591 (25.81) |
|
|
Sex, n (%) |
|
|
|
|
|
|
0.97 |
|
Male |
2630 (40.64) |
646 (40.37) |
554 (41.30) |
215 (41.12) |
285 (39.76) |
930 (40.61) |
|
|
Female |
3841 (59.36) |
954 (59.63) |
787 (58.70) |
307 (58.88) |
432 (60.24) |
1361 (59.39) |
|
|
Race, n (%) |
|
|
|
|
|
|
1.00 |
|
White |
5214 (80.58) |
1291 (80.68) |
1085 (80.94) |
418 (80.14) |
570 (79.48) |
1850 (80.75) |
|
|
Black |
907 (14.02) |
225 (14.07) |
185 (13.80) |
76 (14.47) |
105 (14.67) |
316 (13.80) |
|
|
Other |
349 (5.40) |
84 (5.25) |
71 (5.26) |
28 (5.39) |
42 (5.85) |
125 (5.45) |
|
|
Geographic region, n (%) |
|
|
|
|
|
|
0.87 |
|
Midwest |
1566 (24.21) |
384 (24.03) |
333 (24.84) |
128 (24.53) |
153 (21.38) |
567 (24.77) |
|
|
Northeast |
170 (2.63) |
39 (2.45) |
35 (2.61) |
14 (2.64) |
20 (2.76) |
62 (2.72) |
|
|
South |
4303 (66.50) |
1076 (67.27) |
892 (66.54) |
343 (65.78) |
490 (68.28) |
1501 (65.53) |
|
|
West |
432 (6.67) |
100 (6.25) |
81 (6.01) |
37 (7.05) |
54 (7.58) |
160 (6.98) |
|
|
CCI, mean (SD) |
3.74 (2.51) |
3.74 (2.55) |
3.79 (2.57) |
3.64 (2.30) |
3.84 (2.59) |
3.7 (2.47) |
0.53 |
|
CCI groups, n (%) |
|
|
|
|
|
|
1.00 |
|
0 |
249 (3.85) |
63 (3.91) |
51 (3.78) |
20 (3.89) |
27 (3.77) |
88 (3.86) |
|
|
1 |
774 (11.97) |
201 (12.58) |
154 (11.45) |
59 (11.40) |
79 (11.04) |
281 (12.25) |
|
|
2 |
1137 (17.58) |
274 (17.11) |
241 (17.99) |
92 (17.61) |
127 (17.66) |
404 (17.62) |
|
|
3 |
1341 (20.72) |
330 (20.61) |
273 (20.38) |
107 (20.42) |
158 (22.06) |
473 (20.65) |
|
|
4 |
1214 (18.76) |
301 (18.78) |
255 (19.02) |
103 (19.74) |
126 (17.58) |
430 (18.75) |
|
|
5 |
620 (9.58) |
147 (9.20) |
128 (9.53) |
48 (9.28) |
74 (10.26) |
223 (9.72) |
|
|
≥6 |
1136 (17.55) |
285 (17.80) |
239 (17.85) |
92 (17.66) |
126 (17.63) |
393 (17.15) |
|
|
Comorbidities, n (%) |
|
|
|
|
|
|
|
|
CAD |
4609 (71.23) |
1131 (70.66) |
957 (71.41) |
374 (71.60) |
512 (71.40) |
1635 (71.38) |
0.99 |
|
Atrial fibrillation |
3543 (54.75) |
886 (55.37) |
738 (55.09) |
277 (53.15) |
385 (53.63) |
1256 (54.84) |
0.87 |
|
Hypertension |
6213 (96.01) |
1534 (95.87) |
1290 (96.23) |
502 (96.17) |
689 (96.00) |
2199 (95.96) |
0.99 |
|
Diabetes |
3861 (59.67) |
948 (59.25) |
799 (59.59) |
311 (59.55) |
435 (60.67) |
1369 (59.73) |
0.98 |
|
Hyperlipidemia |
5679 (87.76) |
1402 (87.62) |
1182 (88.15) |
460 (88.22) |
630 (87.74) |
2005 (87.52) |
0.98 |
|
Renal disorders |
3939 (60.88) |
967 (60.44) |
816 (60.84) |
315 (60.30) |
427 (59.58) |
1415 (61.75) |
0.84 |
|
Cancers |
1639 (25.34) |
406 (25.39) |
356 (26.53) |
134 (25.61) |
188 (26.19) |
556 (24.26) |
0.61 |
|
IBD |
582 (8.99) |
140 (8.75) |
119 (8.90) |
52 (10.02) |
63 (8.81) |
207 (9.05) |
0.93 |
|
CAD: Coronary Artery Disease; CCI: Charlson Comorbidity Index; IBD: Inflammatory Bowel Diseases; IPTW: Inverse Probability of Treatment Weighting; IV: Intravenous; SD: Standard Deviation. a) Patient counts were rounded to the nearest whole number. Percentages were calculated based on unrounded patient counts. |
|||||||
|
Parameter, Mean (95% CI) |
Ferric carboxymaltose |
Ferumoxytol |
Ferric gluconate |
Low-molecular-weight iron dextran |
Iron sucrose |
|
HF-related number of visits (1000PPM) |
|||||
|
Total medical |
215 (200, 232) |
221 (204, 240) |
231 (203, 263) |
196 (176, 219) |
221 (207, 235) |
|
Hospital inpatient |
5 (3, 7) |
9 (6, 14) |
13 (7, 24) |
13 (8, 24) |
12 (9, 16) |
|
Skilled nursing facilities |
2 (1, 4) |
2 (1, 5) |
1 (0, 4) |
1 (0, 6) |
2 (1, 4) |
|
ED |
25 (20, 32) |
29 (22, 37) |
35 (23, 53) |
26 (18, 37) |
29 (24, 35) |
|
Outpatient |
72 (60, 87) |
75 (61, 92) |
78 (56, 108) |
52 (40, 69) |
74 (63, 86) |
|
Physician office |
111 (99, 125) |
106 (93, 121) |
105 (85, 128) |
103 (86, 122) |
104 (95, 115) |
|
HF-related LOS (days per 1000PPM) |
|||||
|
Hospital inpatient |
23 (15, 35) |
49 (31, 78) |
64 (30, 134) |
72 (38, 136) |
61 (42, 86) |
|
Skilled nursing facilities |
36 (12, 105) |
34 (11, 111) |
12 (2, 80) |
22 (4, 111) |
33 (14, 82) |
|
HF-related CoC ($US PPPM), |
|||||
|
Total medical |
70 (65, 77) |
94 (86, 103) |
112 (96, 130) |
102 (90, 116) |
100 (93, 107) |
|
Hospital inpatient |
29 (26, 33) |
48 (42, 55) |
77 (61, 97) |
66 (55, 80) |
62 (55, 69) |
|
Skilled nursing facilities |
5 (5, 6) |
5 (5, 6) |
2 (2, 3) |
3 (2, 3) |
4 (4, 5) |
|
ED |
8 (7, 9) |
9 (8, 10) |
11 (9, 13) |
9 (7, 10) |
9 (9, 10) |
|
Outpatient |
17 (15, 19) |
21 (19, 24) |
12 (10, 15) |
16 (14, 19) |
14 (13, 16) |
|
Physician office |
11 (10, 12) |
10 (10, 12) |
9 (8, 11) |
9 (8, 10) |
10 (9, 11) |
|
1000 PPM: 1000 Patients Per Month; CI: Confidence Interval; CoC: Cost of Care; ED: Emergency Department; HCRU: Healthcare Resource Utilization; HF: Heart Failure; IPTW: Inverse Probability of Treatment Weighting; IV: intravenous; LOS: Length of Stay; PPPM: Per Patient Per Month. |
|||||
In the follow-up period, the number of HF-related medical visits was lowest in patients receiving low-molecular-weight iron dextran (mean [95% CI]: 148 [121, 182] 1000PPM), followed by those receiving ferric carboxymaltose (153 [133, 175] 1000PPM) and those receiving ferumoxytol (158 [136, 183] 1000PPM); HF-related medical visits were highest in patients receiving iron sucrose (177 [158, 198] 1000PPM) and those receiving sodium ferric gluconate complex in sucrose (206 [162, 262] 1000PPM; Figure 2A; Supplementary Table S1). Compared to sodium ferric gluconate complex in sucrose (Table 3; Supplementary Table S1), ferric carboxymaltose was associated with a significantly lower number of HF-related visits (ratio [95% CI]: 0.74 [0.56, 0.98]; P=0.0340) as well as fewer hospital inpatient visits (0.20 [0.06, 0.66]; P=0.0087) and ED visits (0.38 [0.19, 0.77]; P=0.0074). Ferric carboxymaltose also had a lower number of HF-related visits compared to ferumoxytol (0.97 [0.79, 1.19]; P=0.7533) and iron sucrose (0.87 [0.72, 1.03]; P=0.1115), though not statistically significant.
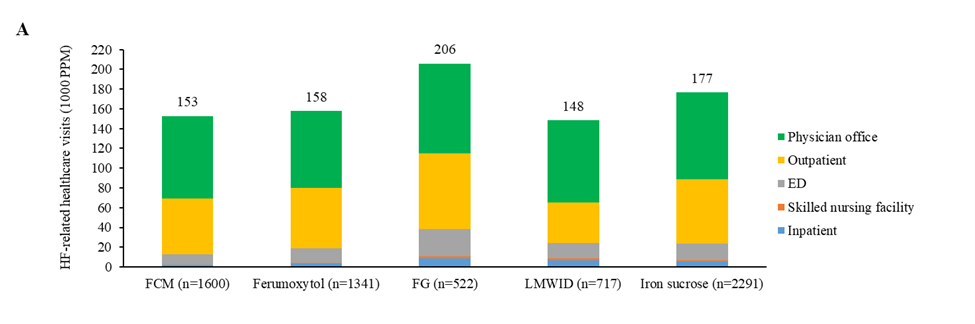
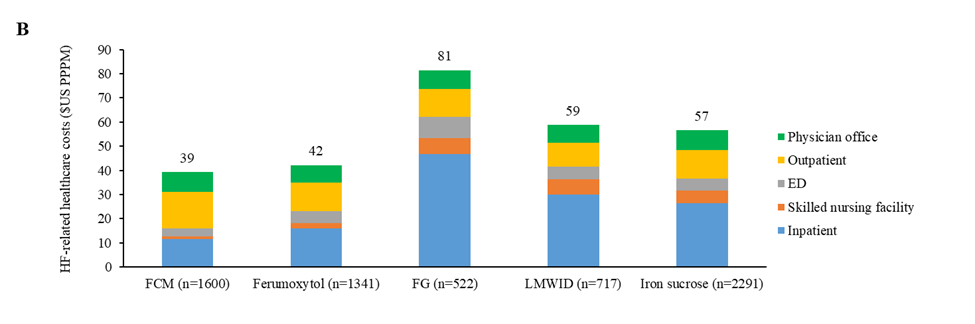
Figure 2. HF-related (A) healthcare visits and (B) CoC in the 12 months after each IV iron administration (total and by category) (IPTW). 1000 PPM: 1000 Patients Per Month; CoC: Cost of Care; ED: Emergency Department; FCM: Ferric Carboxymaltose; FG: Sodium Ferric Gluconate Complex in Sucrose; HF: Heart Failure; IPTW: Inverse Probability of Treatment Weighting; IV: Intravenous; LMWID: Low-Molecular-Weight Iron Dextran; PPPM: Per Patient Per Month.
Ferric carboxymaltose was associated with the shortest LOS of 8 days per 1000 PPM each at hospital inpatient and at skilled nursing facilities (Supplementary Table S1). The hospital inpatient LOS was significantly shorter in patients receiving ferric carboxymaltose versus sodium ferric gluconate complex in sucrose (ratio [95% CI]: 0.20 [0.05, 0.77]; P=0.0200), low-molecular-weight iron dextran (0.27 [0.08, 0.91]; P=0.0353), and iron sucrose (0.32 [0.13, 0.79]; P=0.0128; Table 3). Ferric carboxymaltose also had shorter hospital inpatient LOS than ferumoxytol (0.40 [0.15, 1.11]; P=0.0786), though this was not statistically significant. There were no significant differences in skilled nursing facility LOS in the ferric carboxymaltose cohort versus other cohorts (Table 3).
|
Parameter, Ratio (95% CI) [P value] |
Ferric carboxymaltose vs |
Ferric carboxymaltose vs |
Ferric carboxymaltose vs |
Ferric carboxymaltose vs |
|
HF-related number of visits (1000PPM) |
||||
|
Total medical |
0.97 (0.79, 1.19) [P=0.7533] |
0.74 (0.56, 0.98) [P=0.0340] |
1.03 (0.8, 1.32) [P=0.8176] |
0.87 (0.72, 1.03) [P=0.1115] |
|
Hospital inpatient |
0.52 (0.21, 1.27) [P=0.1515] |
0.2 (0.06, 0.66) [P=0.0087] |
0.27 (0.09, 0.79) [P=0.0173] |
0.34 (0.16, 0.75) [P=0.0075] |
|
Skilled nursing facilities |
0.81 (0.18, 3.59) [P=0.7773] |
0.33 (0.04, 2.56) [P=0.2912] |
0.30 (0.05, 1.82) [P=0.1891] |
0.32 (0.09, 1.21) [P=0.0927] |
|
ED |
0.74 (0.44, 1.23) [P=0.2421] |
0.38 (0.19, 0.77) [P=0.0074] |
0.69 (0.37, 1.29) [P=0.2476] |
0.65 (0.42, 1.03) [P=0.0672] |
|
Outpatient |
0.92 (0.64, 1.33) [P=0.6636] |
0.74 (0.45, 1.22) [P=0.2401] |
1.38 (0.88, 2.16) [P=0.1579] |
0.87 (0.63, 1.2) [P=0.3836] |
|
Physician office |
1.07 (0.84, 1.36) [P=0.5873] |
0.91 (0.66, 1.27) [P=0.5941] |
1 (0.74, 1.34) [P=0.9850] |
0.95 (0.76, 1.17) [P=0.6069] |
|
HF-related LOS (days per 1000PPM) |
||||
|
Hospital inpatient |
0.40 (0.15, 1.11) [P=0.0786] |
0.20 (0.05, 0.77) [P=0.0200] |
0.27 (0.08, 0.91) [P=0.0353] |
0.32 (0.13, 0.79) [P=0.0128] |
|
Skilled nursing facilities |
0.51 (0.08, 3.21) [P=0.4737] |
0.22 (0.02, 2.69) [P=0.2367] |
0.19 (0.02, 1.77) [P=0.1452] |
0.25 (0.05, 1.25) [P=0.0920] |
|
HF-related CoC ($US PPPM) |
||||
|
Total medical |
0.93 (0.81, 1.07) [P=0.3208] |
0.48 (0.4, 0.58) [P<0.0001] |
0.67 (0.56, 0.79) [P<0.0001] |
0.69 (0.61, 0.79) [P<0.0001] |
|
Hospital inpatient |
0.72 (0.60, 0.87) [P=0.0005] |
0.25 (0.19, 0.32) [P<0.0001] |
0.38 (0.30, 0.48) [P<0.0001] |
0.43 (0.37, 0.51) [P<0.0001] |
|
Skilled nursing facilities |
0.58 (0.49, 0.67) [P<0.0001] |
0.19 (0.16, 0.24) [P<0.0001] |
0.2 (0.17, 0.25) [P<0.0001] |
0.25 (0.22, 0.28) [P<0.0001] |
|
ED |
0.62 (0.53, 0.73) [P<0.0001] |
0.36 (0.29, 0.44) [P<0.0001] |
0.59 (0.49, 0.72) [P<0.0001] |
0.63 (0.55, 0.72) [P<0.0001] |
|
Outpatient |
1.30 (1.1, 1.53) [P=0.0018] |
1.31 (1.05, 1.64) [P=0.0168] |
1.57 (1.29, 1.91) [P<0.0001] |
1.29 (1.12, 1.49) [P=0.0004] |
|
Physician office |
1.13 (0.98, 1.3) [P=0.0939] |
1.07 (0.88, 1.29) [P=0.4968] |
1.10 (0.93, 1.31) [P=0.2554] |
1.00 (0.89, 1.14) [P=0.9420] |
|
1000PPM: 1000 Patients Per Month; CI: Confidence Interval; CoC: Cost of Care; ED: Emergency Department; HCRU: Healthcare Resource Utilization; HF: Heart Failure; IPTW: Inverse Probability of Treatment Weighting; IV: Intravenous; LOS: Length of Stay; PPPM: Per Patient Per Month. |
||||
HF-related medical CoC was lowest in patients receiving ferric carboxymaltose ($39 [36, 43] PPPM) and those receiving ferumoxytol ($42 [38, 47] PPPM), followed by patients taking iron sucrose ($57 [52, 61] PPPM) and those receiving low-molecular weight iron dextran ($59 [51, 68] PPPM), and was the highest in patients taking sodium ferric gluconate complex in sucrose ($81 [69, 96] PPPM; Figure 2B; Supplementary Table S1). Significant differences in HF-related medical CoC were found between patients receiving ferric carboxymaltose and those taking sodium ferric gluconate complex in sucrose (ratio [95% CI]: 0.48 [0.40, 0.58]; P<0.0001), low-molecular-weight iron dextran (0.67 [0.56, 0.79]; P<0.0001), or iron sucrose (0.69, [0.61, 0.79]; P<0.0001; Table 3). HF-related medical CoC from hospital inpatient was lowest in patients taking ferric carboxymaltose ($11 [10, 13] PPPM), compared to ferumoxytol ($16 [14, 18] PPPM; ratio [95% CI]: 0.72 [0.60, 0.87]; P=0.0005), sodium ferric gluconate complex ($47 [38, 58] PPPM; 0.25 [0.19, 0.32]; P<0.0001), low-molecular weight iron dextran ($30 [25, 36] PPPM; 0.38 [0.30, 0.48]; P<0.0001), and iron sucrose ($27 [24, 29] PPPM; 0.43 [0.37, 0.51]; P<0.0001;Table 3; Supplementary Table S1). HF-related medical CoC from skilled nursing facilities was lowest in patients taking ferric carboxymaltose ($1 [1, 1] PPPM), compared to ferumoxytol ($2 [2, 2] PPPM; 0.58 [0.49, 0.67]; P<0.0001), sodium ferric gluconate complex ($7 [5, 8] PPPM; 0.19 [0.16, 0.24]; P<0.0001), low-molecular weight iron dextran ($6 [5, 7] PPPM; 0.20 [0.17, 0.25]; P<0.0001), and iron sucrose ($5 [5, 6] PPPM; 0.25 [0.22, 0.28]; P<0.0001;Table 3; Supplementary Table S1). Ferric carboxymaltose had lower HF-related medical CoC from ED ($3 [3, 3] PPPM), compared to ferumoxytol ($5 [4, 6] PPPM; 0.62 [0.53, 0.73]; P<0.0001), sodium ferric gluconate complex ($9 [7, 11] PPPM; 0.36 [0.29, 0.44]; P<0.0001), low-molecular weight iron dextran ($5 [5, 6] PPPM; 0.59 [0.49, 0.72]; P<0.0001), and iron sucrose ($5 [5, 5] PPPM; 0.63 [0.55, 0.72]; P<0.0001;Table 3; Supplementary Table S1). However, ferric carboxymaltose had higher HF-related medical CoC from outpatient care ($15 [14, 17] PPPM), compared to ferumoxytol ($12 [10, 13] PPPM; 1.30 [1.10, 1.53]; P=0.0018), sodium ferric gluconate complex ($12 [10, 14] PPPM; 1.31 [1.05, 1.64]; P=0.0168), low-molecular weight iron dextran ($10 [8, 11] PPPM; 1.57 [1.29, 1.91]; P<0.0001), and iron sucrose ($12 [11, 13] PPPM; 1.29 [1.12, 1.49]; P=0.0004;Table 3; Supplementary Table S1).
Ferric carboxymaltose was associated with a significantly lower proportion of patients having ID during the follow-up period compared with all other IV iron products (ratios ranging from 0.44 to 0.82; P<0.05 for all), and a significantly lower proportion of patients having IDA compared with sodium ferric gluconate complex in sucrose or low-molecular-weight iron dextran and iron sucrose (ratios ranging from 0.55 to 0.76; P<0.05 for all; Figure 3). Ferric carboxymaltose was associated with a lower proportion of patients having IDA than ferumoxytol, though the difference was not statistically significant (ratio: 0.92; P=0.39; Figure 3).
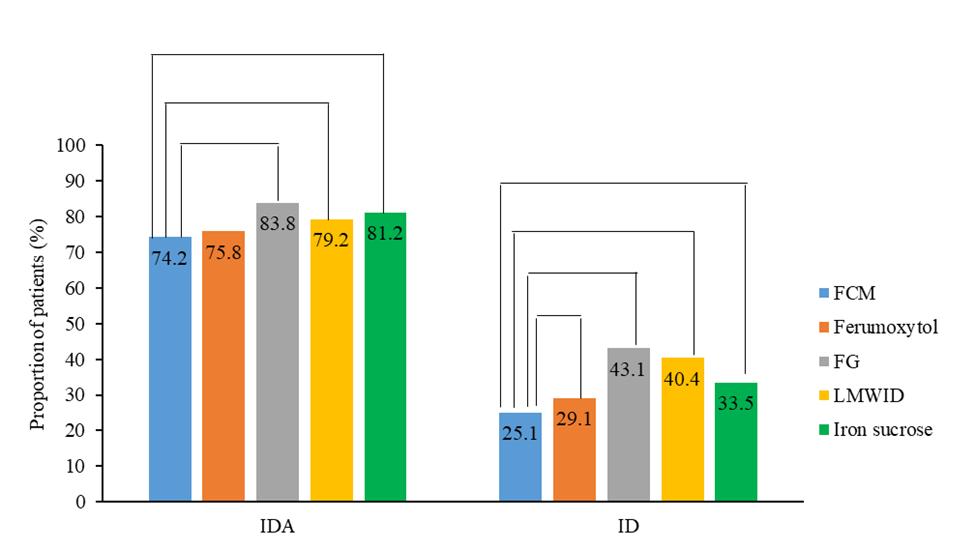
Figure 3. Proportions of patients with ID or IDA in the 12 months after each IV iron administration (IPTW). The proportions of patients with IDA were 74.2% for ferric carboxymaltose, 75.8% for ferumoxytol, 83.8% for sodium ferric gluconate complex in sucrose, 79.2% for low-molecular-weight iron dextran, and 81.2% for iron sucrose. The proportions of patients with ID were 25.1% for ferric carboxymaltose, 29.1% for ferumoxytol, 43.1% for sodium ferric gluconate complex in sucrose, 40.4% for low-molecular-weight iron dextran, and 33.5% for iron sucrose. FCM: Ferric Carboxymaltose; FG: Sodium Ferric Gluconate Complex in Sucrose; ID: Iron Deficiency; IDA: Iron Deficiency Anemia; IPTW: Inverse Probability of Treatment Weighting; IV: Intravenous; LMWID: Low-Molecular-Weight Iron Dextran.
Discussion
There is limited real-world evidence on the impact of different IV iron products on health care outcomes in patients with HF. This study contributes to the existing knowledge base by evaluating the impact of different IV iron products on HF-related HCRU, medical CoC, and proportions of ID/IDA in patients with HF and ID/IDA.
The study results indicate that ferric carboxymaltose is associated with significantly lower HF-related medical CoC and HCRU compared to most of the other IV iron products. This was mainly due to lower CoC and HCRU from hospital inpatient, skilled nursing facilities, and ED, which offset the higher HF-related outpatient cost. The lower inpatient cost was due to significantly shorter hospital stays and significantly fewer inpatient admissions in the ferric carboxymaltose cohort in comparison to the sodium ferric gluconate complex in sucrose, low-molecular-weight iron dextran, and iron sucrose cohorts. Our findings on the low CoC of ferric carboxymaltose are consistent with studies from Europe and Asia showing that ferric carboxymaltose is cost-effective in patients with HF and ID/IDA, by improving patient quality of life/functional status and reducing hospitalization and follow-up costs [30-36].
Despite the CoC in this study being low due to the fact that only costs from claims with HF being the primary diagnosis were considered, the findings of this study are in agreement with several recently published studies comparing ferric carboxymaltose with low-dose IV iron. One study evaluated all-cause HCRU and CoC from 2017 to 2019 in a US commercial population, which found that ferric carboxymaltose was associated with lower number of inpatient admissions (0.68 PPPM vs. 0.94 PPPM and 0.95 PPPM), ED visits (0.11 PPPM vs. 0.15 PPPM and 0.16 PPPM), and outpatient visits (4.13 PPPM vs. 4.52 PPPM and 4.43 PPPM) compared with iron sucrose and low-dose IV iron, respectively [37]. Ferric carboxymaltose had lower all-cause medical CoC than either low-dose IV iron or iron sucrose. The unadjusted total medical CoC was $4081 PPPM for ferric carboxymaltose, $5804 PPPM for low-dose IV iron (adjusted cost ratio=0.78; P<0.0001) and $5691 PPPM for iron sucrose (adjusted cost ratio=0.75; P<0.0001). This study also found that the outpatient CoC was lowest for ferric carboxymaltose than low-dose IV iron (adjusted cost ratio=0.77; P<0.0001) and iron sucrose (adjusted cost ratio=0.73; P<0.0001). Another study investigated healthcare costs in HF patients with IDA and found that ferric carboxymaltose had lower total cost than low-dose IV iron (unadjusted mean cost=$2516 vs. $3434 PPPM) [38]. After adjusting for covariates, total costs were significantly lower for ferric carboxymaltose than low-dose IV iron (adjusted cost ratio= 0.81; P<0.001). Consistent findings have been reported in a third study that assessed the CoC of ferric carboxymaltose and low-dose IV iron [39]. It has been found that all-cause total cost for ferric carboxymaltose was significantly lower than iron sucrose (adjusted cost ratio=0.95; P=0.02) and also lower than low-dose IV iron, though not statistically significant (adjusted cost ratio=0.97; P=0.20). Thus, ferric carboxymaltose demonstrated a benefit for all-cause CoC and HCRU, including in cases where HF was not the primary diagnosis, suggesting that its benefit might extend beyond HF.
In addition to the cost benefit shown for ferric carboxymaltose, it was associated with a significantly lower proportion of patients having ID in the follow-up period, compared with all other IV iron products. A similar benefit in terms of a lower proportion of patients having IDA was observed for patients taking ferric carboxymaltose compared to sodium ferric gluconate complex in sucrose, low-molecular-weight iron dextran, and iron sucrose, further highlighting the value of ferric carboxymaltose in reducing burden of disease and improving patients’ quality of life.
This study has several limitations. First, this study evaluated Medicare patients only because the majority of HF patients are elderly (≥ 65 years of age) [40]. Thus, the results may not be generalizable to patients younger than 65 years old or patients with Medicaid, commercial plans, or no insurance. Future studies may include a more diverse patient population such as younger patients and patients with Medicaid or commercial insurance. A multi-payer approach may provide a more holistic view of HCRU and CoC in patients taking IV iron products. Second, the ID/IDA assessment was based on ferritin and Hb levels, as transferrin saturation values were not available from the database. Therefore, the analysis may not have captured all patients with ID or IDA. Future studies may consider integrating comprehensive laboratory data together with claims data to improve data collection and avoid underestimation of ID/IDA due to lack of transferrin saturation values. There are multiple sources of laboratory data such as electronic medical records and collaboration with clinical laboratories, which may yield more precise patient assessments and outcomes. Third, this study used a follow-up period of 12 months to evaluate HF-related post-treatment HCRU and CoC. This is consistent with a few published studies that evaluated HCRU and CoC of IV iron products from a cross-sectional perspective [37-39,41]. However, future research may use longitudinal study design to follow up patients for a longer period and assess long-term health outcomes, HCRU and CoC of IV iron products. This may provide insights into potential changes in outcomes over time with a more comprehensive understanding of the sustained effects of IV iron products on patient health outcomes and CoC. Lastly, this study did not adjust for unmeasured confounding factors, which may lead to potential biases in results.
During the study period of this analysis, the IV irons assessed were approved only for ID/IDA. The FDA did not approve the ferric carboxymaltose injection for the treatment of ID in adult patients with HF until recently. Future research using databases with larger sample sizes and more patient information (e.g., NYHA functional class) is needed to investigate CoC and HCRU of various IV iron products, with a focus on the period after FDA approval.
Conclusions
Overall, patients taking ferric carboxymaltose had decreased HF-related HCRU, LOS, medical CoC, and proportions of ID/IDA compared to most of the other IV iron products. These findings underline the potential economic benefits of ferric carboxymaltose compared to other IV iron products for health plans and patients with HF and ID/IDA. Future research can target patient populations outside of the Medicare population to further investigate if similar findings are observed.
Conflicts of Interest and Funding Statement
Ethics approval and consent to participate
The ethics committee approval and consent were not required for this study.
Declaration of funding
This work was supported by American Regent, Inc. (Shirley, NY).
Declaration of financial/other relationships
Ye Wang, Jacob Beebe, and Syed Numan are employees of American Regent, Inc.
Author contributions
All authors took part in conception and design of the study; acquisition of data; analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Author contributions statement
All authors meet ICMJE authorship criteria and have made substantial contributions to the study design, data acquisition, analysis, and/or data interpretation and provided intellectual contributions to manuscript development. All authors had full access to all the study protocol and had final responsibility for the decision to submit the manuscript for publication.
Acknowledgments
Catherine Rees (Auckland, New Zealand) and Subhashini Muralidharan (Philadelphia, PA, United States) of inScience Communications, Springer Healthcare, provided medical writing support funded by American Regent, Inc.
References
2. Lindberg F, Lund LH, Benson L, Linde C, Orsini N, Carrero JJ, et al. Iron deficiency in heart failure: screening, prevalence, incidence and outcome data from the Swedish Heart Failure Registry and the Stockholm CREAtinine Measurements collaborative project. Eur J Heart Fail. 2023 Aug;25(8):1270-80.
3. Wong B, Redmond S, Blaine C, Nugent CA, Saiva L, Buckley J, et al. Study of patients with iron deficiency and HF in Ireland: prevalence and treatment budget impact. Br J Cardiol. 2021 Mar 9;28(1):10.
4. Yera HO, Khan A, Akinlade OM, Champsi A, Glouzon VNJ, Spencer C. Improving the Outcome of Patients With Heart Failure: Assessment of Iron Deficiency and Intravenous Iron Replacement. Cureus. 2023 Oct 14;15(10):e47027.
5. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. 2011 Nov;17(11):899-906.
6. Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013 Apr;165(4):575-82.e3.
7. Lee KS, Park DI, Lee J, Oh O, Kim N, Nam G. Relationship between comorbidity and health outcomes in patients with heart failure: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2023 Oct 10;23(1):498.
8. Pan J, Liu M, Huang J, Chen L, Xu Y. Impact of anemia on clinical outcomes in patients with acute heart failure: A systematic review and meta-analysis. Clin Cardiol. 2024 Feb;47(2):e24228.
9. Köseoğlu FD, Özlek B. Anemia and Iron Deficiency Predict All-Cause Mortality in Patients with Heart Failure and Preserved Ejection Fraction: 6-Year Follow-Up Study. Diagnostics (Basel). 2024 Jan 18;14(2):209.
10. Omoomi S, Heidarpour M, Rabanipour N, Saadati M, Vakilbashi O, Shafie D. Prevalence of, association with, severity of, and prognostic role of serum hemoglobin level in acutely decompensated heart failure patients. BMC Cardiovasc Disord. 2023 Oct 4;23(1):491.
11. Allen LA, Anstrom KJ, Horton JR, Shaw LK, Eisenstein EL, Felker GM. Relationship between anemia and health care costs in heart failure. J Card Fail. 2009 Dec;15(10):843-9.
12. Ershler WB, Chen K, Reyes EB, Dubois R. Economic burden of patients with anemia in selected diseases. Value Health. 2005 Nov-Dec;8(6):629-38.
13. Nissenson AR, Wade S, Goodnough T, Knight K, Dubois RW. Economic burden of anemia in an insured population. J Manag Care Pharm. 2005 Sep;11(7):565-74.
14. Solid CA, Foley RN, Gilbertson DT, Collins AJ. Anemia and cost in Medicare patients with congestive heart failure. Congest Heart Fail. 2006 Nov-Dec;12(6):302-6.
15. Nordyke RJ, Kim JJ, Goldberg GA, Vendiola R, Batra D, McCamish M, et al. Impact of anemia on hospitalization time, charges, and mortality in patients with heart failure. Value Health. 2004 Jul-Aug;7(4):464-71.
16. Ogugua FM, Aguilar FA, Gamam A, Maqsood MH, Yoo TK, Kasmi F, et al. Treating Iron Deficiency (ID) Anemia in Heart Failure (HF) Patients with IV Iron: A Meta-Analysis. Cureus. 2023 Jul 14;15(7):e41895.
17. Ponikowski P, Mentz RJ, Hernandez AF, Butler J, Khan MS, van Veldhuisen DJ, et al. Efficacy of ferric carboxymaltose in heart failure with iron deficiency: an individual patient data meta-analysis. Eur Heart J. 2023 Dec 21;44(48):5077-91.
18. Vukadinović D, Abdin A, Emrich I, Schulze PC, von Haehling S, Böhm M. Efficacy and safety of intravenous iron repletion in patients with heart failure: a systematic review and meta-analysis. Clin Res Cardiol. 2023 Jul;112(7):954-66.
19. Hamed M, Elseidy SA, Ahmed A, Thakker R, Mansoor H, Khalili H, et al. Intravenous iron therapy among patients with heart failure and iron deficiency: An updated meta-analysis of randomized controlled trials. Heliyon. 2023 Jun 15;9(6):e17245.
20. Hamza M, Sattar Y, Manasrah N, Patel NN, Rashdi A, Khanal R, et al. Meta-Analysis of Efficacy and Safety of Intravenous Iron in Patients With Iron Deficiency and Heart Failure With Reduced Ejection Fraction. Am J Cardiol. 2023 Sep 1;202:119-30.
21. Martens P, Augusto SN Jr, Mullens W, Tang WHW. Meta-Analysis and Metaregression of the Treatment Effect of Intravenous Iron in Iron-Deficient Heart Failure. JACC Heart Fail. 2024 Mar;12(3):525-36.
22. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
23. Martin-Malo A, Borchard G, Flühmann B, Mori C, Silverberg D, Jankowska EA. Differences between intravenous iron products: focus on treatment of iron deficiency in chronic heart failure patients. ESC Heart Fail. 2019 Apr;6(2):241-53.
24. Loncar G, Obradovic D, Thiele H, von Haehling S, Lainscak M. Iron deficiency in heart failure. ESC Heart Fail. 2021 Aug;8(4):2368-79.
25. Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, et al. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF Randomized Clinical Trial. JAMA. 2017 May 16;317(19):1958-66.
26. Nair R, Mody R, Yu M, Cowburn S, Konig M, Prewitt T. Real-World Treatment Patterns of Glucose-Lowering Agents Among Patients with Type 2 Diabetes Mellitus and Cardiovascular Disease or At Risk for Cardiovascular Disease: An Observational, Cross-Sectional, Retrospective Study. Diabetes Ther. 2022 Dec;13(11-12):1921-32.
27. Place of Service Code Set. Centers for Medicare & Medicaid Services. https://www.cms.gov/medicare/coding-billing/place-of-service-codes/code-sets. Access on Nov 17, 2024.
28. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613-9.
29. Chesnaye NC, Stel VS, Tripepi G, Dekker FW, Fu EL, Zoccali C, et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2021 Aug 26;15(1):14-20.
30. Bourguignon S, Faller M, Champs FO, Moutier H, Levesque K, Caranhac G, et al. Budget impact of intravenous ferric carboxymaltose in patients with chronic heart failure and iron deficiency in France. ESC Heart Fail. 2019 Jun;6(3):559-69.
31. Brock E, Moschovitis G, Maeder MT, Pfister O. Budget Impact of Intravenous Iron Therapy with Ferric Carboxymaltose in Patients with Chronic Heart Failure with Reduced Ejection Fraction (HFrEF) and Iron Deficiency in Switzerland. Pharmacoecon Open. 2022 Sep;6(5):735-43.
32. Gutzwiller FS, Schwenkglenks M, Blank PR, Braunhofer PG, Mori C, Szucs TD, et al. Health economic assessment of ferric carboxymaltose in patients with iron deficiency and chronic heart failure based on the FAIR-HF trial: an analysis for the UK. Eur J Heart Fail. 2012 Jul;14(7):782-90.
33. Hofmarcher T, Borg S. Cost-effectiveness analysis of ferric carboxymaltose in iron-deficient patients with chronic heart failure in Sweden. J Med Econ. 2015;18(7):492-501.
34. Lim EA, Sohn HS, Lee H, Choi SE. Cost-utility of ferric carboxymaltose (Ferinject®) for iron-deficiency anemia patients with chronic heart failure in South Korea. Cost Eff Resour Alloc. 2014 Sep 10;12:19.
35. Rognoni C, Gerzeli S. Ferric carboxymaltose for patients with heart failure and iron deficiency in Italy: cost-effectiveness and budget impact. J Comp Eff Res. 2019 Oct;8(13):1099-110.
36. Theidel U, Väätäinen S, Martikainen J, Soini E, Hardt T, Doehner W. Budget impact of intravenous iron therapy with ferric carboxymaltose in patients with chronic heart failure and iron deficiency in Germany. ESC Heart Fail. 2017 Aug;4(3):274-81.
37. Kwong WJ, Wang K, Wang P, Boccia R. Effect of Ferric Carboxymaltose Versus Low-Dose Intravenous Iron Therapy and Iron Sucrose on the Total Cost of Care in Patients with Iron Deficiency Anemia: A US Claims Database Analysis. Drugs Real World Outcomes. 2024 Jun;11(2):251-61.
38. Kwong WJ, Wang K, Boccia RV. Total Cost of Care in Heart Failure Patients Receiving Parenteral Iron Therapy for Iron Deficiency Anemia: A Retrospective Analysis. Circulation. 2022 Nov 8;146(Suppl_1):A11358.
39. Engel-Nitz NM, Kwong J, Wang K, Tran S, Anderson A. Iron Deficiency Anemia: The Impact of Intravenous Iron Replacement Treatment on Health Care Costs. Blood. 2023 Nov 28;142:3697.
40. Abdel-Kader AK, Eisenkraft JB, Katz DJ. Overview and Limitations of Database Research in Anesthesiology: A Narrative Review. Anesth Analg. 2021 Apr 1;132(4):1012-22.
41. LaVallee C, Cronin P, Bansal I, Kwong WJ, Boccia R. Importance of Initial Complete Parenteral Iron Repletion on Hemoglobin Level Normalization and Health Care Resource Utilization: A Retrospective Analysis. Pharmacotherapy. 2019 Oct;39(10):983-93.