Abstract
Objective: This review article describes the characteristics of published literature using the cisterna magna blood injection mouse model of subarachnoid hemorrhage (SAH) with the aim to define particular standards and identify moderators of mortality rate, SAH grade, and large artery vasospasm.
Methods: We searched for English-original peer-reviewed studies which reported the induction of SAH in mice via single or multiple blood injections into the cisterna magna. The search included studies published until 13th February 2023 on PubMed, Embase and Web of Science. Furthermore, we investigated the reporting of mortality rate, vasospasms by measuring large arteries, and SAH grade in cisterna magna blood injection mouse model.
Results: Seven articles out of 136 identified records matched our inclusion criteria and were therefore included in descriptive analysis. Four articles reported the mortality rate which varied between zero and 22 percent. Five articles displayed vasospasms of large cerebral arteries including basilar artery (BA), anterior cerebral artery (ACA), and middle cerebral artery (MCA). Interestingly, the diameters of the observed arteries started to decrease already within the first hour after blood injection and achieved the lowest values at different times, but mainly between six and twelve hours after SAH induction. The artery diameters reached nearly their pre-SAH (control group) diameters approximately after four to seven days after SAH. However, the SAH severity grade was reported in none of these publications. No uniform model characteristics were observed in current literature.
Conclusion: A systemic overview of the cisterna magna blood injection mouse model of SAH is presented. An important heterogeneity was observed. Hence, standardized model features and study endpoints have to be defined in order to improve reporting frequency and quality to enhance the reproducibility of preclinical SAH research in the future.
Keywords
Subarachnoid Haemorrhage, SAH, Cisterna Magna blood injection, Mouse, Mortality, Reproducibility, Methodological reporting
Introduction
Subarachnoid hemorrhage (SAH) occurs in majority of cases after spontaneous aneurysm rupture [1]. The typical symptoms of SAH are severe headache [2] and consequent loss of consciousness. Intracranial pressure (ICP) increases with the amount of blood entering the subarachnoid space with subsequent impairment of vital functions [3,4]. Up to now, patients suffering SAH display a very high mortality rate, ranging from 32 to 67 % [5], whereas about 15% of the patients die before being hospitalized and as a direct consequence of the bleeding, but in total 40% die within the first month after bleeding [6]. Multiple activated cascades lead to pathophysiological changes occurring already within the first 72 hours of onset (early brain injury, EBI) and might be related to secondary effects or delayed cerebral injuries (DBI) [7,8]. Since vasospasms were ruled out to be exclusively the only contributor to DBI [9,10], researchers start to focus also on different other possibilities. Within all inflammatory processes are the most challenging targets due to its multitude interactions and multiple pathways [11]. Therefore, intensive investigations are urgently needed to defuse dangerousness and improve outcome of SAH patients, both in survival and in long term neurophysiological functions. Researchers predominantly focus on animal models which have been designed to mimic the disease and explore cellular and molecular mechanisms of SAH pathology, each of them having their own advantages and disadvantages.
Barry et al. [12] first described an experimental SAH animal model in 1979 in which SAH was induced via a perforation of an intracranial artery and was modified over the years to improve the model fit for the study of SAH [13]. An additionally introduced method for SAH modelling was, to inject fresh autologous blood into the cisterna magna and prechiasmatic cistern [14,15], or next to an intracranial [16] or an extracranial artery [17] in in vivo models. Among those different methods to investigate SAH, our review focusses on published literature describing experiments using the cisterna magna blood injection model. As missing standards in model characteristics and insufficient reproducibility of results have already been shown for the filament perforation SAH mouse model and for in vivo models of SAH in general [18-20], we analyze whether these concerns are also applicable for the cisterna magna blood injection model and discuss how researchers can face them, if applicable. Moreover, we provide an overview about animal mortality and large artery vasospasm in this model. The overall aim is to increase the value of knowledge gained on preclinical in vivo SAH research, leading subsequently to more efficient clinical studies and ultimately to improvements in patient outcome.
Methods
Search strategy
We searched for English-original peer-reviewed research articles that reported the use of cisterna magna blood injection to induce SAH in mice in the three online databases PubMed, Embase and Web of Science. The search included articles published until 13th February 2023. The following systematic search strategy was used: ("subarachnoid Hemorrhage" OR SAH OR SAH OR "subarachnoid* bleed*" OR "subarachnoid Hemorrhage" OR "subarachnoid* Hemorrhage" OR "subarachnoid* Haemorrhage*") AND (cisterna magna blood injection OR intracisternal injection) AND (mice OR mouse). After removing duplicates from the search results, unique records proceeded to the screening stage.
Inclusion and exclusion criteria
Articles had to fulfil all inclusion criteria to be included in this review. An article had to report at least one experiment using the SAH model of injecting blood into the cisterna magna, other injection locations were excluded. Of these injection model experiments, there had to be reported data for at least one of the outcomes mortality, large artery vasospasm and/or SAH severity grade, all for an untreated cohort. Data for animals with additional treatment (e.g., drug therapy, hypothermia) were not included in the review. Thereby, the experimental animals had to be mice. Additionally, we included only controlled experiments and peer-reviewed original research articles in English language. In general, selections were performed, when there was a consensus of two independently screening reviewers, whereas discrepancies were resolved by a third independent reviewer. All articles were scanned according to our inclusion criteria in two stages. First, we screened only the articles abstracts for obvious failure to match the criteria. Second, articles passing the first stage were screened based on their full-text publications including all data presented in the respective articles.
Data collection
For every article that passed the two screening stages, two independent reviewers extracted data of interest from the full text articles, while discrepancies resolved by a third independent reviewer, too. These data included model characteristics and outcome parameters. The model characteristics included animal specifications, animal housing conditions, anesthetic procedure, and parameters regarding the blood injection and perioperative treatment itself. Outcome parameters include data for mortality rate, large artery vasospasm, and SAH severity score for untreated mice subjected to blood injection and sham-operation. Given an insufficient amount of data from too few articles, we did not conduct a meta-analysis, and instead presented the extracted data descriptively in tabular form. All data are given as mean and standard error of the mean (SEM), unless indicated otherwise.
Results
Literature screening results and study characteristics
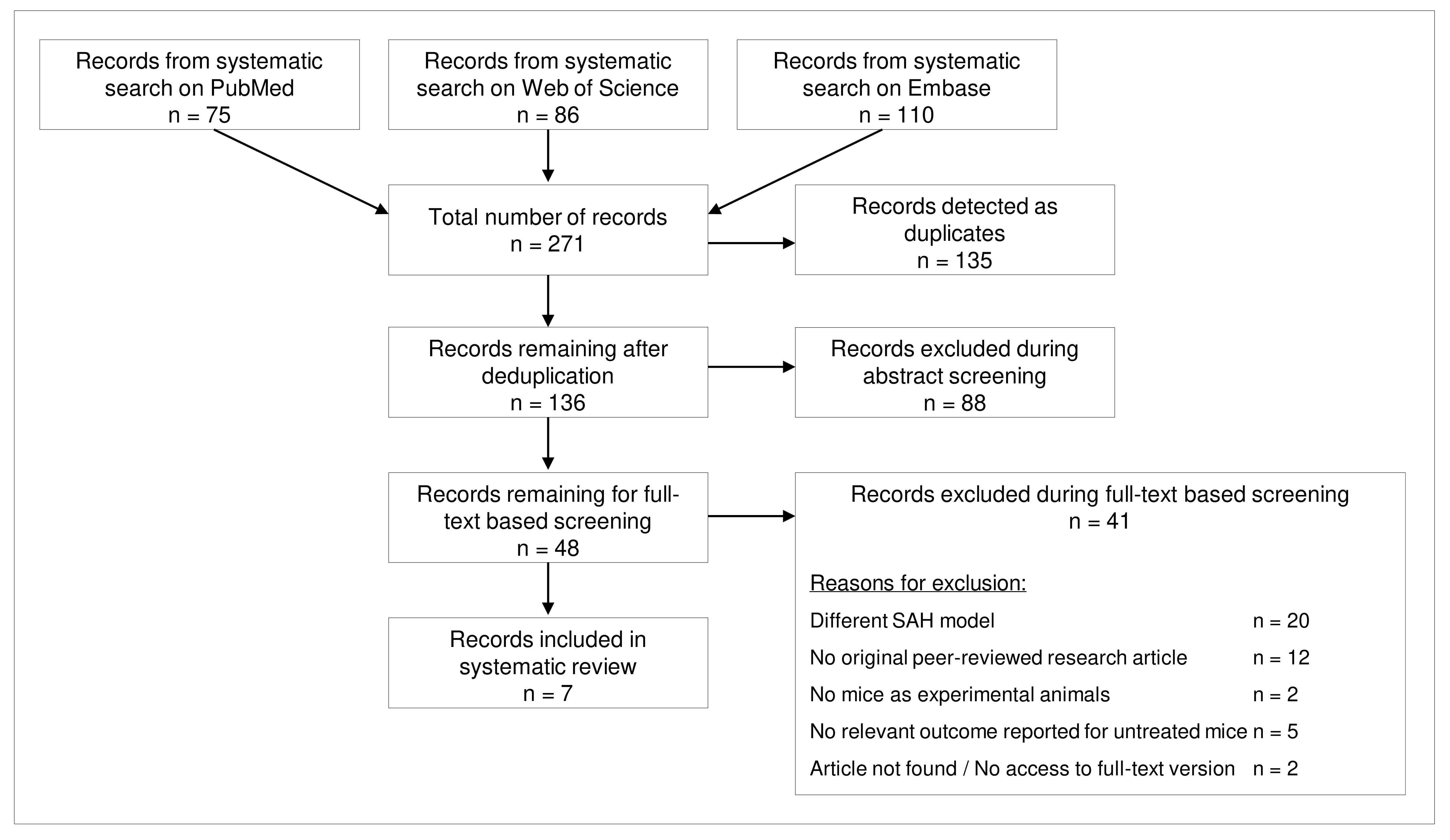
The systematic search on three databases delivered 271 articles, of which 136 remained after deduplication. Of these 136 articles, 48 were selected after abstract screening and of them, seven remained for analysis after screening of full text articles (Figure 1). In the full text screening stage, the most frequent reason for exclusion was the choice of a different SAH model (n=20), mainly prechiasmatic cistern blood injection. Moreover, two authors used rats as experimental animals.
Figure 1. Flowchart for literature search and screening results.
Across the seven relevant articles for our review, the total sample size of untreated mice subjected to SAH cisterna magna blood injection model was at least 149, but the number of mice was unclear in certain studies (Table 1). For the sham-operated mice, the exact group sizes were also often unclear with a total of at least 43 sham-operated mice across all articles (Table 2). In total, four articles reported mouse mortality, five articles reported large artery vasospasm after SAH and none of the studies reported a SAH severity grade.
|
Author (Year) |
Study setting |
Sample size |
Quantitative methods |
Study aim |
|
Lin et al., 2003 |
USA |
59 |
Mortality rate, BA, ACA, MCA vasospasm |
Representing a replicable and inexpensive approach for screening therapeutic candidates |
|
Lin et al., 2005 |
Taiwan |
8 |
Mortality rate, ACA vasospasm |
Anti-E-selectin antibody decreased SAH-induced vasospasm and served as a possible role of E selectin in the pathogenesis of vasospasm after SAH |
|
Kamp et al., 2014 |
Germany |
41 |
Mortality rate |
Murine single-blood-injection SAH model is suitable for pathophysiological and further molecular analysis following SAH |
|
Terpolilli et al., 2016 |
Germany |
6 at least |
BA vasospasm |
Reversing microvascular dysfunction by inhaled nitric oxide might be a promising treatment strategy for SAH |
|
Chaichana et al., 2007 |
USA |
15 |
BA vasospasm |
Hp 2-2 genotype is critical for the development of severe vasospasm |
|
Vecchione et al., 2009 |
Italy |
unclear |
ACA vasospasm |
TNF-α can be used as a novel target of the therapeutic strategy against cerebral vasospasm |
|
Oka et al., 2017 |
USA |
20 at least |
Mortality rate |
Cerebral ischemia is required for spreading depolarization to be triggered after SAH |
Model parameters
We extracted information regarding the different characteristics surrounding this animal SAH model which have been performed by different research groups and summarized their reporting distribution in Table 2. Thereby, the SAH model characteristics displayed recognizable variations and often were not reported at all. Concretely, the age of experimental animals ranged from six to twenty weeks as the weight of mice was between 22 and 35 grams, if reported. Detailed animal housing conditions were reported infrequently and showed single as well as grouped housing. Only two authors reported specific postoperative care procedures including pain management. Regarding anaesthesia, mice were mostly sedated via injection of Chloral hydrate and Xylazine, but huge diversity was observed with five different drug combinations used. If reported, blood was injected into the cisterna magna via a 30-gauge-thick needle. The injected blood volume varied between 40 and 400 µl, while 60 µl was the predominantly chosen amount of blood. An intraprocedural measurement of intracranial pressure (ICP) was reported in two of the seven studies. In the articles that reported data for a sham-operated control group, saline was injected instead of autologous blood.
|
Cisterna magna Injection SAH model characteristics |
|||
|
Experimental animals |
Used drugs for anesthesia |
||
|
Number of mice (all articles) |
Mice (articles) |
Injective anesthesia drugs |
Articles |
|
Total (including all outcomes) |
192 (7) |
Chloral hydrate, Xylazine |
3 |
|
-SAH-injection surgery |
149 (7) |
Ketamine, Xylazine, Midazolam |
1 |
|
-Sham-operation (no SAH) |
43 (5) |
Isoflurane, Medetomidine, Midazolam, Fentanyl |
1 |
|
-Male |
192 (6) |
Ketamine, Xylazine |
1 |
|
-Female |
0 |
Isoflurane |
1 |
|
Strain |
Articles |
Intubation |
Articles |
|
C57/BL6 family |
7 |
Yes |
2 |
|
-C57/BL6 |
2 |
No / Not reported |
5 |
|
-C57/BL6J |
5 |
Cisterna magna blood injection model |
|
|
Age |
Articles |
Thickness of injection needle |
Articles |
|
6 – 8 weeks |
1 |
30-gauge |
5 |
|
10 – 12 weeks |
1 |
Duration of the procedure |
Articles |
|
12 – 16 weeks |
1 |
25-30 min |
1 |
|
15 – 20 weeks |
1 |
1h |
1 |
|
Not reported |
3 |
Description of blood origin |
Articles |
|
Weight |
Articles |
Autologous |
6 |
|
22 – 27 g |
1 |
Homologous arterial blood |
1 |
|
24 – 26 g |
1 |
Blood volume |
Articles |
|
25 – 30 g |
1 |
40 µl |
1 |
|
27 – 32 g |
1 |
50 µl |
1 |
|
30 – 35 g |
1 |
60 µl |
4 |
|
Not reported |
2 |
400 µl |
1 |
|
Housing conditions |
ICP monitoring |
Articles |
|
|
Environment |
Articles |
Yes |
2 |
|
Single cage |
1 |
No |
5 |
|
Group cage |
1 |
Control group |
|
|
Twelve-hour light dark cycle |
2 |
Sham-operated control group |
Articles |
|
Free access to food and water |
3 |
Yes |
5 |
|
Postoperative care procedures |
Articles |
No |
2 |
|
Yes |
2 |
Injected substance in sham-operated group |
Articles |
|
No |
5 |
Saline |
5 |
|
Type of anaesthesia |
Not applicable |
2 |
|
|
Type |
Articles |
|
|
|
Inhalation |
1 |
|
|
|
Injection |
5 |
|
|
|
Inhalation and Injection |
1 |
|
|
Animal mortality
The mortality rate in cisterna magna blood injection model was mentioned in only 4 papers (Table 3). Lin et al. [21] recruited 59 C57Black/6J mice and reported 2 dead mice 20 minutes after blood injection resulting in a mortality rate of about 3,4 %. In another study of Lin et al. [22], they reported that out of eight mice, no mouse died after SAH injection. However, the exact time after injection was unclear. Kamp et al. [23] mentioned rate of dead animals at two different time points. Out of 41 individuals, 4 mice died within the first 15 minutes after injection and one additional individual mouse died in the next 45 minutes, resulting in a one-hour mortality rate of 12.2%. The mortality rates were also reported for two different time points by Oka et al. [24]. Here they reported that 18% deceased after 12 hours and in total 22% of experimental mice after 24 hours after SAH injection. All in all, mouse mortality rates after SAH blood injection into cisterna magna were heterogenous, ranging from 0 to 22% with a trend of higher mortality with increasing observation times.
|
Study |
Strain |
Time (hours) |
Number of individual deceased mouse |
Mortality rate (%)
|
|
Lin et al., 2003 |
C57Black/6J |
0.33 |
2 |
3.4 |
|
Lin et al., 2005 |
C57Black/6J |
- |
0 |
0 |
|
Kamp et al., 2014 |
C57BL/6J |
0.25 1 |
4 5 |
9.8 12.2 |
|
Oka et al., 2017 |
C57BL6J |
12 24 |
unclear |
18 22 |
Large arteries vasospasm
The vasospasm after SAH can be measured as the contraction of cerebral arteries expressed in the change of the arteries diameter with and without SAH injection (sham-operated mice) [25]. Five of the included articles in our review displayed large artery vasospasm in the basilar artery (BA), anterior cerebral artery (ACA), and/or middle cerebral artery (MCA) (Table 4). The most detailed vasospasm data were reported by Lin et al. [21], were the diameters of control groups for the different arteries were 221.3 ± 12.2 µm for BA, 166.2 ± 5.5 µm for ACA and 166.4 ± 16.5µm for MCA (mean ± standard deviation). Thereby, animals’ arteries showed to have their maximum decrease in diameter six hours after SAH for BA (168.4 ± 17.4 µm) and for MCA (131.0 ± 14.0 µm), and twelve hours after SAH for ACA (103.2 ± 25.9 µm). The baseline diameter levels were reached around day four to seven after SAH blood injection into cisterna magna. In another study of Lin et al. [22], they reported that vasospasm levels for ACA after injection were at 78% in comparison to healthy mice. Moreover, Terpolilli et al. [26] made a similar observation comparing the BA diameter of sham operated versus blood injected individuals revealing that one hour after injection, diameters are narrowed to 89.3% and even to 74.8% after twelve hours before regaining 87.8% of the diameter after 24 hours of blood injection. Confirming these values, Chaichana et al. [27] measured the BA after 24 h of SAH induction and could show a general decrease of BA diameter to 88.2 % compared to sham-operated mice. Interestingly, Vecchione et al. [28] reported the highest diameter decrease of ACA at one and two hours post injection to 47.1% ± 22.2% and 50.0% ± 35.8% of the initial diameters, respectively. In summary, vasospasm levels of large cerebral arteries lay between 50 and 90 percent of non-blood-injection control cohort artery diameters, depending on time after injection.
|
Study |
Strain |
Artery |
Time after blood injection (hours) |
Artery diameter† (Mean ± SEM) (µm) |
|
|
|
BA |
0* 1 6 12 24 36 48 72 96 168 |
221.3 ± 12.2 196.0 ± 21.7 168.4 ± 17.4 197.6 ± 3.7 194.0 ± 17.5 193.0 ± 17.0 204.4 ± 24.6 212.3 ± 7.4 212.0 ± 6.9 219.0 ± 6.9 |
|
Lin et al., 2003 |
C57Black/6J |
ACA |
0* 1 6 12 24 36 48 72 96 168 |
166.2 ± 5.5 145.6 ± 21.7 121.1 ± 12.4 103.2 ± 25.9 125.9 ± 17.9 134.8 ± 21.0 139.6 ± 18.7 131.7 ± 11.1 156.8 ± 10.8 150.3 ± 12.0 |
|
|
|
MCA |
0* 1 6 12 24 36 48 72 96 168 |
166.4 ± 16.5 155.4 ± 13.8 131.0 ± 14.0 139.4 ± 23.8 140.8 ± 9.8 146.6 ± 13.2 164.1 ± 20.8 147.7 ± 9.6 160.0 ± 17.2 167.8 ± 12.0 |
|
Lin et al., 2005 |
C57Black/6J |
ACA |
- |
78% |
|
Terpolilli et al., 2016 |
C57BL/6 |
BA |
1 12 24 |
89.3% ± 1.4% 74.8% ± 5.2% 87.8% ± 1.9% |
|
Chaichana et al., 2007 |
C57Bl/6J Hp 1-1 wildtype |
BA |
24 |
88.2% ± 1.1% |
|
Vecchione et al., 2009 |
C57BL6 |
ACA |
1 2 |
47.1% ± 22.2% 50.0% ± 35.8% |
SAH grade
The severity grade of SAH, as described on the scale by Sugawara [29], was not reported in any of the included articles. Therefore, no data are shown for this endpoint.
Discussion
In our systematic review, we provided an overview of the model characteristics, mortality rate, vasospasm, and SAH grade in cisterna magna blood injection SAH mouse model literature of seven articles and a combined number of 192 mice.
Thereby, the mortality rate after different periods of observation after SAH ranged from 0% to 22% at 24 hours after injection procedure into the cisterna magna with autologous blood displaying the overall trend of lower mortality rates compared to the filament perforation model of SAH which is around 20 to 30% [20,30]. As the vasospasm of large cerebral arteries is triggered through blood in the subarachnoid space to prevent the extravasation of a bigger amount of blood and increased ICP, we extracted data of arteries diameters in mice with and without injected autologous blood into the cisterna magna. Indeed, existing literature in this review confirmed this vasospastic effect of blood in subarachnoid space with a reduction of cerebral arteries diameter of 47 to 89% with its peak at six to twelve hours after SAH and the reaching of pre-SAH values after four to seven days. Our systematic review and meta-analysis of the filament perforation SAH mouse model showed similar values for vasospasm from 50 to 90 percent of the non-SAH arteries diameters [20].
However, SAH-grade was not mentioned for the cisterna magna blood injection model in any of the included publication. One reason might be that the controlled injection of a certain volume of blood directly into the cisterna magna could present a sufficiently controlled situation leading to an identical SAH score with known blood distribution in the subarachnoid space possibly making the evaluation of a score for SAH severity redundant. To prove if this is the case and reproducibility is given, we suggest that research labs should conduct a confirmatory SAH grade evaluation study at least once for each SAH model used and present the results in published literature. For such an evaluation, we suggest using the grading system introduced by Sugawara et al. [29], which is already often used in the endovascular perforation model to confirm successful introduction of SAH [20]. They divided the basal cistern into six segments and the final score was calculated by summing up the values of each segment and evaluated it according to the scale of 0-7 for mild SAH, 8-12 for moderate SAH and 13-18 to severe SAH.
Different animal models have been established over the past decades and were used to gain knowledge about SAH which is displaying a life-threatening cerebrovascular disease with high morbidity and mortality [31]. Depending on the main focus of research, scientist can choose between using the endovascular perforation model or blood injection model into prechiasmatic cistern or cistern magna. The later mentioned models cause the presence of blood in the subarachnoid space and thus it is possible to investigate pathophysiological mechanisms and molecular processes of the disease. Thereby each model has certain advantages and disadvantages [32]. The cisterna magna blood injection mouse model of SAH is considered as a replicable, low mortality model compared with the endovascular perforation mouse model [33,34]. Saito and his colleagues [35] and a previous review of our own group [20] identified that the endovascular perforation model resulted in relatively high mouse mortality rates (19-29%) while in the cisterna magna injection model mice had a mortality of mostly under 20%. Possible reasons for the slightly lower mortality in the injection model might be that the quality and quantity of injected blood can be controlled more precisely with a defined volume of blood injected into the subarachnoid space. Moreover, the vascular system itself remains untouched and intact, in contrast to the filament perforation model. The rupture of an artery of the circle of Willis mimics the pathophysiology of SAH more accurate, but there is also the possibility that some mice have more severe hemorrhages with more extensive and lethal courses because of the potentially lower reproducibility of the hemorrhage in the perforation model. Combined with a more invasive and technically more difficult procedure, the mice undergoing filament perforation for SAH modelling are facing a higher mortality.
Delayed cerebral ischemia (DCI) is still considered as one of the most severe complications in patients with aneurysmal SAH [36]. Thereby, cerebral vasospasm is regarded as one of the most accepted theories in the pathogenesis of DCI. Narrowing of the cerebral arteries results in a temporary and continuous interruption of blood flow to the brain parenchyma [37]. Similarly, in animal models, the cerebral vasospasm is detectable by measuring the cerebral arteries diameter [38]. In our study, five articles have measured artery diameters to evaluate vasospasm and subsequently DCI to emphasize the relevance for SAH morbidity and mortality. We showed that vasospasm after SAH is present in the cisterna magna blood injection model with diameter decreases of larger brain vessels (BA, ACA, and MCA) at a maximum to approximately fifty percent compared to non-SAH control mice 6 to 12 hours after blood injection. Exploring this severe complication of SAH, many studies have been published to clarify the pathophysiological mechanisms of post-SAH cerebral vasospasm. They showed post-SAH cerebral vasospasm might be associated with increasing levels of the potent cerebral vasoconstrictor endothelin 1 and reducing nitric oxide production [39,40], activation of calcium channels [41], upregulation of genes involved in inflammation and extracellular matrix remodeling [41,42], triggering oxidative stress and free radical damage to smooth muscle and lipid peroxidation of cell membranes [42,43], sympathetic activation [44,45] and ultimately failure of brain autoregulation, microthrombosis, and cerebral ischemic injury [46]. The complexity and importance of post-SAH effects are widely recognized. Researchers are investigating possible targets at different levels especially neuroinflammation displays an interesting field with its different pathways in pro- and anti-inflammatory effects. Therefore, the cellular and molecular mechanisms need to be further investigated and elucidated, where in vivo models seem to be eligible for such studies because of the presence of large artery vasospasm.
We acknowledge a number of limitations of the present review. The existing literature is sparse with only seven qualifying articles. Hence, we decided to not conduct a meta-analysis but rather present the characteristics of cisterna magna injection SAH model literature descriptively without statistical tests. The data only shows trends, but it cannot be concluded that specific model characteristics lead to a higher mortality rate and vice versa. Furthermore, we emphasize that mortality comparisons between perforation and injection model of SAH have to be interpreted carefully because of shorter observation periods for the cisterna magna injection model than in our review focusing on the filament perforation model [20]. Moreover, sample sizes of animal cohorts were unclear in some studies which presented an obstacle in assessing the impact of their results.
Conclusion
In conclusion, the main purpose of this study was to provide an overview of certain model characteristics in order to improve the comparability of primary outcomes examined with the cisterna magna blood injection SAH model in mice. We showed that there are currently no standards in elementary features of this model, including injected blood volume, age and weight of experimental animals, procedures of anesthesia, ICP monitoring and multiple other characteristics. Standardization and more detailed reporting of model characteristics are needed for sufficient comparability and reproducibility of studies using this model. For cisterna magna blood injection SAH in vivo models, more detailed research has to be done in the future to establish those standards of experimental parameters which can be reliably applicable across research labs to increase the overall comparability of the model.
Author Contributions Statement
The idea for our review was proposed by S Muhammad and S Alpdogan. Literature research in different databases was performed by S Alpdogan and T Sander. S Alpdogan, K Li, and T Sander screened the literature and extracted the data. S Alpdogan prepared the first draft of the manuscript. All authors made contributions to the manuscript, participated in the general conception of the review, read and approved the final manuscript.
Competing Interests
All authors declare that they have no competing interests in this article.
Ethics Approval
Our descriptive review study does not require an ethical approval.
References
2. Maciel CB, Barlow B, Lucke-Wold B, Gobinathan A, Abu-Mowis Z, Peethala MM, et al. Acute Headache Management for Patients with Subarachnoid Hemorrhage: An International Survey of Health Care Providers. Neurocritical Care. 2022 Aug 2:1-2.
3. Dagra A, Williams E, Aghili-Mehrizi S, Goutnik MA, Martinez M, Turner RC, et al. Pediatric Subarachnoid Hemorrhage: Rare Events with Important Implications. Brain and neurological disorders. 2022; 5(1):020.
4. Vyas V, Kanagalingam G, Siddique Z. Pneumococcal Meningitis Complicated by Subarachnoid Hemorrhage and Tonsillar Herniation. Cureus. 2020 Aug 24;12(8):e9994
5. Waweru P, Gatimu SM. Mortality and functional outcomes after a spontaneous subarachnoid haemorrhage: A retrospective multicentre cross-sectional study in Kenya. Plos One. 2019 Jun 12;14(6):e0217832.
6. Hop JW, Rinkel GJ, Algra A, Van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997 Mar;28(3):660-4.
7. Bjerkne Wenneberg S, Odenstedt Hergès H, Svedin P, Mallard C, Karlsson T, Adiels M, et al. Association between inflammatory response and outcome after subarachnoid haemorrhage. Acta Neurologica Scandinavica. 2021 Feb; 143(2):195-205.
8. Zhang Z, Fang Y, Lenahan C, Chen S. The role of immune inflammation in aneurysmal subarachnoid hemorrhage. Experimental Neurology. 2021 Feb 1; 336:113535.
9. Khey KM, Huard A, Mahmoud SH. Inflammatory pathways following subarachnoid hemorrhage. Cellular and Molecular Neurobiology. 2020 Jul; 40(5):675-93.
10. Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nature Clinical Practice Neurology. 2007 May;3(5):256-63.
11. Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). The Lancet Neurology. 2011 Jul 1;10(7):618-25.
12. Barry KJ, Gogjian MA, Stein BM. Small animal model for investigation of subarachnoid hemorrhage and cerebral vasospasm. Stroke. 1979 Sep;10(5):538-41.
13. Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995 Jun;26(6):1086-92.
14. Sehba FA, Pluta RM. Aneurysmal subarachnoid hemorrhage models: do they need a fix?. Stroke Res Treat. 2013; 2013:615154.
15. Prunell GF, Mathiesen T, Svendgaard NA. A new experimental model in rats for study of the pathophysiology of subarachnoid hemorrhage. Neuroreport. 2002 Dec 20;13(18):2553-6.
16. Tsuji T, Cook DA, Weir BK, Handa Y. Effect of clot removal on cerebrovascular contraction after subarachnoid hemorrhage in the monkey: pharmacological study. Heart and Vessels. 1996 Mar;11:69-79.
17. Megyesi JF, Findlay JM, Vollrath B, Cook DA, Chen MH. In vivo angioplasty prevents the development of vasospasm in canine carotid arteries: pharmacological and morphological analyses. Stroke. 1997 Jun;28(6):1216-24.
18. Kamp MA, Lieshout JH, Dibué-Adjei M, Weber JK, Schneider T, Restin T, et al. A systematic and meta-analysis of mortality in experimental mouse models analyzing delayed cerebral ischemia after subarachnoid hemorrhage. Translational Stroke Research. 2017 Jun; 8:206-219.
19. Grüter BE, Croci D, Schöpf S, Nevzati E, d’Allonzo D, Lattmann J, et al. Systematic review and meta-analysis of methodological quality in in vivo animal studies of subarachnoid hemorrhage. Translational Stroke Research. 2020 Dec;11(6):1175-84.
20. Alpdogan S, Sander T, Zhang R, Khan D, Li X, Zhou H, et al. Meta-review on Perforation Model of Subarachnoid Hemorrhage in Mice: Filament Material as a Possible Moderator of Mortality. Translational Stroke Research. 2022 Nov 23:1-4.
21. Lin CL, Calisaneller T, Ukita N, Dumont AS, Kassell NF, Lee KS. A murine model of subarachnoid hemorrhage-induced cerebral vasospasm. Journal of Neuroscience Methods. 2003 Feb 15;123(1):89-97.
22. Lin CL, Dumont AS, Calisaneller T, Kwan AL, Hwong SL, Lee KS. Monoclonal antibody against E selectin attenuates subarachnoid hemorrhage–induced cerebral vasospasm. Surgical Neurology. 2005 Sep 1;64(3):201-205.
23. Kamp MA, Dibue M, Sommer C, Steiger HJ, Schneider T, Hänggi D. Evaluation of a murine single-blood-injection SAH model. PLoS One. 2014 Dec 29;9(12):e114946.
24. Oka F, Hoffmann U, Lee JH, Shin HK, Chung DY, Yuzawa I, et al. Requisite ischemia for spreading depolarization occurrence after subarachnoid hemorrhage in rodents. Journal of Cerebral Blood Flow & Metabolism. 2017 May;37(5):1829-40.
25. Gules I, Satoh M, Clower BR, Nanda A, Zhang JH. Comparison of three rat models of cerebral vasospasm. American Journal of Physiology-Heart and Circulatory Physiology. 2002 Dec 1;283(6):H2551-9.
26. Terpolilli NA, Feiler S, Dienel A, Müller F, Heumos N, Friedrich B, et al. Nitric oxide inhalation reduces brain damage, prevents mortality, and improves neurological outcome after subarachnoid hemorrhage by resolving early pial microvasospasms. Journal of Cerebral Blood Flow & Metabolism. 2016 Dec; 36(12):2096-107.
27. Chaichana KL, Levy AP, Miller-Lotan R, Shakur S, Tamargo RJ. Haptoglobin 2-2 genotype determines chronic vasospasm after experimental subarachnoid hemorrhage. Stroke. 2007 Dec 1;38(12):3266-71.
28. Vecchione C, Frati A, Di Pardo A, Cifelli G, Carnevale D, Gentile MT, et al. Tumor necrosis factor-α mediates hemolysis-induced vasoconstriction and the cerebral vasospasm evoked by subarachnoid hemorrhage. Hypertension. 2009 Jul 1;54(1):150-6.
29. Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. Journal of Neuroscience Methods. 2008 Jan 30;167(2):327-34.
30. Lenz IJ, Plesnila N, Terpolilli NA. Role of endothelial nitric oxide synthase for early brain injury after subarachnoid hemorrhage in mice. Journal of Cerebral Blood Flow & Metabolism. 2021 Jul;41(7):1669-81.
31. Wei YX, Zhang DD, Gao YY, Hang CH, Shi JX. Inhibition of the myeloid differentiation primary response protein 88 reduces neuron injury in the early stages of subarachnoid hemorrhage in an in vitro experimental model. Journal of Physiology and Pharmacology. 2022 Feb 1;73(1).
32. Pedard M, El Amki M, Lefevre-Scelles A, Compère V, Castel H. Double direct injection of blood into the cisterna magna as a model of subarachnoid hemorrhage. JoVE (Journal of Visualized Experiments). 2020 Aug 30(162):e61322.
33. Leclerc JL, Garcia JM, Diller MA, Carpenter AM, Kamat PK, Hoh BL, et al.,. A comparison of pathophysiology in humans and rodent models of subarachnoid hemorrhage. Frontiers in Molecular Neuroscience. 2018 Mar 22;11:71.
34. Kamii H, Kato I, Kinouchi H, Chan PH, Epstein CJ, Akabane A, et al. Amelioration of vasospasm after subarachnoid hemorrhage in transgenic mice overexpressing CuZn–superoxide dismutase. Stroke. 1999 Apr;30(4):867-72.
35. Saito A, Kamii H, Kato I, Takasawa S, Kondo T, Chan PH, et al. Transgenic CuZn-superoxide dismutase inhibits NO synthase induction in experimental subarachnoid hemorrhage. Stroke. 2001 Jul;32(7):1652-7.
36. Diringer MN, Bleck TP, Claude Hemphill JI, Menon D, Shutter L, Vespa P, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society's Multidisciplinary Consensus Conference. Neurocritical Care. 2011 Oct;15:211-40.
37. Velat GJ, Kimball MM, Mocco JD, Hoh BL. Vasospasm after aneurysmal subarachnoid hemorrhage: review of randomized controlled trials and meta-analyses in the literature. World Neurosurgery. 2011 Nov 1;76(5):446-54.
38. Cai J, Sun Y, Yuan F, Chen L, He C, Bao Y, et al.,. A novel intravital method to evaluate cerebral vasospasm in rat models of subarachnoid hemorrhage: a study with synchrotron radiation angiography. PLoS One. 2012 Mar 12;7(3):e33366.
39. Pluta RM. Delayed cerebral vasospasm and nitric oxide: review, new hypothesis, and proposed treatment. Pharmacology & Therapeutics. 2005 Jan 1;105(1):23-56.
40. Seifert V, Löffler BM, Zimmermann M, Roux S, Stolke D. Endothelin concentrations in patients with aneurysmal subarachnoid hemorrhage: correlation with cerebral vasospasm, delayed ischemic neurological deficits, and volume of hematoma. Journal of Neurosurgery. 1995 Jan 1;82(1):55-62.
41. Cahill WJ, Calvert JH, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2006 Nov;26(11):1341-53.
42. Turner CP, Bergeron M, Matz P, Zegna A, Noble LJ, Panter SS, et al.,. Heme oxygenase-1 is induced in glia throughout brain by subarachnoid hemoglobin. Journal of Cerebral Blood Flow & Metabolism. 1998 Mar;18(3):257-73.
43. Armstead WM, Hekierski H, Pastor P, Yarovoi S, Higazi AA, Cines DB. Release of IL-6 after stroke contributes to impaired cerebral autoregulation and hippocampal neuronal necrosis through NMDA receptor activation and upregulation of ET-1 and JNK. Translational Stroke Research. 2019 Feb;10:104-11.
44. Guo ZN, Shao A, Tong LS, Sun W, Liu J, Yang Y. The role of nitric oxide and sympathetic control in cerebral autoregulation in the setting of subarachnoid hemorrhage and traumatic brain injury. Molecular Neurobiology. 2016 Aug;53:3606-15.
45. Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA. Sympathetic control of the cerebral vasculature in humans. Stroke. 2010 Jan 1;41(1):102-9.
46. Viderman D, Tapinova K, Abdildin YG. Mechanisms of cerebral vasospasm and cerebral ischaemia in subarachnoid haemorrhage. Clinical Physiology and Functional Imaging. 2023 Jan;43(1):1-9.