Abstract
Introduction: The effects of the SARS-CoV-2 pandemic continue to disrupt health systems worldwide, leading to population lockdowns in many countries. Preventing hospitalisation, death and long-COVID with repurposed drugs remains a valuable research goal. To respond to this priority, the use of azithromycin (AZM) is one of the most common treatments worldwide, in combination with hydroxychloroquine (HCQ) or as standalone molecule. With the aim of decentralizing early treatment to family practitioners, we address the question: Can early home administration of AZM with zinc help prevent severe COVID-19 disease progression and long-COVID?
Methodology: We conducted a scoping review of articles published from 31 December 2019 to 5 November 2020 in the PubMed, Google Scholar, MedRxiv, and BioRxiv databases, and a review of ongoing clinical trials published in the Clinicaltrial.gov database.
Results: Many studies report on outpatient treatment with a combination of AZM + HCQ versus AZM alone, and a few studies propose the addition of zinc (Zn) to AZM. Studies using HCQ were not considered in this review. We failed to identify any study reporting results of home-based utilisation of AZM administrated by family practitioners. In addition, we identified seven clinical trials currently recruiting individuals for early outpatient treatment with AZM, but results have not yet been published.
Discussion: The antiviral, anti-inflammatory, immunomodulatory benefits of AZM + Zn make this drugs combination a good candidate therapy to treat flu-like COVID-19 and atypical pneumoniae. The antibacterial action of AZM is expected to disrupt the poorly-documented bacteria-virus cooperation. Considering the pros and cons of macrolide use (including antimicrobial resistance), we call for further research on the early use of this therapy by family practitioners for the home treatment of individuals presenting mild or moderate symptoms to prevent hospitalisation, death and long-COVID.
Keywords
COVID-19; Azithromycin; Early treatment; Family practitioners
Introduction
The novel SARS-CoV-2 belongs to the Betacoronavirus family and contains a single-stranded positive-sense RNA genome. Similar to the two other coronaviruses, SARSCoV- 1 and MERS-CoV, SARS-CoV-2 is also likely to have originated in bats, which serve as established reservoirs for various pathogenic coronaviruses [1]. Although it is still unconfirmed, the most commonly admitted hypothesis suggests that SARS-CoV-2 is transmitted from bats to humans via an unidentified intermediate host species (zoonotic transmission). Then, the rapid human-to-human transmission through airborne aerosols occurs, which has been confirmed widely. The first case of COVID-19, which is the disease caused by SARS-CoV-2 [2], was reported in the province of Wuhan, China, on 7th January 2020. On 30th January, following the recommendations of the International Health Regulations (2005) Emergency Committee, the General Director of the World Health Organization (WHO DG) declared the outbreak a Public Health Emergency of International Concern (PHEIC) [3]. At that time, there were 7,818 reported confirmed cases, out of which 7,736 were in China and 82 were in 18 other countries [4]. On 9th March, the WHO DG declared that ‘the threat of a pandemic has become very real’. As of 5 November 2020, over 48 million confirmed cases and 1.2 million deaths had been reported by the World Health Organisation in 215 countries, with high disparities among regions and countries and even at the sub-national level. At that date, the five most affected countries (classified by total number of reported deaths) were the USA, India, Brazil, Russia, and France.
In the absence of effective treatment, only reinforced prevention coupled with early treatment of coinfections with repurposed drugs can disrupt viral transmission. Azithromycin [5], a macrolide antibiotic with antiviral, antiinflammatory, and antibacterial properties has appeared as a valuable treatment candidate. We reviewed studies published from 31 December 2019 to 5 November 2020 addressing the question: Can early home administration of AZM with zinc help prevent severe COVID-19 disease progression and long-COVID?
Five Steps of COVID-19 Disease Progression
The genome of the novel SARS-CoV-2 has been rapidly sequenced to study its host adaptation, viral evolution, infectivity, transmissibility, and pathogenicity [6]. Disease progression has been well described by many authors [6-9]. For the purpose of our study, we have drawn on the classification system proposed by Siddiqi et al. [10] to characterize the infection cycle as follows: Stage 1 – personto- person infection via respiratory droplets produced when an infected individual coughs or sneezes (airborne transmission). Stage 2 – viral penetration to host cells via two receptors: angiotensin-converting enzyme 2 (ACE2) and CD147 [11]. Stage 3 – SARS-CoV-2 inhibits and evades the innate immune response and drives pathogenesis (Viral replication provokes localized inflammation in the lung, leading to viral pneumonia; there is no clear boundary between the viral and inflammatory stages and they may overlap. At this stage, most patients need to be hospitalized for close observation and treatment). Stage 4 – progression to acute respiratory stress associated with increased production of proinflammatory cytokines. Stage 5 – the patient must be admitted to the intensive care unit as he/she is most likely to develop a cytokine storm and autoimmune disorders that can lead to death.
Duration of Infectiousness and Symptoms
The duration of infection from symptom onset to recovery is approximately ten days in non-severe cases [12]. As reported by Matheson and Lehner [13], ‘20% of patients deteriorate 7 to 10 days after symptom onset’. A viral peak appears in the upper respiratory tract within the first week of symptom onset, and later in the lower respiratory tract in both asymptomatic and symptomatic infected individuals. Viral load clearance occurs faster in asymptomatic than in symptomatic patients. Generally, individuals recover within three weeks. However, the postrecovery course of the disease, including its physical and psychological sequelae, presents many unknowns. Around 10% of the patients who tested positive for the SARSCoV- 2 virus remained unwell for more than three weeks, and a small proportion did so for months [14]. Prolonged COVID-19 can induce long-term pulmonary disorders and have adverse effects on the heart, kidneys, digestive tract, and neural system. The most important determinants of disease progression are age, comorbidities, history of smoking, length of hospitalization, severity of the acute disease (such as the need for ICU admission), and the type of medications administered (such as antiviral or corticosteroid therapy) [15]. In addition, the consequences on mental health are underestimated [16].
Preventive Strategies and Therapy
In stage 1, in an epidemic context, defining the basic reproduction number is essential. This ratio represents the expected number of secondary cases caused by an infected individual [17,18]. Estimating the R0 at the country level can be difficult, and studies have reported huge variations in the ratio among regions and countries [19]. From this ratio, the formula R0 = β.c.d, where β = probability of transmission of the virus; c = number of contact cases; and d = generation interval [20], can help decision-makers to take appropriate measures to stop viral transmission:
• β can be reduced by basic protection measures such as regularly washing hands, covering the mouth and nose when coughing and sneezing, not shaking hands, and wearing a mask [21-23]
• c can be reduced by physical distancing, massive testing, contact tracing, and isolation of positive cases [24, 25]
• d can be reduced with antiviral treatment and immunization.
As of 5 November 2020, no study has demonstrated the real added value of any of the experimental treatments, while many clinical trials are underway. Ending the SARSCoV2 epidemic with antivirals and/or a vaccine remains a global priority. In the meantime, however, it is essential to reinforce the ‘therapeutic toolbox’ for controlling the epidemic, to avoid relying only on ‘β’ and ‘c’ measures that have proven to be effective in partially controlling the spread of the epidemic by disrupting person-to-person transmission.
In stage 2, there is a need to develop drugs that can potentially block the host cell receptors ACE2 and CD147. AZM presents this capacity [11]. The combination of (hydroxychloroquine) HCQ + AZM has an in vitro synergic inhibitory effect on the replication of SARS-CoV1 and SARS-CoV2. This can be beneficial in the early stage of COVID-19 infection by reducing the viral load [26]. AZM occupies the ganglioside-binding domain of the spike protein and neutralizes virus binding to lipid rafts, while HCQ covers the ganglioside surface and prevents virus-membrane interaction through a complementary mechanism [27].
In stage 3, efforts have focused on blocking viral replication with antiretroviral drugs such, as those used against human immunodeficiency virus (HIV) or Ebola virus (e.g., Remdesivir®). The most commonly tested drugs were inhibitors of RNA polymerase, such as Darunavir®, Liponavir® and Ritonavir, targeting the transcription of the viral genome, and inhibitors of regulatory proteins, such as Remdesivir, Ribavirin, or Favipiravir®, which target the translation of viral proteins. At the date of this review, only Remdesivir had shown limited efficacy in reducing the length of stay in the ICU from 15 to 11 days. However, on 19 November 2020, due to the high number of reported adverse effects and its high cost, the WHO stated that Remdesivir should not be used to treat hospitalized patients with COVID-19, regardless of disease severity [28]. In parallel, whether corticoid therapy should be initiated to stop the inflammatory process that could be concomitant with viral replication is still unclear. Steroids are considered corrective, anti-inflammatory interventions to be administered later in the diseases course. Thus, the WHO [29] expressed its concern about early steroid use, as there was little or no evidence of its effectiveness at this stage.
In stage 4, it is important to limit the production of cytokines, particularly interleukin 6 (IL6), and of interferons. Anti-inflammatory drugs, such as IL6 inhibitors, corticosteroids, or Tocilizumab®, may potentially remediate severe damage and prevent a cytokine storm. During the SARS-CoV-1 outbreak, an intensive activation of proinflammatory cytokines and chemokines was observed, and researchers used steroids to effectively control the rapid deterioration of clinical conditions by attenuating the immune response [30]. Even if a meta-analysis did not demonstrate a clinical benefit, glucocorticoids have been widely used in syndromes closely related to Covid-19, including SARS, Middle East respiratory syndrome, severe influenza, and communityacquired pneumonia [30]. The RECOVERY open-label trial [31] rapidly proposed to treat COVID-19 hospitalized patients with dexamethasone at a dose regimen of 6 mg daily for up to ten days. On June 2020 preliminary results [32] announced that dexamethasone reduced deaths by one-third in ventilated patients (rate ratio 0.65 [95% confidence interval 0.48 to 0.88]; p=0.0003) and by one fifth in other patients receiving oxygen only (0.80 [0.67 to 0.96]; p=0.0021). There was no benefit among those patients who did not require respiratory support (1.22 [0.86 to 1.75]; p=0.14). Although dexamethasone proved an effective remedy against COVID-19, some severe side effects are associated with corticosteroids use. This should lead physicians to use a risk-benefit ratio and recommend corticosteroids to severely ill COVID-19 patients only.
Why is Azithromycin a Good Candidate Therapy?
AZM, a macrolide antibiotic, has a well-known safety profile, it is easily produced at a low cost as a generic drug, and has been declared an essential medicine by the WHO [33]. It is distributed worldwide, making it compliant with the WHO’s policy for drug repositioning [34]. AZM is effective against gram-positive bacteria, some gramnegative bacteria, and many atypical bacteria. Common side effects include nausea, vomiting, diarrhea, and upset stomach. Allergic reaction, such as anaphylaxis, QT prolongation, or diarrhea caused by Clostridium difficile, is possible. In the search for a safe and effective treatment for early mild or moderate COVID-19, AZM seems to be the most promising option, if administrated early enough.
AZM has antiviral, immunomodulatory, and clinical effects in the treatment of COVID-19 [35,36]. In stage 2, AZM can occupy the ganglioside-binding domain of the spike protein and neutralize virus binding to lipid rafts. It also interferes with the ligand CD147 receptor interaction (antiviral action). In stages 4 and 5, AZM can reduce the synthesis of proinflammatory cytokines, and thus reduce the length of stay or the need for respiratory support during hospitalization (immunomodulatory effect).
Finally, there is a paucity of literature on coinfection with bacterial species in COVID-19 patients. The most frequently isolated species are, in descending order, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella pneumophila, Haemophilus spp., Klebsiella spp., Pseudomonas aeruginosa, Chlamydia spp., S. pneumoniae, and Acinetobacter baumannii [37]. The respiratory symptoms of patients with COVID-19 pneumonia admitted to the hospital with fever and dry cough can mimic those of atypical bacterial pneumonia. This makes it difficult to distinguish COVID-19 pneumonia from hospital-acquired and ventilator-associated pneumonia. Antibiotic treatment should be designed considering the possible side effects (e.g., QT prolongation, diarrhea), the local epidemiology of drug resistance, and the impact of drug resistance on the patient [37]. Macrolide antibiotics, particularly AZM, remain an interesting option in specific conditions.
Why Consider Treatment with Zinc?
Zinc (Zn) is well tolerated and is known for its antioxidant, anti-inflammatory, immunomodulatory, and antiviral activities. Elderly people have an increased probability of zinc deficiency. In the elderly, low Zn status (serum Zn values <0.7 mg/L) represents a risk factor for pneumonia [38]. Inadequate Zn supply may predispose individuals to infectious diseases of the upper and lower respiratory tracts. Although the therapeutic effects of Zn are inconsistent, evidence-based data indicate the efficiency of Zn supplementation in preventing pneumonia and its complications due to the anti-inflammatory properties of zinc [39,40]. Recently, in vitro results indicated that low zinc levels favor viral expansion in SARS-CoV2 infected cells [41].
Why Promote Ambulatory Care?
With the continuous expansion of the pandemic and the resurgence of a second wave in Europe, health systems are facing many disruptions worldwide. In many countries, this situation has led to public lockdowns. To decongest hospitals, McCullough et al. [42] have proposed focusing on early treatment at home’s patient with patients combining drugs, self-quarantine for the control of contagion, and house aeration to reduce self-reinoculation. In addition, frontline health workers and family practitioners can play key roles in triage, early detection, and early treatment of patients with mild and moderate symptoms [43]. Only the most vulnerable patients at risk of severe disease progression should be referred to the hospital.
Methodology: The Review
We adopted Arksey and O’Malley’s [44] five-stage framework for a scoping review: identifying the research question, identifying relevant results, selecting studies, charting data, and reporting results. We defined the following research question: Can early home administration of AZM with zinc help prevent severe COVID-19 disease progression and long-COVID?
The scientific literature review was conducted by searching the online databases of PubMed and Google Scholar using the following search terms and Booleans: (COVID-19 OR SARS-CoV-2 OR coronavirus) AND (azithromycin OR Zithromax) AND (outpatient OR ambulatory OR “early treatment”). Preprints were selected from the bioRxiv, and medRxiv platforms. We accessed grey literature using the Google search engine. Materials published from 31 December 2019 to 5 November 2020 were searched. One researcher (PL) independently searched the databases and conveyed his findings to the co-authors. We also performed an advanced search using the Clinicaltrials.gov database using the keywords COVID, SARS-CoV-2, azithromycin, and Zithromax.
Findings
1. Articles
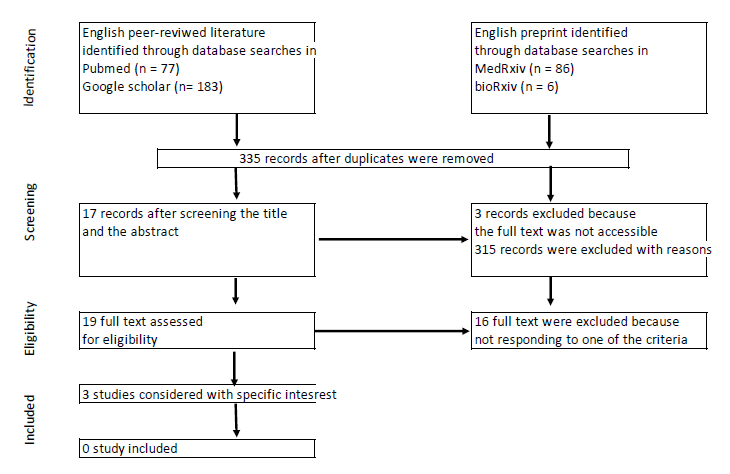
A total of 350 articles were identified, of which 19 (17 peerreviewed articles and two preprints) were selected for full review (Figure 1). The inclusion criteria were outpatient or ambulatory treatment, home treatment prescribed by general practitioners, use of AZM alone or with Zn, and AZM treatment duration ≥ 5 days. We failed to identify any study fulfilling all these criteria, either from both the peer-reviewed literature or preprints, because none of these studies involved family practitioners. Most articles focused on the in vitro action of AZM, reviewing opinions, and the properties of AZM. We paid particular attention to two articles presenting a research protocols [45,46] and one article in which the author [47] recommended formal clinical trials after having successfully treated more than 50 patients presenting flu-like symptoms, with AZM (500 mg on day 1 + 250 mg for the remaining five days). Clinical improvement was observed in all patients 24–48 hours after treatment initiation.
Figure 1. Document selection.
Many excluded articles (peer-reviewed studies, preprints, commentaries, letters, editorials, and so on) reported the effectiveness of a combination of HCQ + AZM administered to patients at different stages of the disease, from outpatient treatment to ICU care. Gautret et al. [48] were the first to highlight the effectiveness of dual therapy, showing better results with HCQ + AZM than with HCQ alone, under rigorous cardiological surveillance, in reducing hospitalization and mortality. Many other studies concluded that given the efficacy of HCQ + AZM in early outpatient treatment, the evidence on the use of HCQ alone or HCQ + AZM in inpatients is irrelevant to its use in high-risk outpatients in early stages of the disease [49,50]. In addition, triple therapy with HCQ + AZM + Zn improved the outcomes and reduced the duration of hospitalization [51]. However, most of these studies had a small sample size, non-robust methodology, different measures of the primary endpoint, and inconclusive results. HCQ was at the center of many debates until the WHO [52] concluded in October 2020 that repurposed drugs such as Remdesivir, Hydroxychloroquine, Lopinavir and Interferon regimens appeared to have little or no effect on hospitalized COVID-19.
2. Protocols for clinical trials
We identified a total of 3,904 NIH registered clinical trials on the ClinicalTrials.gov database for COVID-19. Through an advanced search, we selected 121 studies, out of which 88 were on going. We identified 12 clinical trials on the effect of AZM, with at least one arm comprising patients treated with AZM alone for a minimum duration of five days, and one clinical trial proposing a single dose of AZM. Five trials were hospital-based, and six focused on ambulatory care. In addition, we focused on the PRINCIPLE trial [53], which is a community-based trial in which multiple treatments for the same disease are tested simultaneously, including a AZM arm. This study is registered at the ISRCTN registry [54].
We selected the latter six trials + the PRINCIPLE trial (Table 1).
| Principal investigator | Study | Status | Design | # patients | Remarks |
|---|---|---|---|---|---|
| Alaa Rashad South-Vally university, Egypt | Clarithromycin versus azithromycin in treatment of mild COVID-19 infection | Completed (May to July 2020) No results available | 3 arms double-blinded randomized control trial | 300 participants in total 107 participants aged 45.9 ± 18 in AZM arm |
AZM 500 mg/24h for 7 days Primary outcome: time to complete resolution of fever |
| Paul Little University of Oxford, UK | ATOMIC2: a Multi- centre open-label two-arm randomised superiority clinical trial of azithromycin versus usual care in ambulatory COVID-19 |
Recruiting No results available | Open label, two-arms, randomized superiority trial | Adults (aged 18+) presenting to hospital with clinicallydiagnosed COVID-19 infection managed initially as outpatient 800 participants Currently in phase III |
Daily dose of 500 mg for 14 days Primary outcomes measured at Day 28: proportion progressing to respiratory failure |
| Thomas M. Lietman University of California, USA | ACTION: Azithromycin for COVID-19 treatment in outpatients nationwide. |
Recruiting No results available | Individually randomized telemedicine-based, placebo-controlled trial | 2271 participants In progress | Primary outcome: efficacy of a single 1.2 g dose of ATM for prevention of disease progression to hospitalisation |
| Brandon Web University of Utah, USA | HyAzOUT: Hydroxychloroquine vs. azithromycin for outpatients in Utah: a prospective programmatic trial |
Recruiting No results available | Randomized clinical trial open label with two arms | Adults aged > 44 years 1550 patients Recruitment in process |
ATM 500 mg PO on day 1 plus 250 mg on days 2-5 Primary outcome: admitted to a hospital within 14 days of enrolment |
| Jean- Philippe Lanoix, Centre hospitalier universitaire d’Amiens, France | AMBU-COVID: Proactive care of ambulatory COVID-19 patients |
No results available | Randomised clinical trial open label | 64 participants In progress | ATM 500 mg on day 1, then 250 mg the following 4 days PO Primary outcome: length of symptom duration with ATM treatment |
| Shenoor Azhar University of health science Lahore, Pakistan | PROTECT: Pakistan Randomized and Observational Trial to Evaluate Coronavirus Treatment of Hydroxychloroquine, Oseltamivir and Azithromycin to treat newly diagnosed patients with COVID-19 infection who have no comorbidities like diabetes mellitus |
Recruiting No results available | Double-blinded randomized trial. Seven comparator groups: Each drug (Hydroxychloroquine Phosphate/Sulfate, Oseltamivir and Azithromycin) given as monotherapy (three groups); combinations of each of two drugs (three groups); and a final group on triple drug regimen. | 500 participants Newly diagnosed 18+ patients, either hospitalized or in selfisolation Number of participants not determined | Azithromycin (500 mg orally daily on day 1, followed by 250 mg orally twice a day on days 2-5) alone Primary outcome: turning PCR test to negative on 7 days follow-up |
| Chris Butler University of Oxford | PRINCIPLE: Platform Randomised trial of intervention against COVID-19 in older people | Recruiting No results available | Adaptive clinical trial design intervention arm vs usual care arm | 800 participants People in the community aged ≥ 65, or ≥ 50 with comorbidity, with suspected or confirmed SARS- CoV- 2 infection diagnosed within the last 14 days |
Azithromycin: 500 mg/day during 3 days Primary outcome: time taken to self- reported recovery + hospitalisation and/ or death |
ACTION: This is the most innovative clinical trial on home self-treatment using mobile phones for follow-up. However, in the cohort study, AZM was administered as a single dose. This trial did not study the acceptability and adherence to a six-day home self-treatment regime (AZM 500 mg on day 1 + 250 mg for the five following days).
ATOMIC2: The AZM dose regimen and duration of treatment were similar to those recommended in the UK for Lyme disease (500 mg daily for 14 days).
HyAzOUT: This study involved individuals aged 44 years or older who presented to the hospital. This study aims to compare the benefits of HCQ and AZM.
AMBU-COVID: The Clinicaltrial.gov website has not been updated since the announcement of this trial, and we did not find further information on the PubMed, Google Scholar, medRxiv or bioRxiv databases. Further, the sample size may not allow us to conclusively answer the research question.
PROTECT: This clinical trial provided relevant information from low- and middle-income countries. However, the primary endpoint was a negative PCR results on day 7, which may not be the most relevant endpoint, as HCQ and AZM may delay virus clearance to more than 28 days after symptom onset [55]. This delay in viral clearance is correlated with older age.
Clarithromycin versus azithromycin in treatment of mild COVID-19 infection: This study has been completed. The low size of the AZM arm, AZM dose regime (500 mg), duration of treatment (seven days) and primary endpoint (time to complete resolution of fever) do not allow this study to respond to our research question.
PRINCIPLE: This randomized trial targets people in the community aged ≥ 65, or ≥ 50 with comorbidity, with a suspected or confirmed SARS-CoV-2 infection. In this study, azithromycin is administrated at a dose regimen of 500 mg once daily for three days, to patients with symptoms that appeared between t=0 and t=14 days (zero to 14 days after infection). However, even if PRINCIPLE RCT is conducted out of the hospital, the results might partially answer our research question, if they are not desegregated by date of treatment initiation after symptom onset. Only results from treatment administrated less than seven days after infection should be considered to answer our research question. Furthermore, the trial team conducts the enrolment of and follow-up with participants by telephone, with no involvement of family practitioners.
These trials have proposed different AZM dose regimens, from a single dose to ten days of treatment. If one or more trials provide evidence of AZM’s efficacy or the absence of evidence in a particular population or setting, further studies will be needed to provide conclusive data on the population, settings, and dose regimens [45]. Furthermore, none of these trials involved family practitioners. Hence, we did not find information on the added value of decentralizing early detection and care to family practitioners, who best know their patient’s habits, behaviors, comorbidities, and so on. Further, the trials had different primary outcomes. Hence, the results cannot be compared. Finally, to the best of our knowledge, these trials did not provide information on the prevalence of coinfections before initiating the treatment and/or after recovery.
Discussion
We outlined five stages of COVID-19 disease progression. COVID-19 response strategies should consider these stages and propose appropriate strategies, such as testing, contact tracing, isolation of confirmed cases with homebased early treatment, hospitalization of severe cases, and immunization. In parallel, in the long run, it is essential to maintain a focus on preventive measures. These include washing hands, covering the mouth and nose when coughing and sneezing, refraining from shaking hands, wearing a mask, and physical distancing. In this discussion, we will focus on early home treatments that could significantly reduce the number of infected patients needing hospital-based care. Early action and home-based care are the two essential aspects considered to prevent major disruptions in the health system. Treating patients with COVID-19 as soon as possible and before the seventh day of symptom onset is essential for any potential action of azithromycin.
He Z. et al. [56] analyzed 2,034 COVID-19 studies registered on ClinicalTrials.gov as of 18 June 2020 and reported that the five most frequently tested drugs were HCQ (n = 148), AZM (n = 46), tocilizumab (n = 29), lopinavir (n = 20), and ritonavir (n = 20). We did not conduct a deep review, but of the 3,904 registered trials, as of 5 November 2020, a quick scan showed that HCQ and AZM were still highly preferred, with 262 records mentioning the use of HCQ alone or in combination with AZM. Despite being a promising medication, there is a paucity of data on the use of AZM alone in treating COVID-19. Thirteen studies proposed the use of AZM alone: seven focused on the hospital inpatient level, and six, on the outpatient level. Results of those studies will be deeply scrutinized, once published.
Scientific knowledge of COVID-19 has been increasing rapidly, but as of 5 November 2020, there was still no antiviral with proven efficacy; hence, the world is placing its hope on a vaccine. Press releases announced the start of trials on promising vaccines produced by Pfizer/BioNTech [57], Moderna, Spoutnick, and Sinovac. However, considering the uncertainties of long-term efficacy, the level of protection by age (e.g., protection in older people with a deficient immune response), reactogenicity and side effects of mRNA vaccines, and logistical conditions (e.g., cold storage, production), it is too early to consider these vaccines a ‘magic bullet’. Vaccines and early treatment are complementary and necessary tools in a comprehensive package of prevention aimed at preventing hospitalization, death, and long-COVID. In Europe, and particularly in France, the response strategy for the COVID-19 epidemic failed to involve frontline health workers. A paradigm change is urgently needed to shift from a 100% hospitalbased approach to a family practitioner-based strategy to avoid further disruption of the health system and lockdowns. In addition, recent data suggests focusing on those with long-COVID, also termed as ‘COVID long-haulers’ [58]. The long-term physical and mental consequences of the disease are still unknown.
Dry nose, loss of taste and/or smell, and muscle pain are frequent complaints of COVID-19 patients who visit their family practitioners [59,60]. Meanwhile, hospitalized patients [6] usually complain of fever, dry cough, dyspnoea, chest pain, fatigue, and myalgia. Almost 95% of infected people present mild or moderate symptoms that do not necessitate hospital-based care. Approximately 5% of the patients with COVID-19 and 20% of those hospitalized experience severe symptoms necessitating intensive care [61]. Therefore, family practitioners should play a central role in triage, early treatment of patients with mild and moderate symptoms, and referral to the hospital when early treatment fails and for most-at-risk vulnerable individuals. However, this approach presents two major challenges: the need for a drug with proven effectiveness and early detection of COVID-19 symptoms for early care. As mentioned, HCQ + AZM is the most common drug therapy used for early treatment. However, when decentralizing early care to family practitioners, it might be opportune not to consider HCQ because i) while its efficacy is still controversial, it seems to work only when administered early enough [62], and ii) it can have adverse effects requiring close monitoring. Clinical trials of HCQ or HCQ + AZM arms usually enrolled patients and initiated treatment at very early stages of infection. If administrated to patients in more advanced stages of COVID-19 with mild or moderate symptoms, the use of HCQ could be counterproductive, given its immunomodulating effect. When prescribed at an advanced stage of the disease, dual therapy has little effect; at this stage, only strong antiinflammatory and/or anticoagulant treatments can help the patients [63]. In addition, in these late stages, the HCQ + AZM combination can be toxic in patients whose cardiac status is compromised [64]. HCQ appears to be the main driver of cardiac toxicity and not AZM itself. This is consistent with evidence that macrolides are not associated with an increased risk of cardiac events [35].
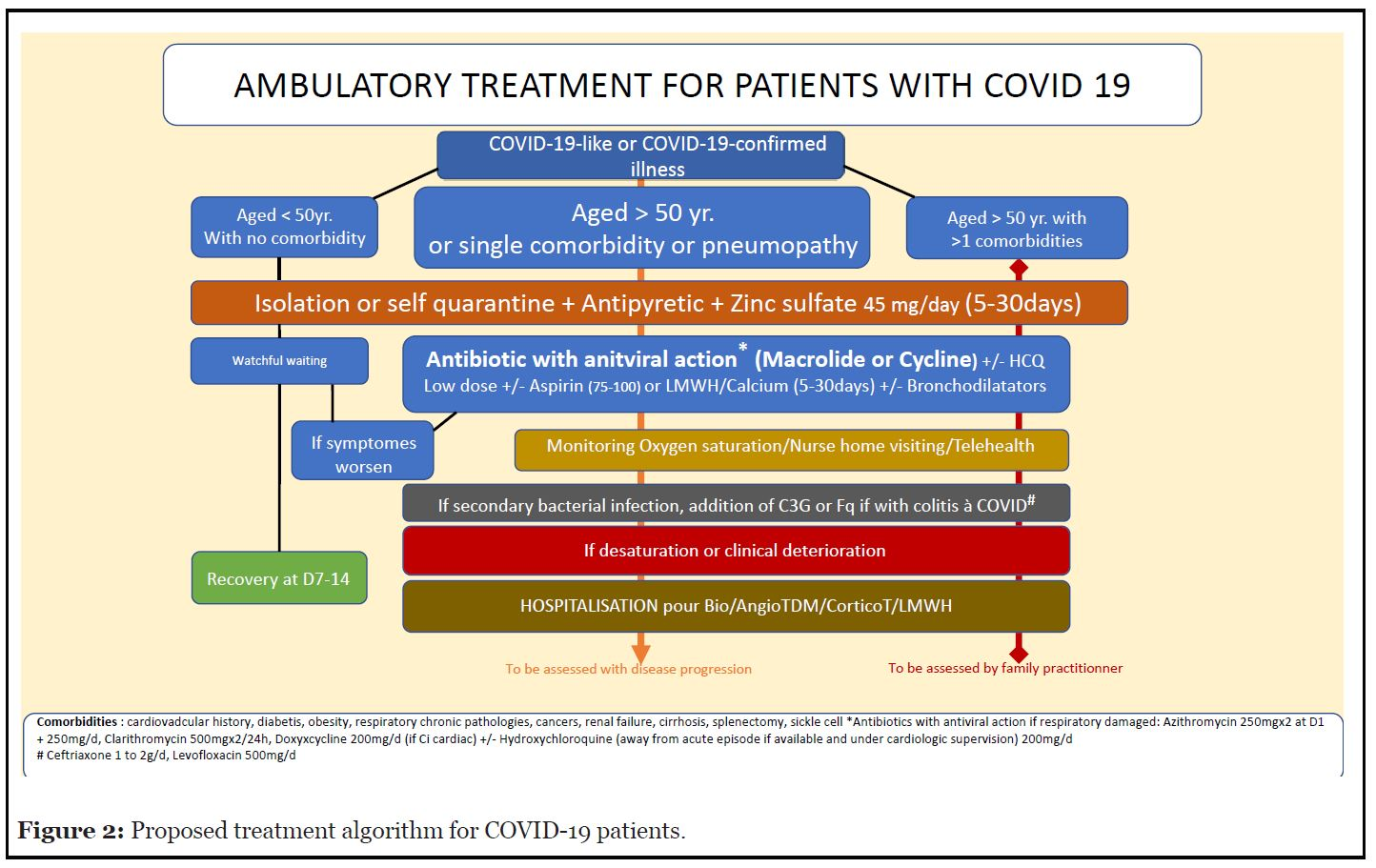
For these reasons, it is appropriate to focus on AZM only, which is considered to be effective if administered less than seven days after viral infection. Moreover, it shows no adverse effects that cannot be monitored at the family practitioner level. In France, several groups of family practitioners, known as the ‘Laissons les médecins prescrire’ and ‘Azi-thro d’hospitalisation’ alliances, claim to have obtained encouraging results with a small number of COVID-19 patients referred to the hospital after AZM treatment. These alliances recommend the involvement of family practitioners and the use of AZM under rigorous scientific guidance. To support these groups of doctors, we (BE) introduce a therapeutic algorithm (Figure 2) based on findings from the literature [35,36,42,65-68]. In parallel, the Association for the Prevention and the Management of Sanitary Crisis (UGPS), which represents individuals who have recovered from COVID-19 or are affected by it, supports these alliances. UGPS is advocating for clinical trials to be designed with the necessary scientific rigor by a research institution. Such a clinical trial would show whether early treatment with AZM significantly reduces i) the number of people infected by SARS-CoV-2 who need to be hospitalized; ii) of these hospitalized people, the number of fatal events; and iii) the number of infected people who suffer from a long-COVID disease. Secondary outcomes could focus on the satisfaction of both, the patient and the family practitioner.
Figure 2. Proposed treatment algorithm for COVID-19 patients.
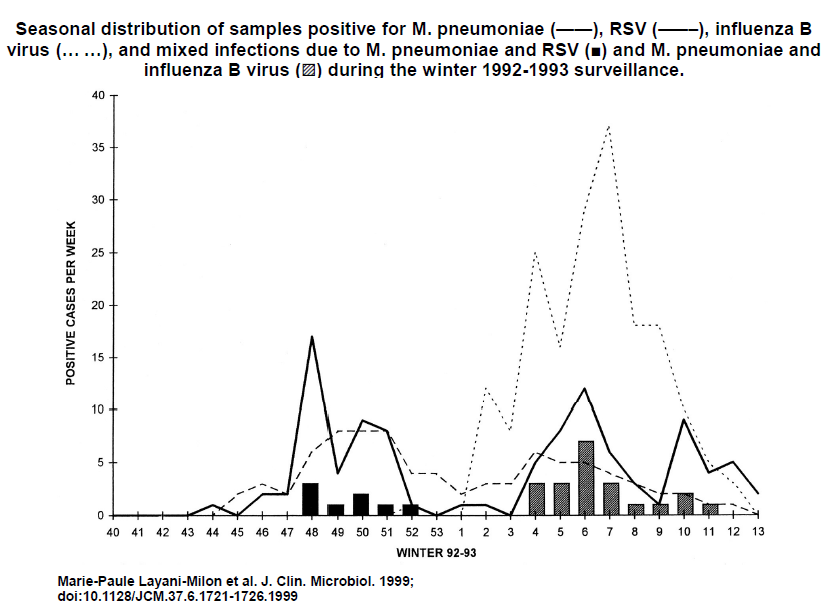
We need to be aware of new data that can be applied to the early ambulatory treatment of COVID-19. The role of Mycoplasma pneumoniae (MP) remains unclear. Lansbury et al. [69] do not recommend the routine COVID-19 infections, because the level of MP–SARSCoV- 2 coinfection is too low. Nicolson and de Mattos [70] suggest that the severity of signs and symptoms in progressive COVID-19 patients could be partly due to MP and other bacteria. We recommend considering the role of use of antibiotics in the management of confirmed MP–SARS-CoV-2 coinfection and the need to address it to prevent severe disease progression [71]. As demonstrated in a previous study (by CE, who obtained the patent EP0349473b1), certain mycoplasmas contribute to the explosive amplification of the replication of certain RNA viruses, such as the respiratory syncytial virus (RSV), in vitro. Bacteria such as Chlamydia pneumoniae (CP), MP, Borrelia burgdorferi (BB), and Legionella pneumophila (LP) are generally present in the pulmonary microbiota, hidden intracellularly in a quiescent state [72]. They participate in the development of local immune disorders, leading to superinfection. Studies on the prevalence of MP, CP, BB, and LP present contradictory findings, considering the difficulty of isolating MP. In studying the seasonal distribution of MP among patients presenting with mild acute respiratory symptoms caused by the influenza virus A or B or RSV, Layani-Milon et al. showed [73] the cocirculation of MP and viral strains of influenza A or B or RSV (Figure 3). The authors noted that every year, at least one peak of MP infection is observed in late autumn (October to December), varying in duration and intensity. Therefore, MP can superinfect patients presenting with viral infections, either in the early stage of infection (the first three days) or in the late stage during the recovery of respiratory cells. To conclude, this hypothesis constitutes a reliable avenue for further research and favors early treatment of COVID-19 patients with AZM.
Figure 3. Seasonal distribution of MP by Layani-milon et al.
Many authors have described the SARS-CoV-2 cell penetration via ACE2 receptors, and the endocytosis facilitated by spike proteins. The viral spike protein binds the host receptor angiotensin-converting enzyme 2 (ACE2) via the receptor-binding domain (RBD). In this process, the potential antiviral action of AZM to block ACE2 receptors is well documented [6,8,10,14,61,74]. However, building on the hypothesis of bacteria-virus cooperation, we think that AZM antimicrobial action is preponderant even if AZM antiviral action cannot be neglected. In our previous work, we (CE) showed that HIV and RSV colonize intracellular bacteria such as mycoplasma in vitro through a bacteriophage-like mechanism. SARS-CoV-2 could develop a similar bacteriophage-like mechanism to colonize MP and other intracellular bacteria [75]. In such a case, it is essential to block the bacteria RNA polymerase responsible for the viral amplification with AZM. Further, Zn can inhibit RNA synthesis activity in vitro of several RNA viruses, including SARS-CoV at the initiation phase of this synthesis. It probably directly affects the enzyme that drives the SARS-CoV-2 genome-encoded RNA-dependent RNA polymerase in RNA replication and transcription [76,77]. If a similar mechanism applies to SARS-CoV-2, it explains why Zn deficiency favors COVID-19 disease progression. Consequently, addition of Zn can enhance the action of AZM, leading to an augmented blockage of viral replication with the association of AZM + Zn.
AZM is known to induce antimicrobial resistance at variable levels in different countries and regions [78], and antimicrobial resistance (AMR) is a matter of major concern [79]. The use of AZM and other antibiotics in the management of COVID-19 should be balanced against the risk of AMR. The paucity of available data makes it difficult to predict the impact that this pandemic may have on antimicrobial stewardship programs and long-term rates of AMR [80]. The deaths from COVID-19 may overtake the deaths from AMR for 2020, but the estimated annual number of deaths from AMR of 10 million by 2050 may be higher than the death toll from the entire COVID-19 pandemic [81]. We encourage adopting a positive approach and considering the impacts of both COVID-19 and AMR. For instance, behavioral interventions, including physical barriers, to prevent the spread of SARS-CoV-2, will likely decrease the spread of other infections and the use of antimicrobials [81]. Such interventions focusing on hand hygiene may have a considerable impact on AMR if adopted in the long term by individuals in their daily lives and health workers in health facilities worldwide.
Family practitioners can promote this. In addition, the high number of hospitalized SARS-CoV-2 infected individuals colonized with carbapenemase-producing Enterobacteriaceae (CPE)/VRE/MRSA/Clostridioides difficile increases the risk of nosocomial transmission within hospitals [80]. Early home treatment with AZM will inevitably reduce the risk of hospital transmission and the future consumption of antibiotics. This risk assessment should be addressed in collaboration with veterinary doctors and patient associations, as AMR is also caused by the overuse of antibiotics in agriculture, and animal and human health. Key monitoring indicators and information to be collected are to be rapidly defined. Considering the interrelated emergencies resulting from COVID-19 and AMR, it may be opportune to involve veterinary doctors and representatives of patient associations in the national task forces that monitor the COVID-19 response at the country level.
Strengths and Limitations
The document selection was conducted by only one researcher (PL), who might have missed or misclassified a few articles; thus, the entire body of research may not be reflected. Nonetheless, we believe we have applied a systematic and rigorous search strategy to retrieve relevant articles responding to the research question. As a result, as per 5 November 2020, we believe we have selected enough relevant articles to draft an overview of the state of utilization of azithromycin at the early stage of disease progression, and that we have been able to answer the research question.
Conclusion
Our scoping review did not reveal evidence of early home treatment with AZM alone or associated with zinc to be a reliable option to prevent severe COVID-19 disease progression, and long-COVID. We failed to identify studies involving family practitioners as frontline healthcare workers administering AZM treatment.
Countries were not prepared to face the SARS-CoV2 pandemic, and a large part of humanity has been or is still locked down. To date, strategies to control the pandemic are mainly based on preventive and restrictive measures, while hope has been pinned on herd immunity acquired through the mass immunization of the population. In the meantime, even if scientific knowledge is evolving at an unprecedent pace, little is known about the longterm health outcomes among people infected by SARSCoV- 2, even for asymptomatic people. It is also too early to assess the extent to which immunization will prevent the occurrence of long-COVID. Persistent symptoms or the development of new symptoms weeks after infection are not well documented but are an evolving problem that could appear as a future public health issue.
With the second wave of the COVID-19 pandemic in Europe, we suggest shifting from a hospital-based approach to a family practitioner-based approach with early home treatment that prevents disease progression to hospitalization and death. In parallel, such an approach could potentially decrease the risk of developing post- COVID-19 symptoms and prevent a future epidemic of chronic disability by reversing the curve before it becomes established. AZM shows the potential to respond to both health issues: mitigating the disruptions of the health system while vaccines are deployed and preventing a new epidemic of ‘COVID long-haulers’. We urge research institutions to provide the necessary support to develop clinical trials in collaboration with family practitioners in providing early home treatment with AZM +/- Zn under rigorous guidance.
Such research studies would benefit from the involvement of veterinary experts through a ‘one health’ approach that includes research components on both antimicrobial resistance and bacteria-virus cooperation. We call for national task forces managing the COVID-19 response and research institutions at the country level to consider the insights of family practitioners, associations of COVID-19 patients who have recovered, and veterinary doctors to develop an appropriate response to COVID-19 and pave the way to prevent the next epidemic.
References
2. Khan M, Adil SF, Alkhathlan HZ, Tahir MN, Saif S, Khan M, Khan ST. COVID-19: A Global Challenge with Old History, Epidemiology and Progress So Far. Molecules. 2021 Jan;26(1):39.
3. The World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) 2020 [Available from: https://www.who.int/news/item/30-01-2020-statementon- the-second-meeting-of-the-international-healthregulations-( 2005)-emergency-committee-regarding-theoutbreak- of-novel-coronavirus-(2019-ncov).
4. The World Health Organization. Novel Coronavirus (2019-nCoV) - Situation Report - 10 2020 [Available from: https://www.who.int/docs/default-source/ coronaviruse/situation-reports/20200130-sitrep-10- ncov.pdf?sfvrsn=d0b2e480_2.
5. Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H, De Ridder D, Petrovic D, Schrempft S, Marcus K, Yerly S. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoVPOP): a population-based study. The Lancet. 2020 Aug 1;396(10247):313-9.
6. Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, Megawati D, Hayati Z, Wagner AL, Mudatsir M. Coronavirus disease 2019 (COVID-19): A literature review. Journal of Infection and Public Health. 2020 Apr 8;13(5):67-73.
7. Bohn MK, Hall A, Sepiashvili L, Jung B, Steele S, Adeli K. Pathophysiology of COVID-19: Mechanisms underlying disease severity and progression. Physiology. 2020 Sep 1;35(5):288-301.
8. Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete- Rouco ME, De-Antonio Cuscó M, Ferrández O, Horcajada JP, Grau S. Azithromycin in the treatment of COVID-19: a review. Expert Review of Anti-infective Therapy. 2021 Feb 1;19(2):147-63.
9. Polak SB, Van Gool IC, Cohen D, Jan H, van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Modern Pathology. 2020 Nov;33(11):2128-38.
10. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. The Journal of Heart and Lung Transplantation. 2020 May;39(5):405-7.
11. Ulrich H, Pillat MM. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Reviews and Reports. 2020 Jun;16(3):434-40.
12. Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. The Lancet Microbe. 2020 Nov 19.
13. Matheson NJ, Lehner PJ. How does SARS-CoV-2 cause COVID-19?. Science. 2020 Jul 31;369(6503):510-1.
14. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis GV, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2004 Jun;203(2):631-7.
15. Salehi S, Reddy S, Gholamrezanezhad A. Long-term pulmonary consequences of coronavirus disease 2019 (COVID-19): what we know and what to expect. Journal of Thoracic Imaging. 2020 Jul 1;35(4):W87-9.
16. Arora T, Grey I. Health behaviour changes during COVID-19 and the potential consequences: A mini-review. Journal of Health Psychology. 2020 Aug;25(9):1155-63.
17. Flahault AA. SARS-CoV: 2. Modeling SARS epidemic. mÈdecine/sciences. 2003;19(11):1161-4.
18. Diekmann O, Heesterbeek JA, Metz JA. On the definition and the computation of the basic reproduction ratio R 0 in models for infectious diseases in heterogeneous populations. Journal of Mathematical Biology. 1990 Jun;28(4):365-82.
19. Rahman B, Sadraddin E, Porreca A. The basic reproduction number of SARS-CoV-2 in Wuhan is about to die out, how about the rest of the World?. Reviews in Medical Virology. 2020 Jul;30(4):e2111-e.
20. Salje H, Kiem CT, Lefrancq N. Estimating the burden of SARS-CoV-2 in France. Science.
21. Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, El-harakeh A, Bognanni A, Lotfi T, Loeb M, Hajizadeh A. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. The Lancet. 2020 Jun 27;395(10242):1973- 87.
22. Tirupathi R, Bharathidasan K, Palabindala V, Salim SA, Al-Tawfiq JA. Comprehensive review of mask utility and challenges during the COVID-19 pandemic. Infez Med. 2020 Jun 1;28(suppl 1):57-63.
23. Wang J, Pan L, Tang S, Ji JS, Shi X. Mask use during COVID-19: A risk adjusted strategy. Environmental Pollution. 2020 Jun 25:115099.
24. Steinbrook R. Contact tracing, testing, and control of COVID-19—learning from Taiwan. JAMA Internal Medicine. 2020 Sep 1;180(9):1163-4.
25. He Z. What further should be done to control COVID-19 outbreaks in addition to cases isolation and contact tracing measures?. BMC Medicine. 2020 Dec;18(1):1-3.
26. Andreani J, Le Bideau M, Duflot I, Jardot P, Rolland C, Boxberger M, Wurtz N, Rolain JM, Colson P, La Scola B, Raoult D. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microbial Pathogenesis. 2020 Aug 1;145:104228.
27. Fantini J, Chahinian H, Yahi N. Synergistic antiviral effect of hydroxychloroquine and azithromycin in combination against SARS-CoV-2: What molecular dynamics studies of virus-host interactions reveal. International Journal of Antimicrobial Agents. 2020 Aug 1;56(2):106020.
28. WHO guideline development group advises against use of remdesivir for COVID-19 [press release]. 20 November 2020 2020.
29. The World Health Organization. Corticosteroids for COVID-19. Geneva: The World Health Organization,; 2020 2 September 2020.
30. Lam CW, Chan MH, Wong CK. Severe acute respiratory syndrome: clinical and laboratory manifestations. The Clinical Biochemist Reviews. 2004 May;25(2):121-32.
31. Randomized Evaluation of COVID-19 Therapy (RECOVERY) [Internet]. 2020. Available from: https:// clinicaltrials.gov/ct2/show/NCT04381936.
32. Low-cost dexamethasone reduces death bu up to one third in hospitalised patients with severe respiratory complications of COVID-19 [press release]. 16 June 2020 2020.
33. The World Health Organization. WHO model list of essential medicines - 21st list, 2019 2019 [Available from: https://www.who.int/publications/i/item/ WHOMVPEMPIAU2019.06.
34. Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK. Drug repurposing approach to fight COVID-19. Pharmacological Reports : PR. 2020;72(6):1479-508.
35. EcheverrÌa Esnal DD, Martin Ontiyuelo CC, Navarrete Rouco MEM, De-Antonio CuscÛ MM, Ferr·ndez OO, Horcajada JPJ, et al. Azithromycin in the treatment of COVID-19: a review. Expert Review of Anti-infective Therapy. 2020:1-17.
36. Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID-19. Clinical Pharmacology & Therapeutics. 2020 Aug;108(2):201-11.
37. Fattorini L, Creti R, Palma C, Pantosti A. Bacterial coinfections in COVID-19: an underestimated adversary.Annali dell’Istituto superiore di sanita. 2020 Sep 11;56(3):359-64.
38. Barnett JB, Hamer DH, Meydani SN. Low zinc status: a new risk factor for pneumonia in the elderly?. Nutrition Reviews. 2010 Jan 1;68(1):30-7.
39. Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, Svistunov AA, Petrakis D, Spandidos DA, Aaseth J, Tsatsakis A. Zinc and respiratory tract infections: Perspectives for COVID-19. International Journal of Molecular Medicine. 2020 Jul 1;46(1):17-26.
40. Pal A, Squitti R, Picozza M, Pawar A, Rongioletti M, Dutta AK, Sahoo S, Goswami K, Sharma P, Prasad R. Zinc and COVID-19: basis of current clinical trials. Biological Trace Element Research. 2020 Oct 22:1-1.
41. Columbia University Kidney Transplant Program. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. Journal of the American Society of Nephrology. 2020 Jun;31(6):1150-6.
42. McCullough PA, Kelly RJ, Ruocco G, Lerma E, Tumlin J, Wheelan KR, et al. Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection. The American Journal of Medicine. 2021;134(1):16-22.
43. de Sutter A, Llor C, Maier M, Mallen C, Tatsioni A, van Weert H, et al. Family medicine in times of ‘COVID-19’: A generalists’ voice. European Journal of General Practice. 2020;26(1):58-60.
44. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005 Feb 1;8(1):19-32.
45. Hinks TS, Barber VS, Black J, Dutton SJ, Jabeen M, Melhorn J, et al. A multi-centre open-label two-arm randomised superiority clinical trial of azithromycin versus usual care in ambulatory COVID-19: study protocol for the ATOMIC2 trial. Trials. 2020 Dec;21(1):1-8.
46. Akram J, Azhar S, Shahzad M, Latif W, Khan KS. Pakistan Randomized and Observational Trial to Evaluate Coronavirus Treatment (PROTECT) of Hydroxychloroquine, Oseltamivir and Azithromycin to treat newly diagnosed patients with COVID-19 infection who have no comorbidities like diabetes mellitus: A structured summary of a study protocol for a randomized controlled trial. Trials. 2020 Dec;21(1):1-3.
47. Schwartz RA, Suskind RM. Azithromycin and COVID-19: Prompt early use at first signs of this infection in adults and children, an approach worthy of consideration. Dermatologic Therapy. 2020 Jul;33(4):e13785-e.
48. Gautret P, Lagier JC, Parola P, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honoré S. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International journal of antimicrobial agents. 2020 Jul 1;56(1):105949.
49. Chaurasia B, Tippetts TS, Mayoral Monibas R, Liu J, Li Y, Wang L, et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science. 2019 Jul 26;365(6451):386-92.
50. Brown SM, Peltan I, Kumar N, Leither L, Webb BJ, Starr N, et al. Hydroxychloroquine vs. Azithromycin for Hospitalized Patients with COVID-19 (HAHPS): Results of a Randomized, Active Comparator Trial. Annals of the American Thoracic Society. 2020.
51. Derwand R, Scholz M, Zelenko V. COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study. International Journal of Antimicrobial Agents. 2020 Dec 1;56(6):106214.
52. Pan H, Peto R, Karim QA, Alejandria M, Henao- Restrepo AM, García CH, et al. Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results. medRxiv. 2020:2020.10.15.20209817.
53. University of Oxford. Platform Randomised trial for INterventions against COVID-19 in older PeoPLE 2020 [Available from: https://www.principletrial.org].
54. University of Oxford. PRINCIPLE: a trial evaluating treatments for suspected COVID-19 in people aged 50 years and above with pre-existing conditions and those aged 65 years and above ISRCTN Registry: BMC; 2020 [Available from: https://www.isrctn.com/ISRCTN86534580].
55. Saleemi SA, Alrajhi A, Alhajji M, Alfattani A, Albaiz F. Time to negative PCR from symptom onset in COVID-19 patients on Hydroxychloroquine and Azithromycin - A real world experience. 2020:2020.08.05.20151027.
56. Okazaki H, Hirata D, Kamimura T, Sato H, Iwamoto M, Yoshio T, et al. Effects of FTY720 in MRL-lpr/lpr mice: therapeutic potential in systemic lupus erythematosus. The Journal of Rheumatology. 2002 Apr 1;29(4):707-16.
57. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England Journal of Medicine. 2020 Dec 31;383(27):2603-15.
58. Baig AM. Chronic COVID Syndrome: Need for an appropriate medical terminology for Long-COVID and COVID Long-Haulers. Journal of Medical Virology. 2020 Oct 23.
59. Sebo P, Tudrej B, Lourdaux J, Cuzin C, Floquet M, Haller DM, et al. Clinical characteristics of SARS-CoV-2 patients: a French cross-sectional study in primary care.
60. Tudrej B, Sebo P, Lourdaux J, Cuzin C, Floquet M, Haller DM, Maisonneuve H. Self-reported loss of smell and taste in SARS-CoV-2 patients: primary care data to guide future early detection strategies. Journal of General Internal Medicine. 2020 Aug;35:2502-4.
61. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 Aug 25;324(8):782-93.
62. Million M, Lagier JC, Gautret P, Colson P, Fournier PE, Amrane S, et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Medicine and Infectious Disease. 2020 May 1;35:101738.
63. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. Journal of the American College of Cardiology. 2020 May 12;75(18):2352-71.
64. Magagnoli J, Narendran S, Pereira F, Cummings TH, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. Med. 2020 Dec 18;1(1):114-27.
65. Association of American Physicians and Surgeons. A guide to home-based COVID-19 treatment. 2020.
66. Min JJ-Y, Jang YJY. Macrolide therapy in respiratory viral infections. Mediators of Inflammation.2012;2012:649570-.
67. Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID-19. Clinical Pharmacology & Therapeutics. 2020 Aug;108(2):201-11.
68. Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. International Journal of Infectious Diseases. 2020 Aug 1;97:396-403.
69. Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and metaanalysis. Journal of Infection. 2020 Aug 1;81(2):266-75.
70. Nicolson GL, de Mattos GF. COVID-19 Coronavirus: Is Infection along with Mycoplasma or Other Bacteria Linked to Progression to a Lethal Outcome?. International Journal of Clinical Medicine. 2020 May 20;11(05):282.
71. Lepere P EB, Yolartiran S, Escarguel C. The Role of Macrolide Antibiotics in the Prevention of Severe COVID-19 Disease Progression Via the Disruption of Bacteria/virus Co-Operation2020. Available from: https://papers.ssrn. com/sol3/papers.cfm?abstract_id=3712423.
72. Gehanno P, Mouton Y, editors. La colonisation microbienne des voies respiratoires. John Libbey Eurotext;1995.
73. Layani-Milon MP, Gras I, Valette M, Luciani J, Stagnara J, Aymard M, et al. Incidence of Upper Respiratory TractMycoplasma pneumoniae Infections among Outpatients in Rhône-Alpes, France, during Five Successive Winter Periods. Journal of Clinical Microbiology. 1999 Jun 1;37(6):1721-6.
74. Hatmal MM, Alshaer W, Al-Hatamleh MAI, Hatmal M, Smadi O, Taha MO, et al. Comprehensive Structural and Molecular Comparison of Spike Proteins of SARSCoV- 2, SARS-CoV and MERS-CoV, and Their Interactions with ACE2. Cells. 2020;9(12).
75. Cristoni S, Brogna C, Petrillo M, Querci M, Piazza O, Van den Eede G. Detection of toxins-likes peptides in plasma, urine and faecal samples from COVID-19 patients. 2020.
76. Te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathogens. 2010 Nov 4;6(11):e1001176.
77. Rahman MT, Idid SZ. Can Zn Be a Critical Element in COVID-19 Treatment? Biological Trace Element Research. 2021;199(2):550-8.
78. Serisier DJ. Risks of population antimicrobial resistance associated with chronic macrolide use for inflammatory airway diseases. The Lancet Respiratory Medicine. 2013 May 1;1(3):262-74.
79. The World Health Organization. Antimicrobial resistance 2020 [Available from: https://www.who. int/en/news-room/fact-sheets/detail/antimicrobialresistance.
80. Rawson TM, Moore LS, Castro-Sanchez E, Charani E, Davies F, Satta G, et al. COVID-19 and the potential long-term impact on antimicrobial resistance. Journal of Antimicrobial Chemotherapy. 2020 Jul 1;75(7):1681-4.
81. Nieuwlaat RR, Mbuagbaw LL, Mertz DD, Burrows LL, Bowdish DMEDME, Moja LL, et al. COVID-19 and Antimicrobial Resistance: Parallel and Interacting Health Emergencies. Clinical Infectious Diseases. 2020 Jun 16.