Abstract
Objective: To Increase the knowledge on Drugs Hypersensitivity Reactions (DHRs); activation, development of reliable diagnostic tests for better: selection of studies, diagnosis, identification of risk groups, prevention, cross-reactions, severs skin drug reactions and alternative therapeutics.
Methods: Review longitudinal and transversal studies about: immune mechanisms, hypersensitivity responses, parameters of methods and diagnostic consistency.
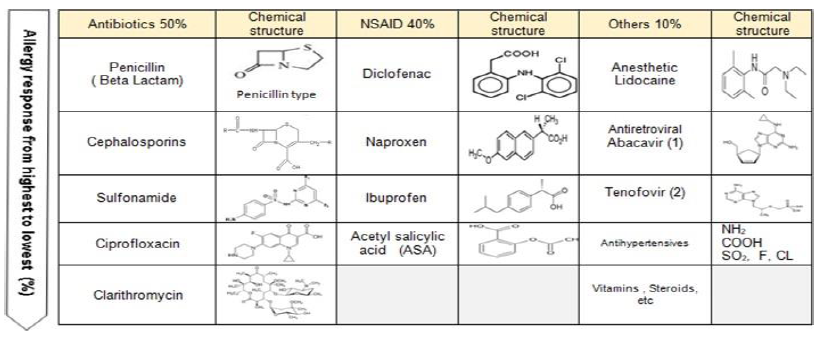
Results: The basophils are the most accessible cells for the study (peripheral blood 0.5%-1%); when they get activated release histamine as a response to allergens; the most used tests are the BAT (IgE- dependent); FcεRI-mediated signaling, the binding to the antigen (bivalent dimers); depends on the concentration (bell curve - ideal form); and more complex antigens present non-ideal dose-response curves (several forms). There are 4 types of evaluating BAT: 1) Secretion of granules 2) Membrane expression of activation markers: CD63, CD69, and CD203c by cytometry 3) the old technique of modified degranulation of basophils (MDB) and 4) Modified Leukocyte Migration Inhibitor Factor (MLIF). Currently there is an increase in the prevalence of DHRs: 7% in older adults, 18% in children (15% -24%) and 5%-15% in hospitalizations. The main cause of allergy are antibiotics: penicillin and β-lactams (50%); more frequently in women (40-60 years).
Conclusions: The BAT and alternative complementary tests with dilutions confirm the diagnosis and suggest the degree of sensitivity of the patient, predicting the response to treatment and reducing the risks in patients with reactions to drugs. These tests are simple, inexpensive and give great support in the diagnosis of drug reactions with coverage of several types of hypersensitivity.
Keywords
Hypersensitivity, Allergy, Adverse reactions to drugs, Antibiotics, NSAID, Anesthetic, BAT, DHRs
Background
Von Pirquet in 1905 described “the serum sickness” in patients using treatment with (diphtheria and tetanus horse serum) and in 1906, introduces the term “allergy” as a special type of defensive or immunological response to foreign substances that normally would not induce a response. In 1930, sulfonamides were also associated with more frequent reactions, which later was denominated as “drug fever” [1-4]. Since 1940, penicillin has been the most used antibiotic and currently remains as the most frequent cause of allergies [5].
The pharmaceutical industry has created new penicillins keeping the β-lactam ring, but changing the side branch without losing any antibiotic capacity; which produce cross-reactivity in allergic reactions and anaphylaxis [6-10]. During the 20th century and the beginning of the 21st century, the increase in the synthesis of drugs (Nonsteroidal anti-inflammatory drug [NSAIDs], analgesics, anesthetics, antihypertensives, steroid contraceptives, chemotherapy, antiretrovirals, etc.) caused a growth in the use of these drugs and increased allergies; some authors consider drug allergy as the epidemic of the 21st century.
The prevalence has increased in all stages of life: adult women (40%), elderly adults (7%), hospitalized patients (5%-15%) and mainly children (18% with a range of 15%-24%) [7,11,12]. Consequently, healthcare services want to improve the strategies for diagnosis, treatment, prevention and lethality.
Classification of Adverse Drugs Reactions (ADRs)
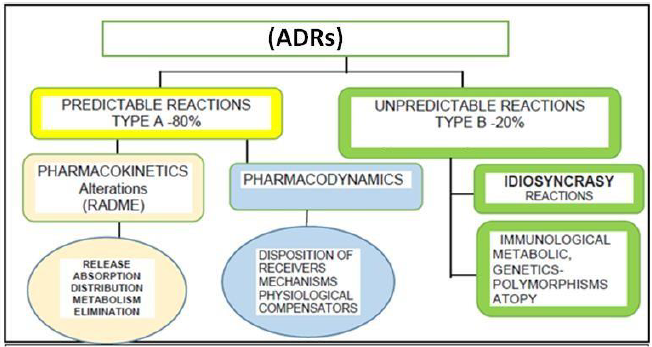
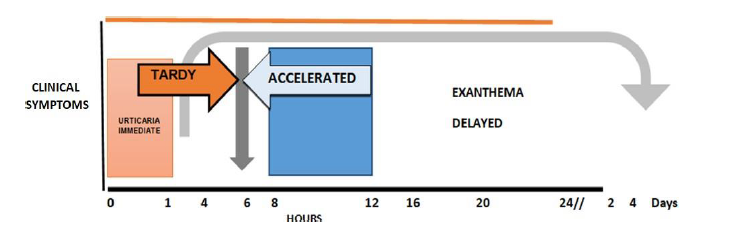
Drugs may produce predictable ADRs (type A, 80%) associated with pharmacological activity, dose dependency, toxicity and slow metabolizers; they can also produce unpredictable adverse reactions (type B, 20%) [13-15] which are dose independent (low concentration) and associated with aggravated intolerance, pseudo-allergies and more severe and lethal allergies. Hypersensitivity is an exacerbated immune response that produces a clinical pattern with systemic and dermal disorders (skin as the most affected organ), anaphylaxis and sudden death [8]. Gell and Coombs created the classification of hypersensitivity which was modified in 1996 [2,7,13,16] to distinguish 4 types of hypersensitivity: I, II, III, IV; later on, Pichler [2,17] subdivided type IV into a, b, c and d [17]. The International Consensus on Drug Allergy (ICON, 2013) defines the drugs hypersensitivity reactions (DHRs) [17] and says that they are caused by activation of the immune adaptive system considering immune mechanisms and activation responses supporting the diagnosis, treatment, follow-up and prevention. This classification is based on the laps of time it takes for the signs and symptoms to occur after the drug administration: 1) immediate (0-1 hour), 2) accelerated (1-72 hours), 3) delayed (more than 72 hours) (Figures 1 and 2, Table 1) [17].
| Type | Type of Immune Response | Clinical symptoms | In vitro Diagnostics | In vivo Diagnostics |
|---|---|---|---|---|
| I | Measured by IgE Eosinophils Mast Cells and Basophils (IMMEDIATE) | Urticaria, Angioedema, Rhinitis, Bronchospasm, Anaphilaxis | IgE specific serum, Triptase Cell stimulation test (CAST) Basophil Activation Technique (BAT): MBD, CD63 | Cutaneous test (prick, intradermal), Challenge test, Proving test [Coombs] |
| II | Citotoxicity dependent on IgG and IGM antibodies, (NOT IMMEDIATE) and Complement | Hemolytic anemia, Thrombocytopenia, Neutropenia, Autoimmunity | Coombs test Antibodies vs platelets Antibodies vs neutrophils | Only challenges to the drug can make diagnosis but are high risk [Coombs] |
| III | Deposit of immuno complexes [IgG and IgM], (NOT IMMEDIATE) Complement or Fc | Serum disease, Vasculitis, LES-Like by medications, Glomerulonephitis, Drug fever | C3, C4, Antinuclear Antibodies (ANA), Antineutrophil cytoplasmic antibodies (ANCA), Cyclic Citrullinated Peptide Antibodies (CCP), Anti-Thyroid, etc. Liver and kidney function tests, Pathological anatomy | Biopsies with Immunofluorescence [Coombs] |
| IVa | TH1 (IFNγ) TNFα, IL-12 and Macrophages (LATE) | Contact dermatitis | Lymphocyte Transformation test or Blastoid Transformation (LTT or BT), Modified Leukocyte Migration Inhibitory Factor (MLIF), Cytotoxic T lymphocyte, Precursors (CTLp), Cytokines by ELISA or PCR | Patch test [Pichler] |
| IVb | TH2 (IL-4, IL-5, IL-13 Eosinophils | Maculo-papular eruptions (MPE) with eosinophilia (DRESS) | Complete Blood Count (CBC) with revision eosinophil cellularity, atypical lymphocytes. MLIF, BT, LTT | Patch test [Pichler] |
| IVc | Cytotoxic T Lymphocytes (CTLs), CD4/CD8 (Perforin, Granzyme B, Fas L) | Contact dermatitis, Maculo-papular and bullous diseases (Steven-Johnson syndrome [SJS]), Toxic Epidermal Necrosis (TEN) | MLIF Liver Function test, CD4/CD8 (death keratinocytes) Activity of IgM vs Herpes Virus, Epstein Barr and Cytomegalovirus (CMV) | Patch test [Pichler] |
| IVd | T Cells, IL-8 CXCL8 in leucocytes Neutrophil inflammation | Acute generalized Exanthematous Pustulosis (AGEP), Pharmacodermias associated with neutrophilia | CBC T cells CD4/CD8 | Patch test [Pichler] |
Mechanisms of Immune Response to Types of Drugs in DHRs: Haptens, Pro- Haptenes, Binding p-i TCR
Prohapten (inactive-reactive). Pharmacogenetic polymorphism
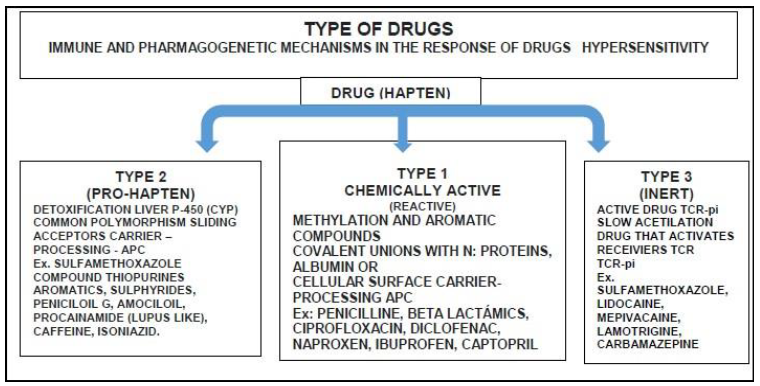
Drugs (generally non-immunogenic haptens) that are chemical substances of low molecular weight (less than 1000 Da) in the form of aromatic, heterocyclic components, -p-NH2Cl, sulfonamides, sulfide, OH components, halogens, with high resonance and instability (β-lactam with low polarity and hydrophily which do not facilitate covalent bonds with autologous proteins). Drugs are eliminated through metabolism with bioactivated detoxifying enzymes (hepatic N-acetylation, oxidation of cytochrome P450-CYP); this occurs mainly in the liver (microsomal), and also in the kidneys, lungs, intestine, plasma and nervous tissue. There are variabilities or ethnic polymorphisms, especially in slow acetylators, in which drugs remain more time in circulation, haptens bind to the protein, become a Hapten-Protein carrier complex (H-P), are englobed and introduced into the Antigen Presenting Cells (APC) for processing and presentation on the membrane with the major Histocompatibility Complex APC-MHC complex to induce a response cellular or humoral with IgE, IgG and IgM [7,13,22].
Active-reactive
Drugs with aromatic, polar groups and nitrogen, facilitate the direct binding or nucleophilic attacks to membranes in order to create a covalent bond with autologous proteins and induce an APC-MHC immune response [7,13,22]
p-I concept (pharmacological interaction with immune receptors)
Drugs without that lack hapten characteristics can bind (non covalently) to TCR and sending signals to create a hypersensitivity response. This explains a fast occurrence of Clinical symptoms without previous sensitizations and sometimes chaotic immune reaction, some crossreactions to the drug or its metabolites [7,14,17,22-24].
HLA restriction in hypersensitivity
HLA class I , mainly HLA-B, described for several sever reactions in DHRs; for example, it has been found that abacavir is strongly associated with the HLA-B * 5701 allele in white population; carbamazepine like an inductor of Steven Johnson Syndrome (SJS) has been associated with the HLA-B * 1502 and HLA-B * 5801 alleles in Chinese patients, while allopurinol has been associated with adverse reactions in SJS and necrolysis epidemical toxic (NET) with HLA-B 5701 (Figures 3 and 4, Table 2) [7,22,25,26].
| Antibiotics | Cross Reaction | NSAID | Cross Reaction | Others | Cross Reaction |
|---|---|---|---|---|---|
| Penicillin (Beta Lactam) | Penicillin derivatives BPO Beta Lactam | Diclofenac | ASA Salicylate Pyrazolones | Anesthetic Lidocaine | Mepivacaine Sulfonamide Allopurinol |
| Cephalosporine | Penicillin derivatives | Naproxen | ASA Salicylate Pyrazolones | Antiretroviral Abacavir (1) | Sulfonamide |
| Sulfonamide | Furozemide Lidocaine Benzocaine Carbamazepine | Ibuprofen | Salicylate Pyrazolones | Tenofovir (2) | Sulfonamide |
| Ciprofloxacin | Metronidazole | Acetyl Salicylic acid (ASA) | Salicylate Pyrazolones | Antihypertensives | PDN Prednisolone |
| Clarithromycin | Lamotrigine Mepivacaine | Vitamins, Steroids, etc |
Atopy
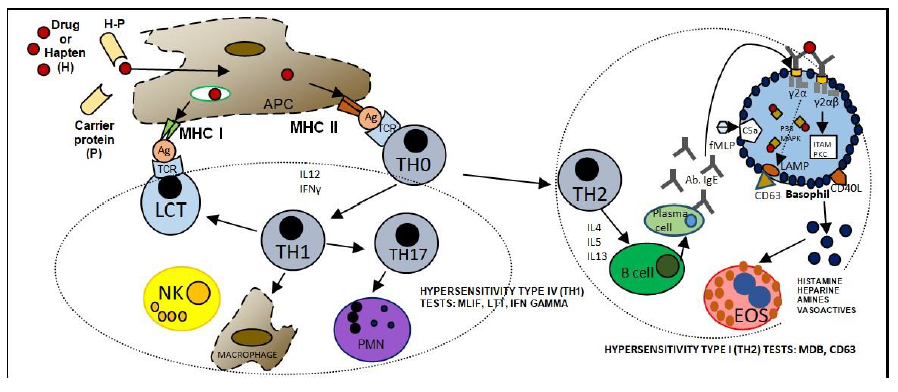
It refers to an individual’s genetic predisposition or susceptibility to develop sensitivity towards an allergen; it requires previous contact (intermittent or continuous) and depends on factors such as environment, temperature, sex (steroidal hormones) and age. This exaggerated immune response is mediated primarily by IgE antibodies, this antibody binds to FCeR1, increasing the half-life of IgE (2 - 60 days). We can find IgE synthesis from fetal stage to end of life. Higher concentrations of high and low affinity receptors have been reported in mast cells and basophils associated with severity, IL-4 inhibits the development of TH1 cells and activating TH2 cells, IL-3 favors the change of the isotype of B lymphocytes for the production of IgE, and the induction of the inflammatory process in DHR (Figure 3) [2,7,31-35].
Diagnosis of Drug Allergy and Diagnostic Tests
Medical history
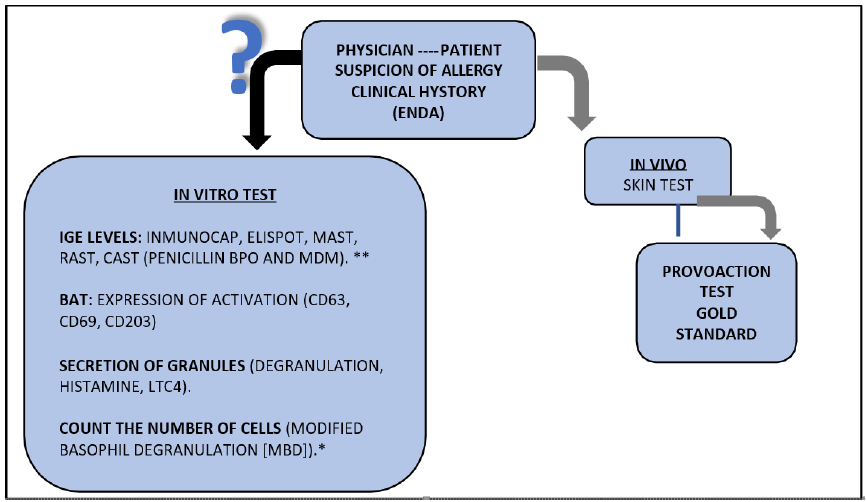
It is the cornerstone of the diagnosis and has a better predictive value. It is based on clinical criteria, anamnesis, diagnostic algorithm, records of clinical history (signs and symptoms), followed by provocation and skin tests, as well as laboratory tests suggested by physicians in some healthcare systems (Figure 5) [7,16,36].
Natural history
IgE antibodies may persist for years. Memory T lymphocytes (TLm) are more intense during nonimmediate DHRs: consequently, it is recommended to avoid the use of drug for long time [16,23].
In vivo tests (provocation tests)
They are considered the gold standard to confirm or rule out a drug as the main inductor of hypersensitivity reactions.
Skin tests is the most frequently used, for better results is used with different dilutions and with controls negative and positive (histamine). Medical monitoring is necessary to avoid risks of anaphylaxis [7,16,18,31,37].
In vitro tests
IgE levels: Most of the DHRs diagnostic methods assess the type I hypersensitivity mediated by IgE and can be demonstrated by measure of peripherical blood levels of this antibody by different technics like Immunocap, MAST, RAST, CAST, Chemo-luminescence, etc.
Basophil activation tests (BAT):
• Evaluating the secretion of granules (degranulation), histamine, LTC4 heparin, vasoactive amines.
• Expression of activation markers (CD63, CD69, CD203c, etc.) in the membrane of basophils.
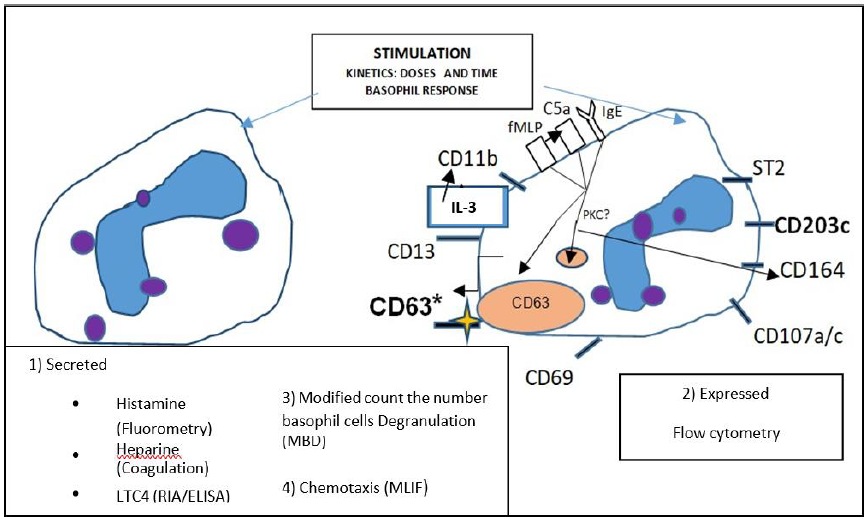
• Count the number of cells can degranulate in exposition to the antigen (drug) (Figure 6).
Secretion methods: In the last five decades of the twentieth century, it was demonstrated that activated leukocytes released histamine and other vasoactive amine molecules as a response to in vivo and in vitro allergens; the fast or slow release by cytokines was also demonstrated, as well as the disappearance of basophils, all evaluated through electronic microscopy [18,31,38].
There are 2 types of participating cells: mast cells and basophils; the latter are more accessible for functional studies, since they are found circulating in peripheral blood (mononuclear cells of 7-9 μm that constitute 0.5%- 1% of the leukocyte total) [7,13,18].
• Histamine: It is a premade mediator immediately released as a response to allergens or drugs. It is among the firstly used elements for in vitro and in vivo tests (erythema evaluation), for fluorometry, spectrophotometry and ELISA. Nevertheless, not all donors or patients release the total amount of histamine, and it may vary between 80% and 95%; therefore, basophil population needs to be spiked. There is also spontaneous release without stimuli that might be associated with higher sensitivity, reactivity and lack of specificity [10,13,18].
• Leukotriene C4 (LTC4): They are other slowly secreted mediators. They are lipid metabolites determined by ELISA and RIA methods [18,10,39].
• Cytokines IL4: They are recently formed, slow release molecules; IL3: they are determined by ELISA [13,18].
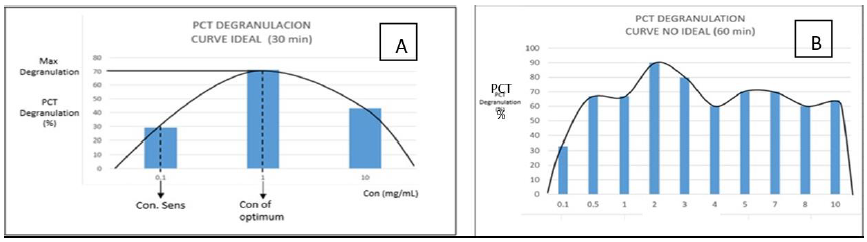
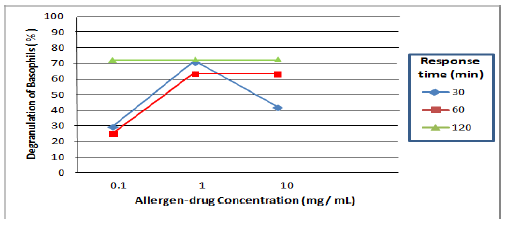
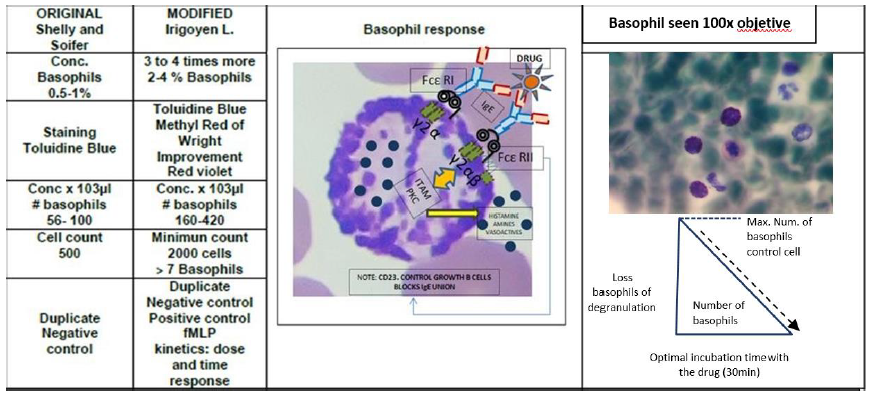
Modified basophil degranulation (MBD): It refers to the disappearance of basophils (count of cells) after activating and releasing their granulations; therefore, losing their morphology. It is determined by microscopy method and specific staining. Basophils are incubated in vitro with the suspected drug, which causes the degranulation and releasing of their content (100% of specificity [Negative Predictive Value, -PV], 84% of sensitivity [Positive Predictive Value, +PV]). The modification consists in increasing the concentration of basophils 3 to 5 times the physiological value in peripheral blood (<1%), cell adjustment (# of basophils 56-100 x 103 μL), standardization (dose of 0.1 to 1.0 mg/ mL and response time of 30 min at 37°C with drugs-bell curve), basophil staining (toluidine blue, Wrigth, methyl red), negative control (physiological saline solution), positive control (formyl-methyl-leucyl-phenylalanine [fMLP]), with a minimum reading of 2000 leukocytes and a minimum value of 7 basophils (execution time <24 h). The reference values (RV) for MBD are 0%-30% of degranulation obtained in non-allergic population (Figures 6 -10) [7, 10,13,18,25,40].
BAT: Mechanisms, Standardize and Improve Test Reliability
Dose-response time curves of basophils in murines and humans with different allergens
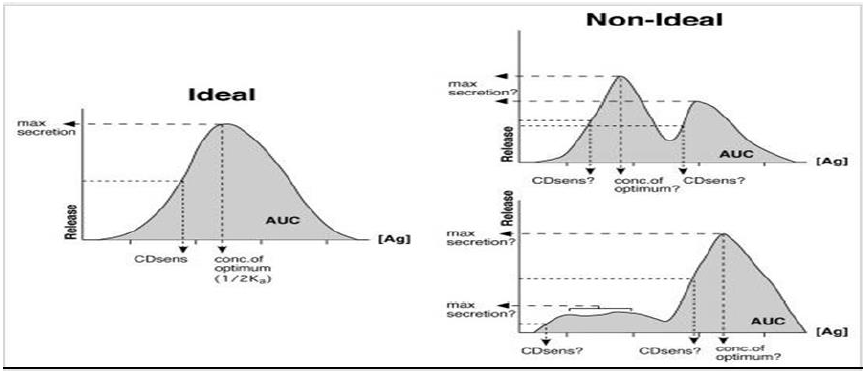
The most common stimulus for basophil studies depends on the activation through antigen-specific IgE (allergen or drug) of the cell surface connected to FcεRI (high affinity) which consists of an α chain and β and γ chains associated with the α chain as αγ2 and αβγ2. The role of β (chromosome 11q and 5q) is to increase the expression of the receptor by promoting the maturation and intracellular transit of the α chain, as well as the survival of IgE. The FcεRI-mediated signaling requires the binding of this receptor to the antigen (simple bivalent dimers inducing signaling); these binding depends on the concentration and affinity, which increases up to an optimal level and later returning to the basal state (bell curve as an ideal form); however, in human and murine models, more complex antigens (drugs) with higher affinity presented non-ideal dose-response curves (several forms). The basophil-IgE-epitope-antigen (drug) reaction can be considered as an antigen-antibody precipitation reaction; this results in the reduction of the receptor’s mobility and RcεI immobilization. The β and γ chains contain ITAM sequences that initiate the activation signaling of MAP kinase. The other receptor for IgE is low affinity Fcε II (CD63), which controls the growth and differentiation of B cells, increases the synthesis of IgE and also blocks the binding between IgE and eosinophils (Figures 3, 6-9) [7,18,39,41-44].
In essence, the binding of the paratope (2 IgE molecules) to the epitope induces intermembrane chain crosslinking and signaling transduction through kinase proteins (PKC), which provokes degranulation and release of histamine, heparin, proteoglycans and vasoactive amines that amplify the allergic reactions (Figures 9,10 and Table 3) [11].
Methods of activation markers expression
Since the last decade of the twentieth century and especially at the beginning of the twenty-first, a wide variety of techniques have been developed at research and commercial kits levels to identify the expression of basophil activation markers that use flow cytometry and monoclonal antibodies. The main clinical experience is with CD63, CD203c and CD69. Pharmacology bases BAT specificity on sign transductions through PKC, which stimulates the expression of gp53 receptor (CD63) (transmembrane lysosomal protein tetraspanin LAMP- 31) on the basophil surface, as CD63 may or may not require IL-3 and its specificity depends more on the epitope (drug)-paratope-IgE complementarity, affinity, avidity, atopy. The other three reported mechanisms are: a) proteins constituting the membrane and that express themselves with fast release of CD11c vesicles, b) recently synthetized proteins which express in higher time frames of hours and require transcription, translation and transportation to the plasma membrane, and c) expression on the basophil surface through the C5a receptor, releasing histamine when activated by a positive formyl-methyl-leucyl-phenylalanine (fMLP) which can be used as positive control for the determination of CD63. Other markers include CD69, CD203c, CD13, CD11b, etc. (Figure 6 and Table 4) [7,9,13,18,38,46,47].
| Acitivity marker | Expresssion | Stimulated | Speed | Location | It depends | Speciftcity |
|---|---|---|---|---|---|---|
| CD69 | Weak | IL-3 | Slow (h) | Does not form part of granule membrane | ARN m | Low |
| CD203c | Moderate | Several including IL-3 | Slow | It can be fused with membrane | Low | |
| CD11b | Various | Fast | Low | |||
| CD63 | High | Associated with Anafilatic degranulation | Fast (min) | It is part of the membrane LAMP3 | PKC fMLP | High Prevent fMLP Activated platelets |
| CD123 | High | Receptor IL-3 | Fast | It is part of the membrane | Non-Specific Avoid dendritic cells |
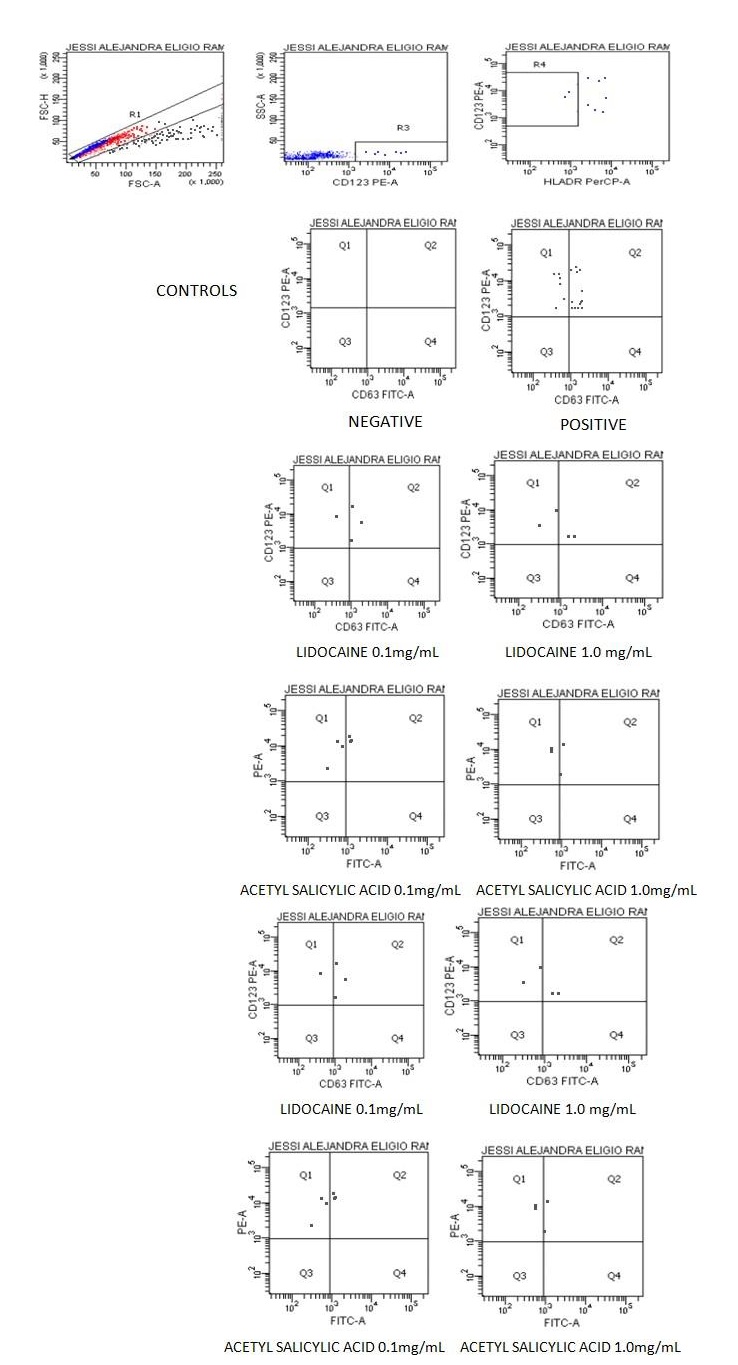
CD63-Flow cytometry: The determination is possible by staining of monoclonal antibodies marked with fluorochromes CD63-FITC, CD123-PE and PerCP HLADR with reading by flow cytometry, a negative control of PBS/Ca-albumin, pH 7.2 and a positive control of fMLP (joined to the C5a receptor). Reliability is determined by specificity and sensitivity (≥ 85%-99.4%); it is reported as CD63 %, as well as the stimulation index (SI); the RV vary depending on the author with CD63 % = 0-5, 0-30, SI or activation >2%-4%. Factors can also vary depending on the ethnicity, C5a receptors, IgE-drug affinity, IgE concentration, LAMP-31 distribution and titration of monoclonal antibodies marked with two-color fluorochrome: green (fluorescein isothiocyanate-FITC) and red (phycoerythrin-PE) (Tables 4,5, and Figure 11) [18,44].
| Results | %CD63 | MFI | %AI | %SI |
|---|---|---|---|---|
| Activation of Basophils CD63 | ||||
| CONTROL (-) | 0 | 0 | 0 | 0 |
| CONTROL (+) | 68.4 | 1,648 | 112.7 | 100 |
| LIDOCAINE 0.1 mg/mL | 75 | 1,284 | 96.3 | 85.4 |
| LIDOCAINE 1.0 mg/mL | 50 | 1,832 | 91.6 | 81.3 |
| ACETYL SALICYLIC ACID 0.1 mg/mL | 50 | 1,188 | 59.4 | 52.7 |
| ACETYL SALICYLIC ACID 1.0 mg/mL | 33.3 | 1,054 | 35.1 | 31.1 |
Functional tests as an alternative to BAT: chemotaxis and type IV hypersensitivity
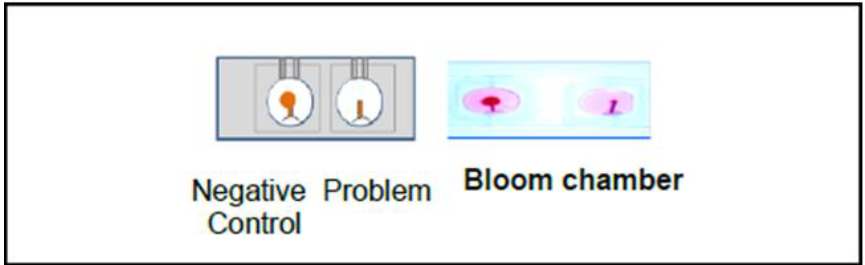
A) Modified Leukocyte Migration Inhibition Factor (MLIF) Type IV a, b and c, associated with anaphylactic degranulation: It has been reported that leukocytes, including basophils (BAT-Chemotaxis) also play a directional chemotaxis role; therefore, when microhematocrits are incubated in Bloom chambers with drugs in two dilutions (1 mg/mL and 0.1 mg/mL) in an RPMI medium, with negative and positive controls, at 37°C, the early (20 min to 2 hrs) and delayed (4, 6 and 18 hrs) migration can be measured; the % of MLIF can also be calculated vs the negative control, as well as the reference values (RV) for MLIF (0%-25% of leukocytes migration inhibition) (Figure 12) [13,37,48,49].
B) Blastoid transformation of lymphocytes (BT): It refers to cultures of incubated lymphocytes (6 days) which were stimulated with drugs at different dilutions, marking the DNA with tritiade thymidine to report the stimulation index in relation to a positive control (PHA) in 48 hrs with a RV (0.83-1.89) (Pathologic Values: 2.1- 6.7) [48-50] and Lymphocyte transformation test (LTT) : It is done with lymphocyte incubation with drugs and DNA concentration with dyes to avoid radioactivity [2,13].
Selection of BAT Diagnostic Methodology
The decision is made based on simple, inexpensive and analysis (manual and automated complexity, equipment, cost and time) [9,18,31]
Advantages and disadvantages of BAT [18,31]
Advantages:
• In vitro methods reduce the risk in hypersensitive patients or patients with severe pharmacodermy, which may lead to anaphylactic events or sudden death.
• Basophils and mast cells have a similar response to crosslinkings for FcεRI and FcεRII activation when increasing the half-life and FcεRI receptors.
• CD63 has a positive regulation in basophils and mast cells, which corresponds to an anaphylactic response.
• The activation of basophils is a fast functional test as a response to an allergen or drug.
• It is possible to separate the intrinsic factors (chemical structure of the drug) from the extrinsic factors (additives, stain, etc.).
• The study may suggest therapeutic alternatives to avoid cross-reactions.
• They have a high predictive value for drugs, allergens (food and inhaled), anesthetics and occupational toxic elements.
• They can determine the degree of sensitivity, reactivity and reading of a thousand events in the flow cytometer in allergic patients.
• There is a follow-up and post-treatment monitoring.
Disadvantages:
• It requires an implementation and standardization process for reliability.
• The concentration of basophils must be increased by separation methods with gradients of Percoll, sedimentation with dextran and only centrifugation.
• There are specific conditions, such as type of anticoagulant, preservation, transportation (5-8°C) in horizontal position, neutral pH of the solvent (pH of 3.7 allows the IgE to dissociate from the FcεRI in 10 to 30 seconds), drug stability.
• Reaction kinetics must be performed (concentration and response time), which include viability and functionality response time.
• The determination is by secreted substances (histamine, LTC4 and cytokines) or by fluorometry, spectrophotometry, ELISA (20,000 to 50,000 basophils).
• The requirement of: sampling, anticoagulant, viability (preservation and transport) to perform the BAT should not be greater than 48 hours.
• There are non-responding or secretagogue individuals.
• There is methodology complexity (equipment, reagents [expensive], laboratory staff’s experience and interpretation).
• There is a need for standardization, precision, coefficient of variation (CV) and reference values (RV) obtained in normal population (without infection), as well as for age groups and preventive culture.
• The skin provocation test and BAT may not correspond to the diagnosis (some cases of chronic spontaneous urticaria and neurodermatitis).
Discussion
Currently the synthesis and consummation of drugs has increased; in a collateral manner also an increase prevalence DHRs (urticaria, anaphylaxis, SJS, TEN, AGEP) [4,7,13,51], as well as to autoimmunity and malignancy processes [25] since postnatal stage to the old age (7% in elderly adults, 18% in children with an interval of 15%-24%, 5%-15% in hospitalized patients); more frequent in women between 40 and 60 years age. Trigger drugs include, in first place: antibiotics like penicillin, beta-lactams and cephalosporins (50%), followed by NSAIDs and analgesics (40%), and finally anesthetics, antihypertensive, hormones, antiretrovirals, among others (10%). Therefore, it is necessary to select a reliable methodology (BAT and complementary alternatives test with dilution, controls negative and positive) for better improvement in the diagnosis and public health [7,12,13,18,16,52,53]. The selection of studies according to the type of DHRs, associated with the activation of the adaptative immune system; facilitated by Gell and Coombs classification using a diagnostic algorithm [16].
Due to all of the above and since discovery in 1906 of “Allergy “and their association with mast cells and basophils: during several decades of the 20th century and the beginning of the 21st century, have been an important focus of study and research models of human species, murine and other; for the development and implementation of in vivo and in vitro tests. Based on mechanisms of adaptive immune response that provoke the release of histamine, heparin, vasoactive amines and activation markers (CD63, CD203c), Mac Glashan Jr. [18] has focused on the BAT studies in murine models; these models represent a good strategy to acquire knowledge about the functions of the effects, mechanisms of molecular and immunological activation, the development of techniques that they can be applied in humans. Several researchers, including Hoffman et al. and others [7,13,18,31,38,46,47] propose that, at present time, the most developed and widely used tests at clinical and research level are the IgE-dependent BAT; there are two principal types: 1) secretion of granules (degranulation), histamine, heparin, vasoactive amines, and 2) activation markers expression (CD63, CD69, CD203c, etc.) [18]; when comparing the essays in murine and human models, similar responses to the stimulus with allergens and drugs, two more were found during the development and standardization of BAT methods for anaphylactic degranulation (3.-MDB-Microscopy and 4.-MLIF-chemotaxis) in our laboratory (Figures 6 -10) [18].
Mac Glashan Jr reflects: What do we know? 1) If flow cytometry methods and Kits provide us with a reasonable alternative to traditional tests, especially CD63 (is more closely associated with degranulation) [18] over CD203c. We think there may be a problem with target because the basophil has a c5a receptor which can be activated via the complement (infectious or autoimmune inflammation); consequently, It loses some specificity? Although our article does not consider it. 2) Is intrinsic sensitivity of basophils a useful measure to know the sensitivity, we probably think that it is in accordance with the sensitivity and reactivity of the basophils since some patients show greater degranulation, a higher %CD63 and a %SI (responders, atopy) of the allergic patient, in addition to the reactivity and severity increase; 3) How is BAT diagnostic methodology selected? This decision is based on the clinical study requested by the physician (DHRs situation), these tests are simple, inexpensive and give great support in the diagnosis of drug reactions with coverage of several types of hypersensitivity, time, as well as basophil viability and report urgency) [13,18].
Giner-Munoz is especially concerned about children and other age group; because the first worldwide cause of allergy is infections and this also with antibiotics (50%), mainly penicillin and β-lactams, prescribed by physicians due to their wide spectrum and low toxicity, therefore it is considered that BAT and alternative tests are important to avoid cross reactions and give future therapeutic options [6,7,54].
At present there are several groups of patients that require special attention in order to promote the implementation and development of BAT and other alternatives, like chemotaxis (MLIF- Type IV) [13] that may help to reduce the prevalence, risk factors, severity, lethality, and assist in solving a public health issue:
1) The prevention of allergies to penicillin and β-lactams: improve during the diagnostic algorithm, the epidemiology, history of previous anaphylactic allergic reactions, atopic family, as well as microbiological cultures with antibiograms as a preventive measure before prescribing antibiotics in attention primary services and hospitals (public and private); in this manner, 65% of allergic patients would be identified and avoid the use of lidocaine as a solvent in lyophilized antibiotic [13,55]; 2) Patients with surgeries, accidents, autoimmune diseases or traumatological events require concomitant prescription of antibiotics and NSAIDs, which may cause DHRs in many cases; for example, diclofenac (TH1 and TH2) (39%) [13,56], and anesthetics [57] causing perioperative and operative anaphylaxis (60%) [13] hypnotic drugs (2 -10%); 3) Patients with HIV [58] make frequent use of antiretroviral treatment in the form of abacavir dose (nucleoside analogue that inhibits retrotranscription) and sulfonamide antibiotics which, in severe DHRs, activate TH1 and TH2 (80%), especially in slow acetylators [53,57,59-62]; 4) For symptoms associated with SJS (such as erythema, general discomfort, fever, nausea and vomiting), 78% of the patients had the HLA-B*57:01 allele [25]; hypersensitivity to sulfonamides and carbamazepine was also found [31,63,64]; 5) Neurological patients with epilepsy and bipolar disorder treated with carbamazepine developed SJS and had the HLA-B*15:02 allele [17,55,65]. 6) Patients with gout, persistent renal lithiasis, even leishmaniasis, as well as patients with chemotherapy and control therapy for high uric acid levels, treated with alopurinol, had the HLA-B*58:01 allele [17,24,54,63,64,66].
Conclusions
The selection of studies according to the type of DHRs is important. Nowadays, BATs are the most used tests at clinical level; however, other alternatives based on the specific stimulation of IgE-dependent basophils should be considered. The BATs (CD63-cytometry with correlation of degranulation (MBD by Microscopy) and unidirectional chemotaxis against a stimulus (MLIF-IV) read at 2 hours and 18 hours to determine hypersensitivity I and IV, this would improve diagnostic value, treatment, monitoring, prevention, identification of risk groups, prevent cross-reactions and severe pharmacodermy, creating therapeutic alternatives. The future proposed studies would be Fcε I, IFN γ, IgE in umbilical cord blood, genogram, HLA, and acetylator phenotype, all of which would help to improve the diagnosis, prevention and treatment; as well as the education for the population in this area, quality of life and health.
Acknowledgement
We appreciate the technical and administrative staff of the LCEIL Laboratory as well as our mentors in the area of Immunology: Ramon Cruz-Rodriguez, Maria de los Angeles Ortega-Hernandez and Emilio Garcia Procel. The participation of the laboratory of Hematopathology of the National School of Biological Science, IPN (Instituto Politecnico Nacional) by the Phd Elba Reyes- Maldonado and the MSc Erika Rosales-Cruz is gratefully acknowledged.
References
2. Rojas-Espinosa O. Immunology from memory. (forth edition)Médica Panamericana, Mexico 2017.
3. Montes-Montes J, Barrón-Enrique A, del Alva-Cruz- Leyva J, Flores-Flores J (2004). Reflections on the allergic and cross reactions of sulfonamides and drugs with sulfamidic radical. Revista Alergia Mexico. 2004; 51(2): 66-72.
4. Dibbern Jr DA, Montanaro A. Allergies to sulfonamide antibiotics and sulfur-containing drugs. Annals of Allergy, Asthma & Immunology. 2008 Feb 1;100(2):91-101.
5. Dreser A, Wirtz VJ, Corbett KK, Echaniz G. Antibiotic use in Mexico: review of problems and policies. Salud Publica de Mexico. 2008;50:S480-7.
6. Giner-Muñoz MT. Hypersensitivity to medications. Pediatría Integral. 2009;13:819-834.
7. Giner-Munoz MT. Allergy to medicines. Basic concepts and attitude to be followed by the pediatrician. Protoc Diagn Ter Pediatr. 2013; 1:1-24.
8. Romano A, Blanca M, Torres MJ, Bircher A, Aberer W, Brockow K, et al. Diagnosis of nonimmediate reactions to ?-lactam antibiotics. Allergy. 2004 Nov;59(11):1153-60.
9. Romano A, Guéant-Rodriguez RM, Viola M, Pettinato R, Guéant JL. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Annals of Internal Medicine. 2004 Jul 6;141(1):16-22.
10. Torres MJ, Romano A, Celik G, Demoly P, Khan DA, Macy E, et al. Approach to the diagnosis of drug hypersensitivity reactions: similarities and differences between Europe and North America. Clinical and Translational Allergy. 2017 Dec 1;7(1):7.
11. Padilla MS, Arias AC, Weinmann AM, González SD, Galindo GR, García CC. Prevalence of allergy to drugs in a group of asthmatic children and adolescents of northeast of Mexico. Revista Alergia Mexico (Tecamachalco, Puebla, Mexico: 1993). 2006;53(5):179-82.
12. Becerril-Ángeles M, Aranda-Jan A, Moreno-Quiróz J. Survey of adverse reactions to drugs in hospitalized patients. Revista Alergia Mexico (Tecamachalco, Puebla, Mexico: 1993). 2011;58(4):179-84.
13. de Lourdes Irigoyen-Coria M, Rojo-Gutiérrez MI, Meyer-Gómez RH, Leyva-Carmona I, Zendejas-Buitrón VM, García-Ruiz AD, et al. Modified tests basophil degranulation and leukocyte migration inhibition factor in drug allergy. Study 2009-2014. Revista Alergia de Mexico. 2016 Oct 1;63(4):342-350.
14. Lares-Asseff I, Trujillo-Jiménez F. Pharmacogenetics and its Clinical Significance. Gaceta Médica de México. 2001;137(3):227-236.
15. Silbergeld K, Nebert DW, McKinnon RA. Genetics Determinants of the Toxic reponse, Chapter 33 Toxicology [Spanish].Encyclopedia of health and safety at work (Thirth edition) Ministry of Labor and Social Affairs General Subdirectorate for Publications. Madrid, Spain. 1998;33.21-33.28.
16. Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International Con sensus on drug allergy. Allergy. 2014 Apr;69(4):420-37.
17. Pichler WJ. The pi concept: pharmacological interaction of drugs with immune receptors. World Allergy Organization Journal. 2008 Dec 1;1(6):96-102.
18. MacGlashan Jr DW. Basophil activation testing. Journal of Allergy and Clinical Immunology. 2013 Oct 1;132(4):777-87.
19. Gibaldi M. Pharmacogenetics: Part I The Annals of Phannacotherapy. 1992; 26: 121-26.
20. Pichler WJ. Pharmacological interaction of drugs with antigen-specific immune receptors: the pi concept. Current Opinion in Allergy and Clinical Immunology. 2002 Aug 1;2(4):301-5.
21. Mayorga C, Sanz ML, Gamboa P, Garcia-Aviles MC, Fernandez J, Torres MJ. In vitro methods for diagnosing nonimmediate hypersensitivity reactions to drugs. Journal of Investigational Allergology and Clinical Immunology. 2013 Jan 1;23(4):213-5.
22. Hewitt RG. Abacavir hypersensitivity reaction. Clinical Infectious Diseases. 2002 Apr 15;34(8):1137-42.
23. Ticse-Aguirre R,Huayanay-Falconi L, Malaga- Rodriguez G, Ferrufino-Llach JC, Ramos-Aguilar C. Síndrome de hipersensibilidad por uso de Trimetoprim/ sulfametoxazol: Reporte de un caso. Revista Medica Herediana. 2006 Apr;17(2):109-14.
24. Pichler WJ, Adam J, Watkins S, Wuillemin N, Yun J, Yerly D. Drug hypersensitivity: how drugs stimulate T cells via pharmacological interaction with immune receptors. International Archives of Allergy and Immunology. 2015;168(1):13-24.
25. Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomažič J, Jägel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P. HLA-B* 5701 screening for hypersensitivity to abacavir. New England Journal of Medicine. 2008 Feb 7;358(6):568-79.
26. Li Y, Ye D. Molecular biology for formyl peptide receptors in human diseases. Journal of Molecular Medicine. 2013 Jul 1;91(7):781-9.
27. Guzman MA, Salinas J, Toche P, Afanis A. Allergy to betalactams. Revista chilena de infectología. 2004;21(4):285-98.
28. Torres MJ, Barrionuevo E, Kowalski M, Blanca M. Hypersensitivity reactions to nonsteroidal antiinflammatory drugs. Immunology and Allergy Clinics. 2014 Aug 1;34(3):507-24.
29. Allende MB, Izuel MR, Urbieta ES, Villar IF, Carcelén JA. Cross-hypersensitivity syndrome between antiepileptic drugs: report of a case. Farmacia hospitalaria: organo oficial de expresion cientifica de la Sociedad Espanola de Farmacia Hospitalaria. 2004;28(1):56-8.
30. Gall H, Kaufmann R, Kalveram CM. Adverse reactions to local anesthetics: analysis of 197 cases. Journal of allergy and clinical immunology. 1996 Apr 1;97(4):933-7.
31. Hoffmann HJ, Knol EF, Ferrer M, Mayorga L, Sabato V, Santos AF, Eberlein B, Nopp A, MacGlashan D. Pros and cons of clinical basophil testing (BAT). Current allergy and asthma reports. 2016 Aug 1;16(8):56.
32. Hartwig IR, Sly PD, Schmidt LA, van Lieshout RJ, Bienenstock J, Holt PG, et al. Prenatal adverse life events increase the risk for atopic diseases in children, which is enhanced in the absence of a maternal atopic predisposition. Journal of Allergy and Clinical Immunology. 2014 Jul 1;134(1):160-9.
33. Mora N, Rosales C. Funciones de Receptores Fc en mecanismos de defensa y regulación inmunológica. Revista de Investigación Clínica. 2009;61(4):313-26.
34. Baillieau F. Immunoglobulin E: review and update their role in health and disease. Archives Allergy and Immunology clinical. 2015; 46(2):54-66.
35. Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nature Reviews Immunology. 2003 Sep;3(9):721-32.
36. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy and Clinical Immunology. 2004 May 1;113(5):832-6.
37. Rangel CH, Montero-Mora P, Espinoza-Larranaga F, Castillo FJ. Inhibitor factor of leukocyte migration and degranulation of basophils in drug reactions. Revista Alergia Mexico. 1991; 38(4):105-9.
38. McGowan EC, Saini S. Update on the performance and application of basophil activation tests. Current Allergy and Asthma Reports. 2013 Feb 1;13(1):101-9.
39. Sanz ML, García-Avilés MC, Gamboa PM, Weck AL. Antigen-specific leukotrienes production in the in vitro diagnosis of allergy using CAST (Cellular Assay Stimulation Test). Archives of Allergy and Immunology Clinical. 2006; 37:12-33.
40. Maeda S, Yanagihara Y. Inflammatory cytokines (Il-4, IL-5 and IL-13). Nihon rinsho. Japanese journal of clinical medicine. 2001 Oct;59(10):1894-9.
41. Sánchez-Mejorada G, Rosales C. Signal transduction by immunoglobulin Fc receptors. Journal of Leukocyte Biology. 1998 May;63(5):521-33.
42. Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of Fc?RIIB modulates B-cell receptor signalling. Nature. 1994 Mar;368(6466):70-3.
43. Sanz ML, Garcia MC, Caballero MR, Dieguez I, Gamboa PM. Basophil activation test in the diagnosis of allergy to medicines. InAnales del sistema sanitario de Navarra 2003 26(2):39- 47.
44. Fernandez P, Chaparro P, Pereira P, Castillo JL. Expression of CD63 in basophils as indicator of latex-induced degranulation, diclofenac sodium or acetylsalicylic acid. Latin American Journal of Biomedical News. 2008; 2(1): 6-10.
45. Kumar V, Cotran RS, Robbins S. Robbins: Structural and Functional Pathology (sixth edition) Mc Graw-hill Interamericana , Spain 2000
46. Steiner M, Huber S, Harrer A, Himly M. The evolution of human basophil biology from neglect towards understanding of their immune functions. BioMed Research International. 2016; 1-16.
47. Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. The Journal of experimental medicine. 1982 Jan 1;155(1):264-75.
48. Rocklin RE. Mediators of cellular immunity, their nature and assay. Journal of Investigative Dermatology. 1976 Sep 1;67(3):372-80.
49. Williams WR, Davies BH. Modulation of lymphocyte adrenergic receptors and transformation responses by therapeutic drugs. Journal of Clinical & Laboratory Immunology. 1984 Jan;13(1):29-34.
50. Garcia P, Castillo V, Felix F, Irigoyen C, Gayol V. Indices of increased blastoid transformation in drug allergy. V Coference of Clinical Chemists in IMSS, Mexico 1984.
51. Akdis CA. Allergy and hypersensitivity: mechanisms of allergic disease. Current opinion in immunology. 2006 Dec;18(6):718.
52. Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003 Sep;58(9):854-63.
53. Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003 Sep;58(9):854-63.
54. Gonzalez-Cervera J. Allergy to medicines attention primary. Practical Approach.VI Primary care pediatrics Forum of Extremadura, Spain.2009; 27-37.
55. Venegas-Montoya E, López-Pérez GT, MendozaHernández D. Transcendence of the activation of basophils test in allergy to anesthetics. Are the skin tests enough? Case report and literature review. Alergia, Asma e Inmunología Pediátricas. 2017 Jan 15;25(3):106-10.
56. Eberlein B, Santos AF, Mayorga C, Nopp A, Ferrer M, Rouzaire P, et al. Basophil activation testing in diagnosis and monitoring of allergic disease–an overview. Allergo Journal International. 2016 Jun 1;25(4):106-13.
57. Flores J, Armijo JA, Mediavilla A. Local Anesthetics. In Human Pharmacology; Hurle MA chapter 18. (Third edition) Masson S.A. Barcelona, Spain. 1997; 295-302.
58. Pirmohamed M. HIV and drug hypersensitivity. InDrug Hypersensitivity 2007: 84-94.
59. Arnedo MV. HLA-B* 5701 and hypersensitivity reactions to abacavir. Study methods and clinical relevance. Enfermedades Infecciosas Y Microbiologia Clinica. 2008 May;26:34-9.
60. Huerta-Lopez JG, Pedroza-Melendez A, Rivas- Larrauri FE, Lopez-Valentin E. Allergy to Trimethoprim with sulfamethoxazole in pantiens with HIV desensitization schemes at the National Institute of Pediatrics. Report of 2 cases. Pediatric Allergy, Asthma and Immunology. 2007;16 (3) 81-85.
61. De Leon-Nava MA, Morales-Montor J. Immune sexual dimorphism: Can sex steroids affect the Th1/ Th2 cytokine profile?. Revista de Investigación Clínica. 2006;58(2):161-9.
62. Barañao RI. Sex hormones and immune response. Revista SAEGRE. 2009;16(1):20-30.
63. Lee HY, Pang SM, Thamotharampillai T. Allopurinolinduced Stevens–Johnson syndrome and toxic epidermal necrolysis. Journal of the American Academy of Dermatology. 2008 Aug 1;59(2):352-3.
64. Halevy S, Ghislain PD, Mockenhaupt M, Fagot JP, Bavinck JN, Sidoroff A, et al. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. Journal of the American Academy of Dermatology. 2008 Jan 1;58(1):25- 32.
65. Chung WH, Hung SI, Hong HS. Medical genetics: a markerfor Stevens-Johnsonsyndrome. Nature2004.;428:486.
66. Hernandez-Zaragoza DI, Barquera R. HLA and pharmacogenetics in adverse drug reactions. PLM Dictionary of pharmaceutical specialties (sixty-one edition). French and European Publications Inc 2016.