Abstract
Bacterial metabolism affects the effectiveness of antibiotics. Bacterial metabolism is linked to the ability of an antibiotic to be bactericidal or bacteriostatic because a bacterium can metabolize carbohydrates that affect its pH and its ability to use the proton motive force (PMF). When the pH is low, there is more availability of protons that can help to power the proton motive force needed for the efflux of antibiotics. Antibiotics increase the internal pH of a bacterial cell, but when the external pH is low or acidic, the lethality of the antibiotics dwindles. Adding an efflux inhibitor (EI) can block the efflux of antibiotics; however, the pH also affects the effectiveness of the efflux inhibitor. At a low pH the efflux inhibitor cannot block the efflux of antibiotics. This is important for the effectiveness of EIs to block efflux in acidic bacterial environments such as in the stomach or in the small intestines where the pH is highly acidic and low. However, in the colon the pH is highly alkaline and higher leading to a lesser availability of protons, in which the bacterial cells must rely on carbohydrate metabolism to expel any noxious agent such as an antibiotic via the ATP activation of the ABC transporter. As a consequence, for an efflux inhibitor to be effective the pH and the metabolism of carbohydrates to power the ABC transporter must be considered in the design of potential efflux inhibitors. This commentary will offer support for the arguments made in the article, Reducing bacterial antibiotic resistance by targeting bacterial metabolic pathways and disrupting RND efflux pump activity, by presenting the results of experiments that prove the gene inhibition of the AcrAB-TolC subunits of AcrB and TolC as a potent and effective EI design.
Keywords
Antibiotics, Efflux Inhibitors, Protons, pH, Bacterial Metabolism, Glucose, Efflux
Introduction
In this commentary, the arguments presented in the review article called Reducing bacterial antibiotic resistance by targeting bacterial metabolic pathways and disrupting RND efflux pump activity will be further extended and supported. According to Bartek et al., the metabolism of bacterial cells plays a major role in antibiotic cell death [1-3]. Metabolism, bacterial growth, the balance of energy expenditures, and cell division are some of the many cellular mechanisms responsible for cell death. Cellular respiration processes nutrients to energize ATP synthesis. NADH carries electrons from these cellular respiration reactions to the electron transport systems. The electron transport system transfers and orients protons through the inner cytoplasmic membrane, which creates a gradient of protons. Energy is extracted from this proton gradient by using ATP synthase to allow the re-entry of protons into the cell. A great number of ATP molecules can be hydrolyzed when nucleic acids, cell walls, and proteins are synthesized. However, if cellular respiration is delayed or stagnated, antibiotic sensitivity decreases and antibiotic tolerance increases [1]. Antibiotic tolerance occurs when there is a reduction in the translocation of protons [1-3]. By inhibiting cellular respiration, the decreased flow of electrons lessens the formation of the pH gradient [1].
Bartek et al. confirmed and showed that cell death caused by antibiotics is possible when there is a higher intracellular pH [1]. The proton motive force (PMF) includes the electrochemical proton gradient that occurs throughout the cytoplasmic inner membrane. PMF powers the essential processes and mechanisms inside of cells. PMF drives synthesis of ATP, which helps to move substrates, such as ions, metabolites, and produce motility. PMF also leads to cell division and mediates cell-to-cell signaling. PMF powers the influx of an antibiotic named aminoglycoside, but if PMF is diminished, through the presence of protonophores, aminoglycosides can become less of a potent bactericidal and more bacteriostatic, which helps give rise to new generation of antibiotic-tolerant persister cells [4]. Efflux pumps, which cause multidrug resistance, need PMF to energize its pumping of antibiotics from cells [4-6].
Antibiotics and pH
Bartek et al. showed that cell death by antibiotics weakens when the intracellular pH is decreased, but when increasing the pHIN the antibiotics become more lethal [1]. For example, when the extracellular pH of cells was acidic with the presence of multiple protons, the lethality of the antibiotic named chlorpromazine was quenched [1]. Increasing the alkalinity of a bacterial cell population of bacilli caused increased cell death by chlorpromazine application, which was the result of the dwindling of cytoplasmic protons [1]. All antibiotics displayed a decrease in cell killing when at lower pHEX as more cell killing occurred at higher levels of pHEX. For example, a low and acidic pHEX decreased the killing of Mycobacterium smegmatis via antibiotics but there was no change in the Minimum Inhibitory Concentration (MIC) for the antibiotics of kanamycin and chlorpromazine [1]. When adding a protonophore, the cells became more tolerant of antibiotics and the MIC was not increased [1].
The bacterial cells were able to reduce internal alkalization. However, the many processes for preventing alkalization became a failed attempt through the duration of time [1]. This led to an increase in pHIN and increased cell death of many subpopulations. Bartek et al. found a trend that proved that more antibiotics efficient at increasing alkalization inside the cell produces a higher rate of cell killings [1]. Bartek et al. was able to demonstrate that an increased pHIN of M. smegmatis cells caused a subpopulation to also alkalize intracellularly. The rate of antibiotic killings was proportional to the rate of the bacterial cell population to become alkalized during increased antibiotic pressure [1]. Lowered pHEX or protonophores caused amplified proton influx and more antibiotic tolerance [1]. However, if proton influx was reduced by a high pHEX, then increased antibiotic sensitivity would occur [1]. Without antibiotics a high pHIN can cause extreme lethality in bacterial cells [1]. Bacteria can prevent alkalization pressure through multiple mechanisms that serve to continue the balance of protons. However, when these homeostatic processes and the reentry of protons via ATP synthesis cannot occur at a faster rate than proton pumping, the pHIN will become alkalized [1]. This alkalization internally of the cell will cause the hydrogen bonds located between the lipids of membranes to degrade, which will inhibit other necessary cellular processes [1].
Efflux and pH
Proton influx powers the efflux of an antibiotic termed rifampin, which causes increased rifampin resistance [1]. This may be possible through the decrease of pHIN, which is caused by the increased influx of protons that power the efflux pumps. Because efflux pumps’ expulsion of antibiotics relies upon proton antiporters, the efflux pumps can effectively inhibit cell killing by reducing the pHIN [1]. When external pH is equal to the internal pH, PMF determines the membrane potential [4]. Bacteria use efflux pumps to expel protonophores and many other toxic components, which in turn can prevent the negative effects of these harmful compounds [4]. However, efflux pumps are energized by PMF [4]. The pH of the cytoplasm controls the rate of efflux because the pH affects PMF, which alters the mechanisms of efflux systems [4].
A Proposed Model of Antibiotics, Efflux, and pH
The review, Reducing bacterial antibiotic resistance by targeting bacterial metabolic pathways and disrupting RND efflux pump activity, place much focus on stagnating efflux via gene inhibition of acrB, which codes for expression of the AcrB subunit a part of the acrAB-TolC RND efflux pump complex. AcrB depends on PMF in order to operate as a subunit of the multidrug efflux pump complex. The AcrB operates as a drug to proton antiport efflux system. The drug molecules are able to move externally out of the cell when protons flow into the cells’ cytoplasm, which powers efflux [7-10]. Therefore, Bartek et al. presented a hypothesis that postulated that the lethality of antibiotics is possible when antibiotics inhibit biosynthetic processes while metabolic processes continue to force proton efflux even as ATP synthase decreases proton influx [1]. Bartek et al. concluded that if there is no reduction in proton efflux via cellular respiration, then the high intracellular alkalinization will cause amplified cell death [1]. Hence, this commentary works to extend the possible outcome and application proposed in the aforementioned review article. This commentary serves to propose a model, as shown in Figure 1, for the possible outcomes of using the application presented in the review article. The proposed model simulates the possible effects on pHIN and pHEX when applying a glucose adjuvant with antibiotics while inhibiting efflux pump activity either with an efflux inhibitor (EI) or with gene-based efflux inhibition.
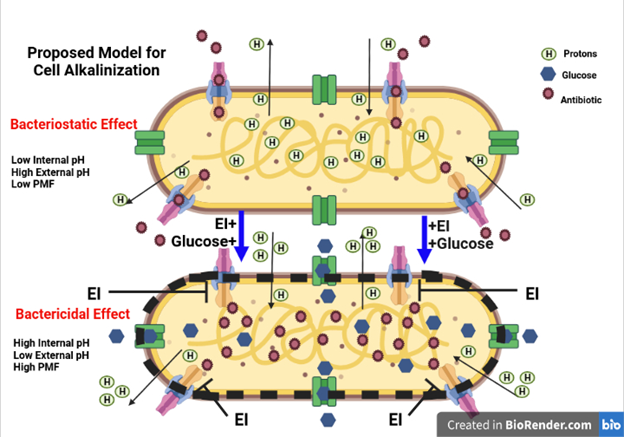
Figure 1. A Proposed Model for Induced Cell Alkalinization. Applying antibiotics with glucose as an adjuvant can increase the metabolic increase of proton efflux via PMF. Adding an efflux inhibitor can block the efflux of the antibiotics and increase antibiotic retention while the pH internal of the cell is greatly impacted by inducing a higher cytoplasmic pH needed for complete alkalinization of the cells, which amplifies the lethality of the antibiotics.
The model incorporates a visual representation of a possible application of the Bartek et al. antibiotic lethality hypothesis. The model proposes that cell death caused by high internal alkalinity will occur when antibiotic efflux is inhibited because the antibiotics inhibit ATP-consuming biosynthetic processes, and thereby, cause the stoppage of proton influx via ATP synthase as the continued cellular respiration sustains the efflux of protons. Simultaneously, as included in the aforementioned review article, while the antibiotic’s efflux is inhibited, the glucose adjuvant combined with the antibiotics will continue the metabolic efflux of protons via PMF, as noted by Bartek et al., necessary for amplifying the influx of antibiotics as well. When relying on the Bartek et al. hypothesis [1], the reduction of PMF powered efflux combined with the antibiotic-to-glucose adjuvants’ continuation of metabolic PMF should cause an increasing PHIN unto complete alkalization that leads to cell death.
Martins et al. added an efflux inhibitor called the PMF un-coupler carbonyl cyanide m-chlorophenylhydrazone CCCP to block the efflux of Ethidium Bromide (EtBr) from bacterial cells [11]. The internal bacterial cell’s pH was increased to 8, and Martins et al. added glucose to the culturing medium of the bacterial cells. Martins et al. found that the bacterial cells showed a higher fluorescence of the EtBr, which was indicative of a lesser efflux of the EtBr from the AcrAB-TolC efflux pumps, after adding the CCCP in a bacteria cell pH of 8 [11]. Martin’s et al. concluded that the external and internal pH must be taken into consideration when formulating efflux inhibitors for RND efflux pumps because Martins et al. found that at a lower pH of 5 efflux of EtBr sill occurs in the presence of an efflux inhibitor [11]. However, Martins et al. also discovered that at a higher pH of 8 efflux of EtBr was stopped because the CCCP acquired the remaining small amounts of available protons, which lowered the PMF needed to lessen efflux [11]. Martins et al. added glucose to the medium for the purpose of showing how carbohydrate metabolism can affect the effectiveness of an efflux inhibitor and to mimic the environment of bacteria present in the colon. Martins et al. argued that glucose metabolism can increase the availability of protons in the bacterial cell via the process of converting ADP to ATP that is bound to an ABC transporter, which activates the ABC transporter to expel a noxious agent [11]. Martins et al. explained that for an efflux pump to be effective against a food-borne pathogen such as Escherichia coli, it must combat the action of the ABC transporter as well. Therefore, Martins et al. concluded that the pH, regulating an efflux pump, is greatly important when designing an efflux pump inhibitor [11].
In conclusion, pH can affect the effectiveness of a bacterial RND efflux pump inhibitor. The bacterial cell pH is affected by antibiotics, the bacterium’s metabolism of carbohydrates, and the proton motive force that powers antibiotic efflux. This commentary serves to further support the arguments in the article, Reducing bacterial antibiotic resistance by targeting bacterial metabolic pathways and disrupting RND efflux pump activity, by showing that the lethality of antibiotics may be altered and increased by combining efflux inhibitors with antibiotics that contain glucose adjuvants, which can maintain metabolic PMF while containing antibiotic retention through less efflux as proven by Bartek et al. The combination of disrupting efflux pump activity with antibiotics and glucose adjuvants may reduce antibiotic resistance via increasing the intracellular pH, causing the alkalinization of target bacterial cells. This alkalinization can lead to cell death of target colonic E. coli cells. A higher pH is present in the colon of a human host and this higher pH leads to a lesser amount of protons to power PMF for the efflux of antibiotics from bacterial cells such as E. coli. Instead, the E. coli cells can rely on its ABC transporter that is activated by glucose or carbohydrate metabolism when bound to ATP. The ABC transporter must be taken into much consideration when designing an efflux inhibitor. Consequently, this commentary offers additional support for the arguments in aforementioned review article, which presented a mechanism to inactivate the AcrB and TolC subunits of the AcrAB-TolC efflux pump via gene inhibition. Gene inhibition of AcrB and TolC can block efflux to the threshold of overriding the action of the ABC transporter. Martins et al. showed this by increasing the pH to 8, adding CCCP, which is an AcrB efflux inhibitor, and including glucose in the bacterial cell medium, all of which displayed continuous efflux inhibition of the EtBr under high glucose conditions. Bacterial metabolism and pH levels must be considered when designing efflux inhibitors. Gene inhibition of the AcrB and TolC subunits of the AcrAB-TolC efflux pump may be a potent efflux inhibitor design that can help eliminate potential food-borne pathogens such as E. coli. Future research is needed to show the effectiveness and limitations of inhibiting the bacterial genes acrB and tolC in vivo.
Acknowledgments
Many thanks are given to my mentors who provided lectures, workshops, seminars, and research readings, which helped to establish my understanding of the fundamentals for microbiology. I also received hands-on experience in applying common and current novel microbiological techniques.
Conflicts of Interest
The authors declare no conflict of interest.
Funding
This research received no external funding.
References
2. Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797-810.
3. Rao SP, Alonso S, Rand L, Dick T, Pethe K. The proton motive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2008;105:11945-11950.
4. Dai Le EK, Sinjab F, Pilizota T, Kim M. Active Efflux Leads to Heterogeneous Dissipation of Proton Motive Force by Protonophores in Bacteria. mBio. 2021;12(4).
5. Terradot G, Krasnopeeva E, Swain PS, Pilizota T. The proton motive force determines Escherichia coli's robustness to extracellular pH. bioRxiv. 2021.
6. Valderrama K, Pradel E, Firsov AM, Drobecq H, Bauderlique-le Roy H, Villemagne B, et al. Pyrrolomycins are potent natural protonophores. Antimicrobial Agents and Chemotherapy. 2019;63(10):e01450-19.
7. Su CC, Li M, Gu R, Takatsuka Y, McDermott G, Nikaido H, Yu EW. Conformation of the AcrB multidrug efflux pump in mutants of the putative proton relay pathway. Journal of Bacteriology. 2006;188(20):7290-6.
8. Takatsuka Y, Nikaido H. Threonine-978 in the transmembrane segment of the multidrug efflux pump AcrB of Escherichia coli is crucial for drug transport as a probable component of the proton relay network. Journal of Bacteriology. 2006;188(20):7284-9.
9. Yu EW, Aires JR, McDermott G, Nikaido H. A periplasmic drug-binding site of the AcrB multidrug efflux pump: a crystallographic and site-directed mutagenesis study. Journal of Bacteriology. 2005;187(19):6804-15.
10. Seeger MA, von Ballmoos C, Verrey F, Pos KM. Crucial role of Asp408 in the proton translocation pathway of multidrug transporter AcrB: evidence from site-directed mutagenesis and carbodiimide labeling. Biochemistry. 2009;48(25):5801-12.
11. Martins A, Spengler G, Rodrigues L, Viveiros M, Ramos J, Martins M, et al. pH modulation of efflux pump activity of multi-drug resistant Escherichia coli: protection during its passage and eventual colonization of the colon. PloS one. 2009;4(8):e6656.
