Abstract
Adenosarcoma is a rare tumor composed of benign glandular epithelium and a malignant mesenchymal component. Here we present a case of 62-year-old female, with irregular post-menopausal bleeding. On histological diagnosis, Uterine adenosarcoma with rhabdomyoblastic differentiation with sarcomatous overgrowth was made, which carries poor prognosis and reduces the survival period of a patient. Her post – operative course was uneventful.
Keywords
Adenosarcoma, Rhabdomyoblastic, Sarcomatous overgrowth
Introduction
Uterine adenosarcoma is a rare malignancy. It is defined as a biphasic tumor composed of both sarcomatous stroma and benign epithelial components. When the sarcomatous component occupies more than 25 % of the tumor then it is referred to as the sarcomatous overgrowth which accounts to about 10% of uterine adenosarcoma cases [1]. Usually, adenosarcoma is considered a tumor of low malignant potential. But the sarcomatous overgrowth variant presents as a high-grade sarcoma and so reduces the survival rate of the patient.
In 1974, Clement and Scully were the first to describe this rare entity of ‘adenosarcoma of the uterus’. It occurs in all the age groups with peak incidence in 50s and 60s [1,2]. Common clinical presentation is irregular uterine bleeding. According to current WHO definition, Mullerian adenosarcoma are not graded histologically. In its typical form, comparable to the low – grade endometrial stromal sarcomas, adenosarcomas are considered of low malignant potential [2].
The epithelial component exhibit ER and PR receptors expression. The sarcomatous component stains for CD10 which may be lost in the cases of sarcomatous overgrowth variants. The rate of recurrence for adenosarcoma without sarcomatous overgrowth is between 15 & 25% and with sarcomatous overgrowth is as high as 45-70%. Distant metastases have been described in about 5% of affected patients [1]. Mortality of the patient of adenosarcoma with sarcomatous overgrowth is around 75%.
Here we present a case of 62 yrs. old female presented with irregular menstrual bleeding. On histopathologic examination, a diagnosis of Adenosarcoma with sarcomatous overgrowth and rhabdomyoblastic differentiation was made, indicating poor prognosis for the patient and warranting definitive empirical management.
Case Report
A 62-year-old post-menopausal woman, gravida 3 para 2, presented with chief complaint of an irregular post-menopausal bleeding. On ultrasonography, a large heterogenous mass occupying the whole uterine cavity was observed.
Clinically, a diagnosis of degenerated myoma, leiomyosarcoma or endometrial stromal sarcoma was suspected. Her serum CA-125 levels were slightly elevated to 42U/ml while CA19.9 levels were within normal limits.
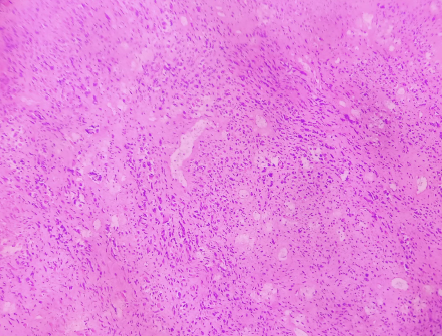
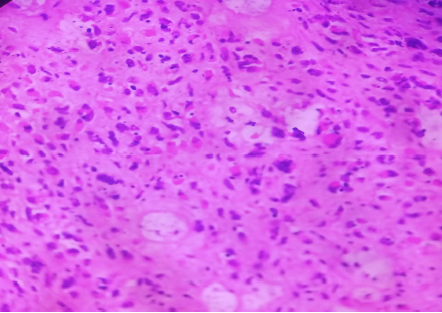
She underwent diagnostic dilatation and curettage. The endometrial biopsy was then sent in formalin to the Department of Pathology. Microscopically, sheets of round to polygonal cells having eccentric hyperchromatic nucleus, with marked pleomorphism, prominent nucleoli and moderate amount of eosinophilic cytoplasm along with few pleomorphic spindle cells were seen on H&Estained sections (Figure 2 & 3). It was diagnosed as poorly differentiated malignant tumor with Rhabdoid and focal spindle cell morphology (Figure 4). Immunohistochemistry was advised for definitive typing of the tumor.
Figure 2. H & E photograph showing malignant mesenchymal component (X100).
Figure 3. H & E photograph showing malignant mesenchymal component (X400).
Figure 4. H& E photograph showing rhabomyosarcomatous component (X400).
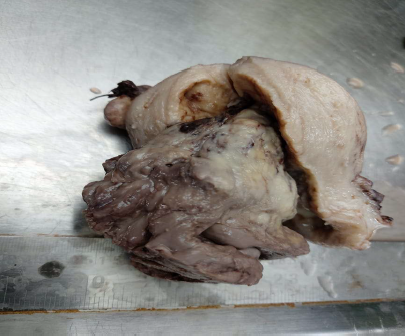
The patient was admitted and she underwent total abdominal hysterectomy with bilateral salpingooophorectomy. Grossly, uterus with cervix measured 8.2×5.4×4.5 cm and weighed approximately 200 gms. Cervical length was 3.5 cm. On cut section, a polypoidal growth was seen protruding into the uterine cavity and measuring 9.1×5.6×4.5 cm (Figure 1). On serial sectioning, a polypoidal grey-white fleshy mass with hemorrhagic and necrotic areas was seen arising from the fundus of uterine corpus and filling most of the uterine cavity. Cervix was unremarkable.
Figure 1. Gross photograph of uterine adenosarcoma – showing a polypoidal growth.
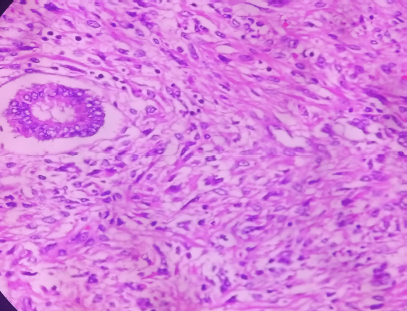
On histopathological examination, a biphasic tumor was identified with intimately admixed bland looking glands comprising of single layered endometrial type lining and surrounded by prominent atypical stroma throughout. The glandular component was evenly dispersed with intraglandular growth pattern imparting a phyllode-like architecture. The stroma around the glands was cellular and markedly atypical with 4-5 mitotic figure/hpf and with frequent areas of necrosis. Individual stromal cells were rhabdoid looking with focal spindling and fascicular like arrangement at places. Sarcomatous component was comprising of >25% of the tumor. Myometrial invasion of the tumor was superficial and involved only <20% of myometrial thickness. Heterologous element of rhabdomyosarcomatous differentiation contributed to the high-grade morphology in the setting of sarcomatous overgrowth.
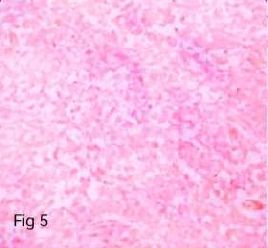
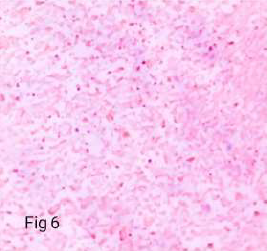
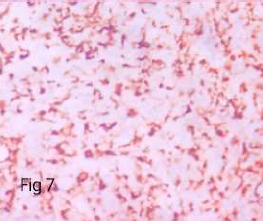
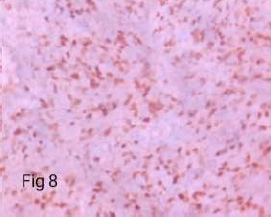
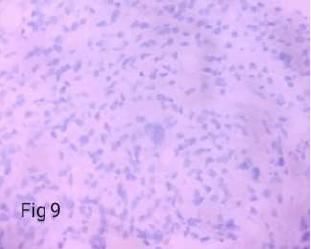
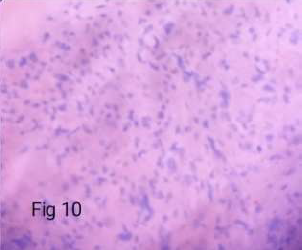
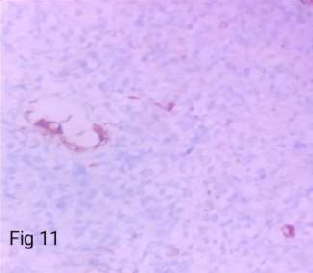
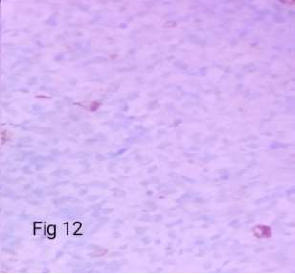
On Immunohistochemistry, the sarcomatous component was immunoreactive for Vimentin, CD 10 (variable), CD 117, cyclin D, Desmin and Myogenin while nonimmunoreactive for S-100, caldesmon and SMA. The glandular component was immunoreactive for ER, PR, Pancytokeratin (CK-AE3), while non-immunoreactive for all the mesenchymal markers mentioned above (Table 1) (Figure 5-12).
Figure 5. Immunoreactive in benign epithelial component (CK - X400).
Figure 6. Immunoreactive 4+ in sarcomatous component (Desmin – X400).
Figure 7. Immunoreactive 4+ in sarcomatous component (vimentin – X400).
Figure 8. Immunoreactive 4+ in rhabdomyo sarcomatous component (Myogenin – X400).
Figure 9. Non - Immunoreactive in glandular & sarcomatous component (SMA – X400).
Figure 10. Non - Immunoreactive in glandular & sarcomatous component (caldesmon – X400).
Figure 11. Immunoreactive in 40-50% cells in glandular component (ER – X400).
Figure 12. Immunoreactive in 40-50% cells in glandular component (PR- X400).
| Antibody | Clone | Tumor cells Immunoreactivity |
|---|---|---|
| CK | AE3 | Immunoreactive in benign epithelial component |
| SMA | Ia4 | Non-Immunoreactive in glandular & sarcomatous component |
| Desmin | Gm007 | Immunoreactive 4+ in sarcomatous component |
| CD10 | 56c6 | Immunoreactive 1+ (patchy) in sarcomatous component |
| Vimentin | V9 | Immunoreactive 4+ in sarcomatous component |
| Myogenin | mgn185 | Immunoreactive 4+ in rhabdomyosarcomatous component |
| S-100 | 4c4-9 | Non-Immunoreactive in glandular & sarcomatous component |
| Caldesmon | Ep-19 | Non-Immunoreactive in glandular & sarcomatous component |
| ER | Sp-1 | Immunoreactive in 40-50% cells in glandular component |
| PR | 1er | Immunoreactive in 40-50% cells in glandular component |
| CD117 | t-595 | Immunoreactive 1+ in sarcomatous component |
| Cyclin D1 | Sp4-r | Immunoreactive 2+ in sarcomatous component |
A diagnosis of Adenosarcoma with sarcomatous overgrowth with rhabdomyoblastic differentiation, based on morphology (Rhabdoid cell morphology) and confirmed on immunohistochemistry (Desmin & Myogenin both +ve) with FIGO STAGE-IB (Tumor confined to uterine corpus with <50% of myometrial invasion) was made. No evidence of malignant epithelial or malignant glandular component was seen. Her postoperative course was uneventful. She was subsequently discharged from the hospital.
Discussion
Mu?llerian adenosarcoma composed of malignant stromal and benign epithelial components, being a biphasic tumor. Adenosarcoma is characterized by a broad leaflike architecture along with peri-glandular cuffing of the stromal cells around the glands. The mesenchymal component is low-grade spindle cell sarcoma, whereas the epithelial counterpart is commonly endometrioid with frequent squamous or mucinous metaplasia and may, show mild to moderate atypia. It is important to assess for the presence of sarcomatous overgrowth and myometrial invasion, for prognostication . In this brief review, we present the clinical, histopathologic, and immunohistochemical features of adenosarcoma with sarcomatous overgrowth and rhabdomyoblastic differentiation. In 1974, Clement and Scully described these tumors as adenosarcoma in which, sarcomatous component is malignant and epithelial component is benign.
A subsequent study, [3] in 1979, added a few cases to the original series, and the term Mu?llerian adenosarcoma has since become universally recognized. The uterine corpus is the most common primary site of adenosarcoma, although they may also arise in the cervix, ovary, fallopian tube, or vagina. Some studies suggest that the use of tamoxifen may have a role in the pathogenesis of adenosarcoma. The differential diagnosis includes primarily benign entities, such as adenofibroma, adenomyoma (benign), endometrial polyps with unusual features, and atypical polypoid adenomyoma. In the presence of high-grade sarcoma and/or sarcomatous overgrowth, the main diagnosis to rule out is carcinosarcoma, although undifferentiated uterine sarcoma, Endometrial stromal tumor, embroyonal rhabdomyosarcoma and leiomyosarcoma should also be considered in the differential diagnosis. The most challenging differential diagnosis is carcinosarcoma, which infers both the component are malignant. Contrarily adenosarcoma typically demonstrates cytologic atypia in the sarcomatous component only. In most of the carcinosarcomas, the sarcomatous component is less predominant [4] .
The molecular mechanism of adenosarcoma with sarcomatous overgrowth is quite unclear but some studies reported the karyotype of adenosarcoma with sarcomatous overgrowth revealed a hyper diploid karyotype with structural and numerical abnormalities involving many chromosomes [5] Low-grade Endometrial Stromal Sarcoma (ESS) may resemble adenosarcoma with sarcomatous overgrowth, particularly if glandular elements and/or entrapped benign endometrial glands are present. In ESS, the tumor cells are monomorphic and having round to ovoid nuclei and fine chromatin with indistinct cell borders, scanty cytoplasm. Thin-walled blood vessels surrounded by whirling of tumor cells is characteristics. CD10 cannot distinguish ESS from Adenosarcoma as both shows immunopositivity for it.
The immunohistochemical profile varies, nonreactive for CD10, estrogen receptor, and progesterone receptor, in contrast to adenosarcoma. High-grade ESSs are positive for cyclin D1, which indirectly reflects the presence of a YWHAE-FAM22 gene fusion product created by the characteristic translocation t (10;17).24 Chemotherapy regimen such as doxorubicin, ifosfamide and gemicitabine are recommended and newer drugs like trabectedin is prescribed in cases with sarcomatous overgrowth cases.
Conclusion
Müllerian adenosarcoma is a relatively uncommon entity in gynecologic pathology that has distinctive clinical and histologic characteristics. It is important for pathologists to be aware of the morphologic features of these neoplasms to distinguish them from other biphasic (epithelial and mesenchymal) tumors, both benign and malignant. Adenosarcoma is of generally low malignant potential, except when accompanied by sarcomatous overgrowth and my invasion. Patients with adenosarcoma with sarcomatous overgrowth have poor prognosis and low survival rate.
References
2. Hodgson A, Amemiya Y, Seth A, Djordjevic B, Parra- Herran C. High-grade Müllerian Adenosarcoma: Genomic and Clinicopathologic Characterization of a Distinct Neoplasm with Prevalent TP53 Pathway Alterations and Aggressive Behavior. The American Journal of Surgical Pathology. 2017 Nov;41(11):1513-1522.
3. Valdez VA, Planas AT, Lopez VF, Goldberg M, Herrera NE. Adenosarcoma of uterus and ovary. A clinicopathologic study of two cases. Cancer. 1979 Apr;43(4):1439-47.
4. McCluggage WG. Mullerian adenosarcoma of the female genital tract. Advances in Anatomic Pathology. 2010 Mar 1;17(2):122-9.
5. Lee CH, Ali RH, Rouzbahman M, Marino-Enriquez A, Zhu M, Guo X, et al. Cyclin D1 as a diagnostic immunomarker for endometrial stromal sarcoma with YWHAE-FAM22 rearrangement. The American Journal of Surgical Pathology. 2012 Oct;36(10):1562.