Abstract
Introduction: Radiofrequency (RF) ablation of cavotricuspid isthmus (CTI) is an effective treatment for atrial flutter (AFL). This prospective, randomized study aimed to compare cryoablation (CRYO) with 10 mm tip RF catheter ablation (10RF) and 4.0 mm tip RF irrigated catheter ablation (IRRF) regarding the efficacy, safety, and long-term prognosis.
Materials and methods: Two hundred sixteen patients (51 CRYO, 84 10RF, and 81 IRRF) with CTI dependent AFL (median age 62 years) were randomized to CRYO, 10RF or IRRF catheters. The primary endpoint was the long-term efficacy defined as no symptomatic recurrences of AFL during the 12-month follow-up. The ablation endpoint was bidirectional CTI block.
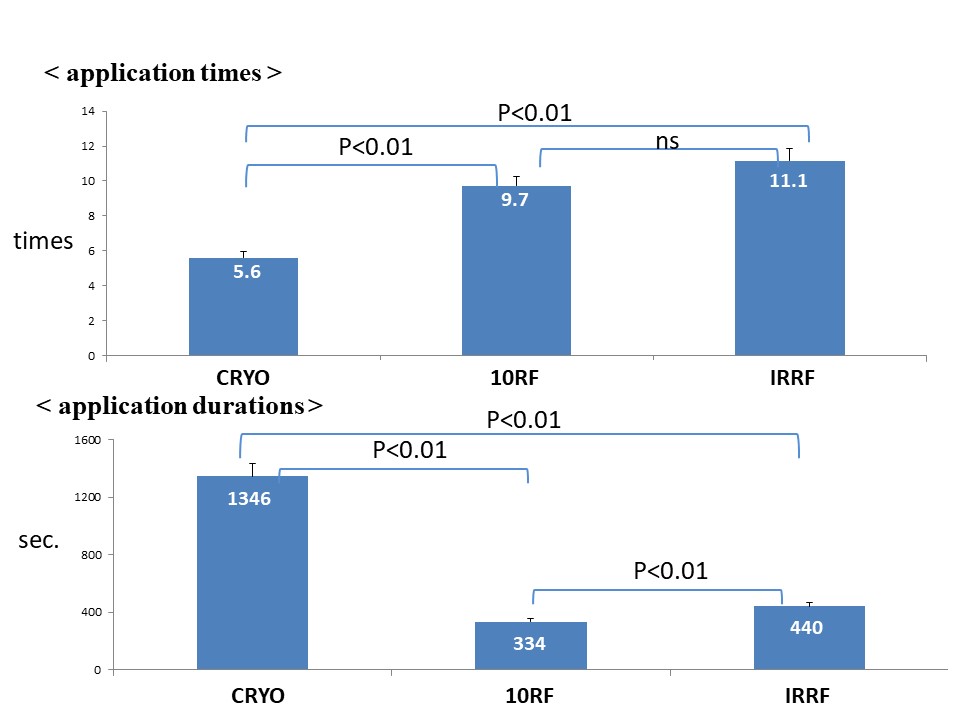
Results: The acute success rates were 98% for CRYO, 96% for 10RF, and 96% for IRRF (p=0.99). The application duration was longer with CRYO (1346 ± 49 sec) than 10RF (334 ± 37) and IRRF (440 ± 53) (p<0.01). The application time was significantly shorter with CRYO (5.6 ± 1.6) than 10RF (9.7 ± 2.2) and IRRF (11.1 ± 3.1) (p<0.01). The success rates during 12-month followup were 9% for CRYO, 96% for 10RF, and 97% for IRRF (p=0.86). No major adverse events occurred. Pain perception was not recognized for CRYO different from the 10RF and IRRF.
Conclusion: CRYO of AFL was not inferior to 10RF or IRRF but had a significantly longer application duration and a smaller number of application time.
Keywords
Catheter ablation, Atrial flutter, Radiofrequency current energy, Cryo-ablation, Large tip ablation catheter, Irrigation ablation catheter
Introduction
Various catheter ablation technologies have evolved to improve the procedural success and safety of cavotricuspid isthmus (CTI) block. Numerous studies have compared the different energy types, catheter tip sizes, and energy settings [1-3]. Irrigated or large-tip catheters have a theoretical advantage of creating wider and deeper lesions than the conventional catheters [4,5]. Disadvantageous aspects of radiofrequency (RF) energy include pain, overheating with popping, char formation, and a risk to the coronary arteries [6]. Cryoablation (CRYO) is a relatively recent addition to the transvenous ablation armamentarium and has been shown to be comparable to RF. The development of 10 mm tip RF (10RF), 4.0 mm open-irrigated-tip RF (IRRF), and 8 mm tip cryothermy ablation catheter has increased the interest in this technology for atrial flutter (AFL) ablation. There have been no randomized comparative studies published comparing CRYO, 10RF and IRRF.
Methods
Patient population
A total of 216 patients with ongoing symptoms and documented AFL with or without atrial fibrillation were included between July 2018 and September 2019. This study was a prospective, single-blinded, randomized and controlled single-center trial. At least one recent episode of AFL (within the last 6 months) was documented on a 12 lead ECG. Patients with drug induced AFL could be included and had their drug therapy continued after ablation procedure. Patients were excluded if they had undergone a previous AFL ablation, thrombus was present in the atria, or after previous cardiac surgery for valvular or congenital heart disease. The study was approved by the ethics committee of our institution. All patients signed written informed consent.
Study design
Primary endpoint: The primary endpoint was clinical success defined as the freedom from AFL evaluated at the 12-month follow-up.
Secondary endpoint: The secondary endpoints were acute ablation success defined as bidirectional CTI block, safety assessed by the rate of periprocedural complications, and the procedure and fluoroscopy times.
Baseline Electrophysiological Study
The patients were studied in the fasting state with light sedation under local anesthesia. Heparin at a dose of 5000 IE was intravenously given to the patients. Diagnostic electrode catheters were positioned in the coronary sinus (CS) and at the His recording site. One 7 Fr duodecapolar Halo catheter (2-10-mm interelectrode spacing, Livewire TM, Abbott, USA) was positioned around the tricuspid annulus for sequential activation mapping to demonstrate counterclockwise activation around the tricuspid annulus and through the CTI. Additionally, concealed entrainment during pacing in the inferior CTI confirmed the isthmus dependence of the arrhythmia.
Ablation procedure
The right atrial angiography was performed prior to catheter ablation in all patients. The length of the CTI was measured.
Cryoablation group: A long 15 Fr deflectable sheath (FlexCath Advance, Medtronic, MN, USA) was used for angiography of the CTI region and introduction of a 9 Fr, 8 mm tip cryoablation catheter (Freezor MAX, Medtronic, MN, USA), which was positioned on the tricuspid annulus (TVA). Cryoablation was then performed using a sequential application technique point-by-point from the TVA to the inferior vena cava (IVC). Cryoablation was performed at a target temperature of -80°C. Each application lasted for 240 seconds. Crossover to RF was allowed at the physician’s discretion if 10 or more cryoenergy applications failed to create CTI block.
4.0 mm open-irrigated-tip RF ablation group: RF ablation was performed using a 7 Fr 4.0 mm openirrigated- tip catheter (Navister Thermocool, Biosense Webster, Diamond Bar, CA, USA) to create an ablation line between the TVA and IVC. An RF ablation catheter was introduced through the steerable long guiding sheath (Agilis NXT, Abbott, MN, USA) into the right atrium. Continuous RF energy was delivered with a 40 W power, with a target temperature between 40 and 42°C and cutoff limit of 50°C.
10 mmm tip RF ablation group: The other RF ablation was performed using a 7 Fr 10 mm-tip catheter (Blazer-II Xp EPT-10mm, Boston Scientific, MN, USA). Ablation was performed by point-by-point RF applications along the line between the TVA and IVC for a maximum duration of 60 seconds. A temperature-controlled RF delivery was performed with a maximum power output of 70W and temperature limit of 60°C.
Three-dimensional mapping system was not used in all patients during AFL ablation.
Ablation endpoint
Acute success was defined as complete bidirectional conduction block (BCB) in the CTI, that lasted 30 minutes after the final application. The BCB was verified by pacing at a cycle length of 600 ms on each side of the ablation line using a duodecapolar Halo catheter positioned around the TVA. The differential pacing method was utilized to ascertain the BCB [7]. Additionally, the presence of BCB was confirmed by recording widely spaced double potentials along the entire ablation line.
Follow-up
All patients underwent post-procedural follow-up at 3, 6, 9, and 12 months post-ablation. Twelve-lead electrocardiograms were performed at the time of each clinic visit and Holter monitoring was performed between visits. After a blanking period of 3 months, any documented episodes of AFL lasting longer than 30 seconds were considered a recurrence. All antiarrhythmic drugs were withdrawn at 3 months after the ablation procedure. A second ablation procedure was scheduled for patients with a relapse of symptomatic and documented AFL.
Statistical analysis
Data are expressed as the mean ± SD or median and range, where appropriate. Continuous data were compared using a Bonferroni test. In the two-tailed test, a p value of <0.05 was regarded as significant. JMP Statistics version 10.0 software (SAS, Cary, NC) was used for the descriptive and inferential statistical analyses. The study procedures followed were in accordance with the “Declaration of Helsinki” and the ethical standards of the responsible institutional committee on human experimentation.
Results
Patients characteristics
The mean age was 64, 61, and 61 for the CRYO, 10RF, and IRRF groups, respectively, and 157 out of 216 (73%) were male. The mean left atrial dimension was 23.7 ± 6.4 mm and mean left ventricular ejection fraction was 63.7 ± 5.9%. Hypertension was observed in 104 (48%) patients. Table 1 shows the demographic characteristics of the patients in each group. There was no significant difference among the groups in terms of the clinical characteristics including the length of the CTI.
|
Clinical Characteristics |
||||
|
|
CRYO |
10RF |
IRRF n=81 |
P value |
|
Age |
64 ± 8 |
61 ± 11 |
61 ± 12 |
0.70 |
|
male (%) |
34 (65%) |
62 (73%) |
61 (76%) |
0.80 |
|
BMI |
23 ± 4 |
24 ± 3 |
24 ± 3 |
0.53 |
|
Left atrial diameter (mm) |
38 ± 5 |
39 ± 7 |
40 ± 7 |
0.29 |
|
Left ventricular EF % |
65 ± 9 |
63 ± 8 |
63 ±10 |
0.26 |
|
Hypertension |
30 (57%) |
41 (48%) |
33 (41%) |
0.98 |
Ablation results
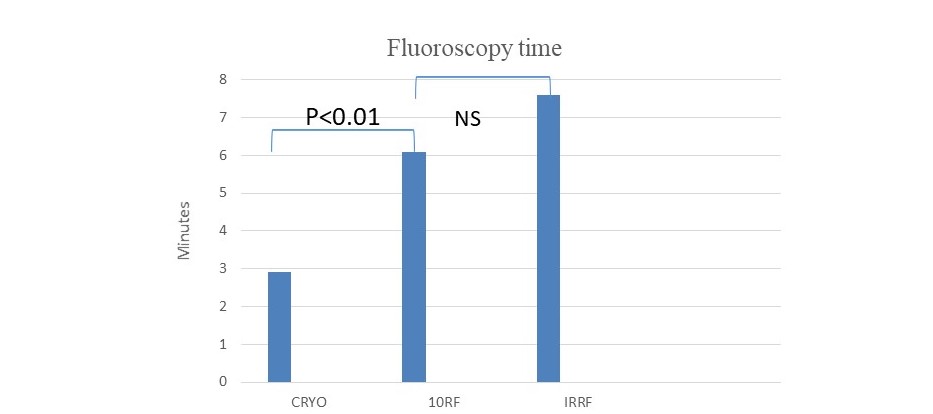
Ablation was performed during the CS paging in all study patients. Assessment of the acute results showed that BCB at the CTI, using the criteria mentioned above, was achieved in 50 out of 51 (98%) of the CRYO group patients, 81 out of 84 (96%) of the 10RF group, and 78 out of 81 (96%) of the IRRF group (p=0.99). In successful patients, the number of applications to ensure the BCB in the entire group was 8.8 ± 2.6, and 5.6 ± 1.9 in the CRYO group, 9.7 ± 2.2 in the 10RF group, and 11.1 ± 2.4 in the IRRF group. There was a significant difference between CRYO and 10RF (p<0.01) and CRYO and IRRF (p<0.01). The total ablation duration was 1346 ± 118 in the CRYO group, 334 ± 87 in the 10RF group, and 440 ± 95 in the IRRF group (p<0.01) (Figure 1). Regarding the total procedure duration, the 10RF exhibited a significantly shorter time than the CRYO and IRRF (3239.8 ± 245.1, 7537.6 ± 356.2, and 4884 ± 299.7 sec [p<0.05], respectively). In those in whom the BCB could not be achieved, the total number of applications was 27 ± 9 for CRYO, 31 ± 10 for 10RF, and 29 ± 9 for IRRF (NS) with a total application time of 2724 ± 984 seconds for CRYO, 4672 ± 1225 for 10RF, and 3241 ± 776 for IRRF (NS). The fluoroscopy duration in the CRYO, 10RF, and IRRF groups were 16.2 ± 3.8, 20.2 ± 9.3, and 18.5 ± 8.3, respectively (p=0.07) (Figure 2).
Figure 1: Application and total procedure times.
Figure 2: Total fluoroscopy time.
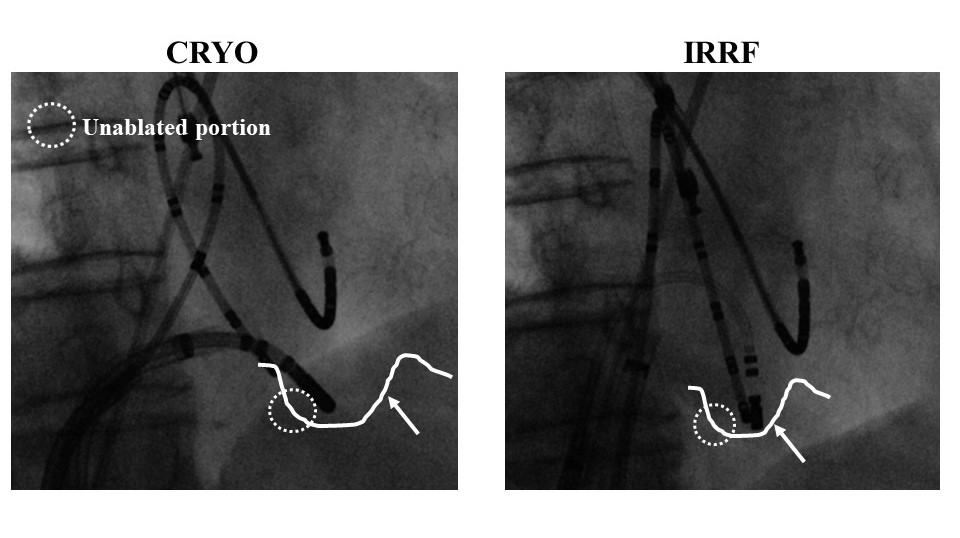
In all study patient groups, a single line at approximately 6 o’clock was drawn. In one patient in the CRYO group, the BCB could not be obtained due to the deep pouch-like recess preventing sufficient contact of the cryoablation catheter at the target tissue, which might have been associated with the inherent configuration of the cryoablation catheter (Figure 3). In four patients in the 10RF group and three in the IRRF group, the conduction recurred later during the waiting period (with a median time of 18 minutes) requiring further applications (NS). Of interest, the CRYO was able to create BCB at the CTI immediately after an unsuccessful 10RF ablation in one patient.
Figure 3: Angiographic view of cases of isthmus morphologies relative to the catheter design comparison between CRYO and IRRF.
Pain perception
The pain perception was markedly higher for 10RF and IRRF than CRYO both regarding the mean and peak pain during the ablation procedure. Further analgesic and sedative medications were required more often for 10RF and IRRF than CRYO. Actually, the pain perception was not recognized during the CRYO ablation at all.
Complications
There were no cases of prolonged AV conduction or block, transient or permanent, with the ablation positions used in this study. The only observed clinical complication was a groin haematoma in three patients in the CRYO group, two in the 10RF, and three in the IRRF group (NS).
Follow-up
After a mean follow-up of 15.4 ± 6.2 months (range 10.6 to 25.4 months), which was similar in all three groups (NS), ECG documented recurrent AFL occurred in one patient in the 10RF group and one in the IRRF group. Those recurrences were observed at 5- and 9-months post ablation, respectively. We could not exclude asymptomatic arrhythmias. No previously undocumented atrial tachycardias were observed in any of the study patients.
Discussion
The present study provided evidence, for the first time, of the long-term safety and efficacy of CRYO for the treatment of AFL as compared to the 10RF and IRRF ablation modalities.
Main findings
We compared the efficacy and safety of the CRYO, 10RF, and IRRF for CTI-dependent AFL in a randomized trial. The CRYO was not inferior to the 10RF and IRRF in terms of the acute and long-term success and no major adverse events occurred in any group. The CRYO group required significantly fewer applications to achieve success associated with a significantly shorter fluoroscopic time. However, 10RF demonstrated the shortest total procedure time among the groups. Arrhythmia recurrences in the initially successful patients were similar with a very low AFL recurrence rate.
Previous reports
In the present study, acute efficacy was close to 100 %, which was in accordance with the published cryoablation studies and RF ablation of AFL [8-10]. Bastani et al., demonstrated that CRYO ablation of CTI-dependent AFL was not inferior to RF, and of interest, CRYO was less associated with procedure-related pain [11]. In fact, CRYO was perceived as nearly pain free by the study patients in that study. Four patients underwent a successful CRYO ablation following a failed RF ablation due to a severe pain perception [11]. This matter can be regarded as one of the clinical advantageous aspects of CRYO. In addition, a lower requirement of analgesia in patients undergoing CRYO ablation might be associated with the lack of a need for general anesthesia.
Procedural parameters
The procedure time was compared between CRYO and 10RF and IRRF, which was in accordance with the other studies [9,10,12]. This matter was presumably associated with a requirement for a longer time for the lesion creation with CRYO compared to RF. Of interest, the application time of CRYO was significantly less than that for 10RF and IRRF. This matter seemed to be related to the tip size, however, the 10RF catheter has a 10 mm tip that is larger than the CRYO tip. This finding might be caused by the “cryo-adherence”, which makes the CRYO catheter firmly attached to the cardiac tissue during each CRYO application, resulted in a lesser application time with CRYO. The 10RF and IRRF catheters might move during energy deliveries due to cardiac contractions and respirations, which might cause unnecessary overlap of the lesions resulting in a larger number of applications. The CRYO catheter firmly adheres to the endocardial tissue during freezing, and therefore, there is no need to observe the catheter position using fluoroscopy. This aspect of the CRYO seems to be associated with a significantly shorter total fluoroscopic time. In other words, cryo-adhesion may be a crucial factor in reducing the fluoroscopy time.
No procedural complications were observed, such as temporary ST elevation in the inferior leads caused by coronary spasms as previously reported [10]. Pop was felt and audible in one patient with a 10RF catheter, however, no adverse effects were observed in that case.
Limitations
This study was a single center study involving a relatively limited number of patients. Isthmus conduction recovery was possible in asymptomatic cases. Recurrence of AFL was diagnosed based on the evaluations of patient symptoms and surface ECGs during the follow-up. In asymptomatic patients, electrophysiology studies were not routinely performed, so the potential differences in the longevity of the BCB could not be assessed. The results included a learning curve with each catheter for the operators performing these procedures at our center, which may have influenced the results. The operators were not blinded to the isthmus angiography, which might have influenced the catheter maneuvers to fit the isthmus morphology resulting in the differences in the results [13].
Conclusion
In this prospective, randomized, and single center study, we showed that CRYO of isthmus-dependent AFL was not inferior to 10RF and IRRF. The results showed that CRYO may provide not only short-term efficacy, but also efficacy in the long-term, and was associated with less procedurerelated pain.
References
2. Feld G, Wharton M, Plumb V, Daoud E, Friehling T, Epstein L. Radiofrequency catheter ablation of type 1 atrial flutter using large-tip 8-or 10-mm electrode catheters and a high-output radiofrequency energy generator: results of a multicenter safety and efficacy study. Journal of the American College of Cardiology. 2004 Apr 21;43(8):1466- 72.
3. Calkins H, Canby R, Weiss R, Taylor G, Wells P, Chinitz L, et al. Results of catheter ablation of typical atrial flutter. The American journal of cardiology. 2004 Aug 15;94(4):437-42.
4. Nakagawa H, Yamanashi WS, Pitha JV, Arruda M, Wang X, Ohtomo K, et al. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline-irrigated electrode versus temperature control in a canine thigh muscle preparation. Circulation. 1995 Apr 15;91(8):2264-73.
5. Jai¨s P, Hai¨ssaguerre M, Shah DC, Takahashi A, Hocini M, Lavergne T, et al. Successful irrigated-tip catheter ablation of atrial flutter resistant to conventional radiofrequency ablation. Circulation. 1998 Sep 1;98(9):835-8.
6. Thornton AS, Janse P, Alings M, Scholten MF, Mekel JM, Miltenburg M, et al. Acute success and short-term follow-up of catheter ablation of isthmus-dependent atrial flutter; a comparison of 8 mm tip radiofrequency and cryothermy catheters. Journal of Interventional Cardiac Electrophysiology. 2008 Apr 1;21(3):241-8.
7. Shah D, Hai¨ssaguerre M, Takahashi A, Jai¨s P, Hocini M, Cle´menty J. Differential pacing for distinguishing block from persistent conduction through an ablation line. Circulation. 2000 Sep 26;102(13):1517-22.
8. Jai¨s P, Shah DC, Hai¨ssaguerre M, Hocini M, Garrigue S, Le Metayer P, Cle´menty J. Prospective randomized comparison of irrigated-tip versus conventional-tip catheters for ablation of common flutter. Circulation. 2000 Feb 22;101(7):772-6.
9. Timmermans C, Ayers GM, Crijns HJ, Rodriguez LM. Randomized study comparing radiofrequency ablation with cryoablation for the treatment of atrial flutter with emphasis on pain perception. Circulation. 2003 Mar 11;107(9):1250-2.
10. Manusama R, Timmermans C, Limon F, Philippens S, Crijns HJ, Rodriguez LM. Catheter-based cryoablation permanently cures patients with common atrial flutter. Circulation. 2004 Apr 6;109(13):1636-9.
11. Bastani H, Drca N, Insulander P, Schwieler J, Braunschweig F, Kennebäck G, et al. Cryothermal vs. radiofrequency ablation as atrial flutter therapy: a randomized comparison. Europace. 2013 Mar 1;15(3):420-8.
12. Collins NJ, Barlow M, Varghese P, Leitch J. Cryoablation versus radiofrequency ablation in the treatment of atrial flutter trial (CRAAFT). Journal of Interventional Cardiac Electrophysiology. 2006 Jun 1;16(1):1-5.
13. Saygi S, Bastani H, Drca N, Insulander P, Wredlert C, Schwieler J, Jensen-Urstad M. Impact of cavotricuspid isthmus morphology in CRYO versus radiofrequency ablation of typical atrial flutter. Scandinavian Cardiovascular Journal. 2017 Mar 4;51(2):69-73.