Abstract
Background: Visceral leishmaniasis (VL), a potentially devastating neglected tropical disease is a major health concern. Despite recent advances made in diagnosis of VL, an accurate and a reliable diagnostic biomarker is still needed.
Objective: This study primarily aims to characterize the 51 kDa protein of Leishmania donovani promastigote membrane antigens (LAg) for diagnosis of VL and post kala-azar dermal leishmaniasis (PKDL).
Methods: One of the antigenic components of LAg of molecular weight 51 kDa which was previously identified as elongation factor1-α (EF1-α), was electroeluted and confirmed with anti-EF1-α antibody through immunoblot assay. Sero-diagnostic potential of 51 kDa antigen and one of its B cell epitopes was accessed through ELISA. The 51 kDa was further elucidated for urine-based diagnosis of PKDL.
Results: In the present study 51 kDa antigen was investigated for reactivity with VL patients’ sera. A strong immuno-reactivity of this antigen was observed in ELISA demonstrating 98.27% sensitivity with VL sera and 93.33% specificity with endemic and nonendemic healthy controls as well as other diseases. Further a B cell epitope of 51 kDa antigen was found to be 100% sensitive and 93.33% specific in ELISA. Additionally, in another ELISA 51 kDa antigen showed 100% sensitivity and specificity with PKDL patients’ urine.
Conclusion: The data obtained from this study leverage the potential of 51 kDa antigen in diagnosis of VL and PKDL which can be developed as a rapid diagnostic test.
Keywords
Diagnosis, Visceral leishmaniasis, Post kala-azar dermal leishmaniasis, Antigen, Immunological assays, ELISA, Electroelution
Introduction
Visceral leishmaniasis, a life threatening protozoan parasitic disease, is mainly caused by Leishmania donovani complex and is prevalent in the Indian subcontinent, East Africa and Brazil. VL patients even after successful treatment might show other clinical revelations of skin called post kala-azar dermal leishmaniasis (PKDL) [1].
Although there is substantial decline in the incidence of VL in some areas, the disease is still prevalent in most of the endemic regions with newer areas of infection being continuously reported [1]. Classical VL diagnosis relies on microscopic observation of the parasite from splenic and bone marrow aspirates [2,3]. For PKDL, slit-skin smear or biopsy specimens derived from skin lesions are examined for presence of the parasite [4,5]. These procedures are quite painful, risky and require expertise for their operation. Acute infection of VL results in production of antigen-specific antibodies which remain in the blood of patients for several years. Various serological methods such as ELISA, direct agglutination test (DAT), western blot etc. are available to detect Leishmania specific antibodies in patients. However, these assays are limited to laboratory settings [6]. Development of a rapid diagnostic test (RDT) based on recombinant antigens makes diagnosis in a field setting comparatively easier. rK39 antigen based RDT are routinely used for diagnosis of VL patients especially in the Indian subcontinent. However, this test is not equally effective for all endemic regions [1,7,8]. Recently number of serological tests using kinesin-based recombinant antigens such as rK28, rKE16, rKLO8 have been described [9-11]. Out of these, rK28 based RDT has better performance in Sudan whereas sensitivity of rK39-based RDT is low [1,12]. Earlier we have reported the diagnostic importance of Leishmania membrane antigens (LAg) and its components through various techniques such as ELISA, immunoblot assay and strip test in the Indian subcontinent [13-15] and other parts of the world [16]. Recently, we have investigated the antigenicity and protective efficacy of nine antigenic components of LAg and indicated 34 and 51 kDa native antigens as potential leads for VL diagnosis.
In the current study we have validated the sero-reactivity of 51 kDa antigen and one of its immunogenic epitopes with VL patients in the indirect ELISA. We have also characterized the potential of 51 kDa antigen for the noninvasive diagnosis of PKDL through urine.
Materials and Methods
Study cohort
Archive serum samples from VL patients (n=58) and urine of PKDL patients (n=23) were included in this study. All the VL patients were initially confirmed with routine rK39 strip test. Parasites were detected from the splenic or bone marrow aspirates of VL patients and from skin slits of PKDL patients. A total of 10 serum and 12 urine samples were used from endemic healthy controls. Besides, 10 serum and 12 urine samples of nonendemic healthy individuals were included as controls. Samples from diseases other than VL were from 3 malaria, 4 tuberculosis, 2 viral fever and 1 typhoid patients.
Electroelution and western blotting
Leishmania promastigote antigens (LAg) as described previously [17] were separated in 10% SDS-PAGE. Protein band of 51 kDa was excised from the gel and electroelution was done as per the manufacturer’s protocol (Model-422; Bio-Rad). Electroeluted fraction of the protein was dialyzed against PBS and the purity was checked on SDS-PAGE.
For western blotting, LAg (5μg/lane) was resolved by SDSPAGE and transferred onto nitrocellulose membrane. The membrane was blocked in bovine serum albumin (5%) and incubated overnight in primary anti-EF1-α polyclonal antibody (1:1000 dilution). Next day the membrane was incubated in secondary antibody (HRP-anti human) at room temperature for 2 h and the blot was developed using Image Lab software (Bio-Rad, CA, USA).
Indirect ELISA for antibody detection
Antibody capture ELISA was performed in 96-well bottom plates (Maxisorp Nunc; Thermo Fisher Scientific, MA, USA) as described previously [17]. Briefly, 51 kDa electroeluted antigen (1μg/well) in phosphate buffer (100μl/well) was used to coat the plates overnight in 4°C. Next day, the coated plate was washed in PBS with Tween-20 (0.01%) and blocked with 1% BSA in PBS for 1 h at 37°C. Subsequently serum samples with a dilution of 1:2000 and urine sample (1:10) was added for 2 h at 37°C followed by secondary antibody (peroxide conjugated antihuman IgG; 1:4000) for 1 hour. Finally, TMB (3,3′,5,5′-Tetramethylbenzidine) substrate was added and the reaction was stopped using 2 N Sulfuric acid. The absorbance was recorded at 492 nm wavelength using ELISA plate reader (RS232C; Thermo Fisher Scientific, MA, USA).
Statistical analysis
Nonparametric statistical analysis was performed using the Mann-Whitney U test for unpaired samples. GraphPad Prism software 7, San Diego, CA, USA was used for all the statistical analysis. The P<0.05 was taken as significant consideration. ROC curves were drawn for each ELISA and cut off values were determined where maximum sensitivity and specificity was achieved.
Results
Electroelution of 51 kDa protein from LAg and its reactivity in immunoblot assay
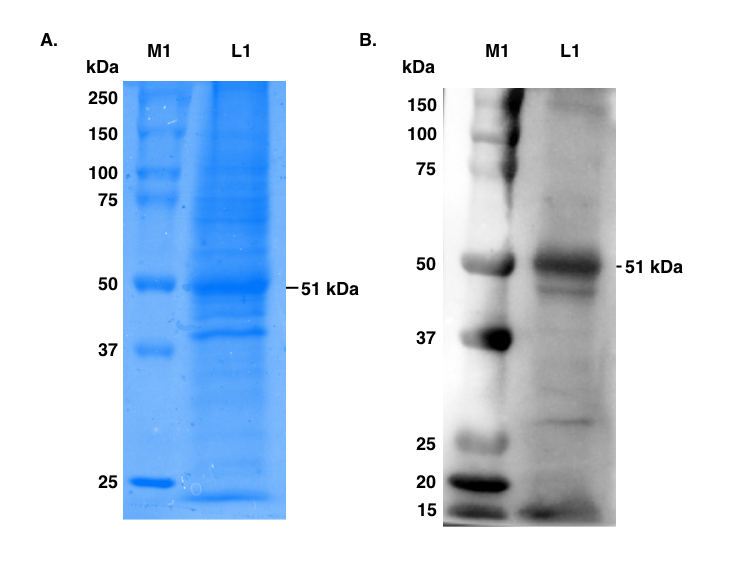
Earlier we have reported the 51 kDa antigen of LAg to be an excellent bonafide candidate antigen for diagnosis of VL [17]. Further in the previous study we have identified this antigen as elongation factor1-α (EF1-α) through mass spectrometry [15]. In this study LAg was purified from the promastigote membranes of the L. donovani. 51 kDa protein was excised from the Coomassie stained SDSPAGE gel of LAg and subjected to electroelution (Figure 1A). To validate the identity of this antigen, 51 kDa protein was accessed by the immunoblot assay of LAg with anti EF1-α polyclonal antibody raised in mice (Figure 1B). Reactivity of polyclonal antibody with 51 kDa antigen of LAg confirmed its identity as EF1-α.
Figure 1. SDS-PAGE of Leishmania promastigote membrane antigens (LAg) and Immunoblot assay with anti-elongation factor1-α (EF1-α) polyclonal antibodies.
A. LAg (5μg) was run on 10% SDS-PAGE and visualized by Coomassie blue. Lane M1 corresponds to molecular weight markers and lane L1 depicts different components of LAg including 51 kDa.
B. LAg was run on SDS-PAGE and gel was electro-transferred to nitrocellulose membrane. The blot was probed with anti-elongation factor1-α (EF1-α) polyclonal antibodies. Lane M1, molecular weight markers (150-15 kDa), lane L1; LAg probed with anti-EF1-α (51 kDa).
Sero-reactivity of electroeluted 51 kDa protein in ELISA
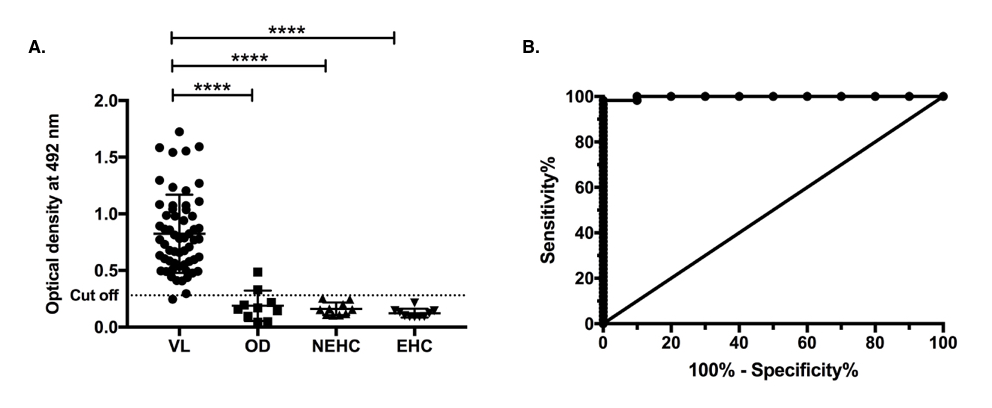
In our previous study we demonstrated the reactivity of native 51 kDa antigen with 23 VL sera [17]. In this current study we extended the experiment with 58 more serum samples along with 10 samples each from endemic and nonendemic healthy individuals as well as from diseases other than VL. We found a substantial increase (P<0.0001) in the serum antibody titre of VL patients as compared to healthy controls and symptomatically similar diseases (Figures 2A & 2B). Out of the 58 VL samples examined only one showed absorbance below the cutoff line. The antigen did not show any cross reactivity with endemic and nonendemic healthy controls. However, it did cross react with two other diseases sera, one from patient of malaria and another having viral fever. The overall ELISA experiment showed 98.27% sensitivity and 93.33% specificity. ROC curve of the experiment is depicted in Figure 2B.
Figure 2. Indirect ELISA using purified electroeluted 51 kDa antigen of LAg with VL sera. A. IgG immune-reactivity of 51 kDa antigen with serum samples from 58 VL patients (VL; n=58), 10 symptomatically similar other diseases (OD; n=10) including 3 malaria, 4 tuberculosis, 2 viral fever and 1 typhoid patient serum samples, 10 nonendemic healthy controls (NEHC; n=10) and 10 endemic healthy controls (EHC; n=10). The dotted line shows the cut off value obtained from the ROC curve where sensitivity and specificity was maximum. Results are shown as mean ± SD and were tested for significance at p<0.05. Mann-Whitney U test was performed to calculate the statistical significance. B. Receiver Operating Characteristic (ROC) curve corresponding to the ELISA.
Immunoreactivity of VL patients’ sera with B cell epitope of 51 kDa protein in ELISA
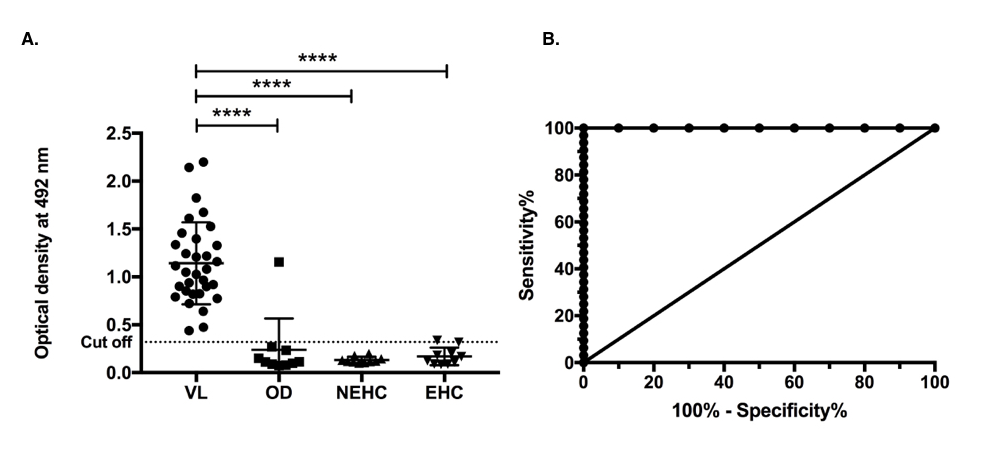
The native 51 kDa protein of LAg and one of its B cell epitopes (VCGNSKNDPPKEAAD) which was identified and synthesized from EF1-α was studied previously for their diagnostic potential using urine samples [15]. After evaluating the 51 kDa antigen in sera, we next evaluated the performance of its B cell epitope. Similar to the results obtained for native 51 kDa antigen, we found highly significant increase in the antibody titre of VL patients when tested with the epitope (p<0.0001). Clearly, 100% sensitivity and 93.33% overall specificity was observed with healthy controls and other diseases (Figure 3A). One of the malaria serum sample showed absorbance above the cutoff line thus giving a false positive response with one sample. The ROC curve of the ELISA is shown in Figure 3B. The results obtained with 51 kDa antigen and its B cell epitope tested with patients’ serum samples prove their usefulness for diagnosis of VL through ELISA.
Figure 3. Indirect ELISA using synthetic peptide of 51 kDa antigen of LAg. A. IgG immune-reactivity of synthetic peptide of 51 kDa antigen with serum samples from 32 kala-azar VL patients (VL; n=32) , 10 symptomatically similar other diseases (OD; n=10) including 3 malaria, 4 tuberculosis, 2 viral fever and 1 typhoid patients serum samples , 10 nonendemic healthy controls (NEHC; n=10) and 10 endemic healthy controls (EHC; n=10). The dotted line shows the cut off value obtained from the ROC curve where sensitivity and specificity was maximum. Results are shown as mean ± SD and were tested for significance at p<0.05. Mann-Whitney U test was performed to calculate the statistical significance. B. Receiver Operating Characteristic (ROC) curve corresponding to the ELISA.
Immunoreactivity of PKDL urine with 51 kDa protein
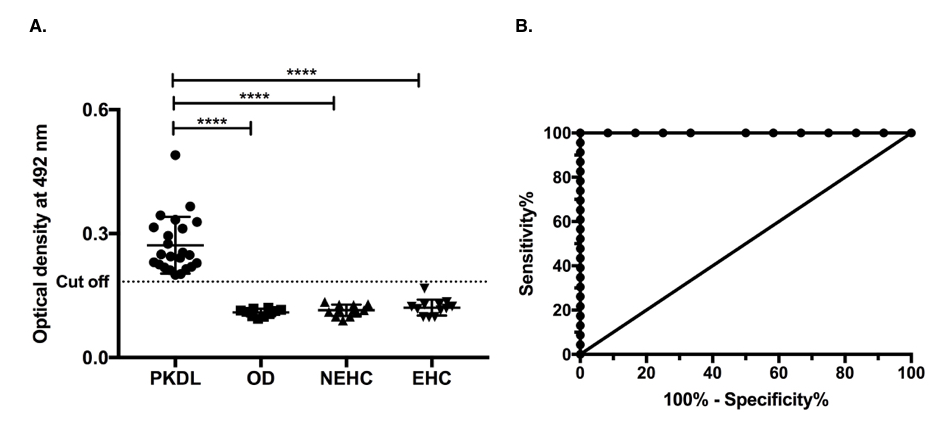
The results obtained for VL diagnosis with serum samples prompted us to further evaluate the immunoreactivity of this 51 kDa antigen for the noninvasive diagnosis of PKDL. We found a significant (p<0.0001) difference in urine antibody titre of PKDL patients to distinguish from healthy and other diseases controls (Figure 4A). Interestingly we did not find cross reactivity with any group of control urine samples, healthy or diseased. The overall sensitivity and specificity of the ELISA was found to be 100% for PKDL diagnosis with urine. The ROC curve obtained from this study is depicted in Figure 4B.
Figure 4. Indirect ELISA using purified electroeluted 51 kDa antigen of LAg with PKDL urine. A. IgG immune-reactivity of 51 kDa antigen with urine samples of 23 post kala-azar dermal leishmaniasis patients (PKDL; n=23) , 10 other diseases (OD; n=10) including 3 malaria, 4 tuberculosis, 2 viral fever and 1 typhoid urine samples , 12 nonendemic healthy controls (NEHC; n=12) and 12 endemic healthy controls (EHC; n=12). The dotted line shows the cut off value obtained from the ROC curve where sensitivity and specificity was maximum. Results are shown as mean ± SD and were tested for significance at p<0.05. Mann-Whitney U test was performed to calculate the statistical significance. B. Receiver Operating Characteristic (ROC) curve corresponding to the ELISA.
Discussion
VL is one of the oldest, often neglected tropical disease for which there is still no suitable diagnostic test available for all endemic regions. Microscopic observation of the amastigote stage of the parasite from splenic and bone marrow aspirates is considered to be the gold standard for Leishmania diagnosis [1]. However, these procedures are painful and cannot be used in a field setting. VL is characterized by Leishmania specific antibodies that remain in patients’ sera for several years even after treatment. Our laboratory has characterized the immunoglobulin G and its sub classes in kala-azar patients before and after treatment where strong reactivities of different component proteins of LAg were reported with patients’ serum [18]. In recent years attention has been directed towards recombinant antigens either single or in combination for serological diagnosis of VL. A major problem with the recombinant antigen is that they are largely expressed in the bacterial system which evades post-translational modifications. Thus, these antigens do not have carbohydrate and lipid moieties which are present in the native antigen. This might be the reason why performances of many recombinant antigens are not equal in all the VL endemic area [19]. Earlier we have evaluated the major constituents of LAg, including 51 kDa, for the diagnostic purpose using both serum and urine samples. In our recent study, we reported 51 and 34 kDa proteins of LAg as excellent candidates for VL diagnosis [17]. However, the number of serum samples used for the assay was limited [17]. Here we have extended our previous work using a larger number of samples for further validation of the study. Furthermore, a B cell epitope of 51 kDa antigen was evaluated through ELISA for VL sero-diagnosis. In addition, using 51 kDa antigen, we demonstrated a noninvasive diagnostic method for detection of PKDL infection through urine samples.
Elongation factor1-α (EF1-α), is a ubiquitously expressed protein and plays an important role in protein translation [20]. Apart from its role in protein synthesis, noncanonical functions of this protein have also been reported. In Leishmania, EF1-α is reported to be a critical virulence factor involved in modulation of various cellular signaling networks [21]. Also, EF1-α, is involved in eliciting humoral response and induces antibody production during disease [22]. Earlier we have identified EF1-α as a component of LAg through mass spectrometry MALDI ms/ms analysis. EF1-α has been found in LAg either as 51 kDa protein [15] or as a 36 kDa truncated form [22]. In this study we validated the identity of 51 kDa antigen as EF1-α through western blotting.
To investigate the reactivity of this antigen we electroeluted the antigen and performed indirect ELISA using patients’ serum samples. We found particularly good 98.27% sensitivity and 93.33% specificity upon comparison with endemic and nonendemic healthy controls and other diseases. Cross reactivity was found to be exceptionally low and only with other diseases that included one malaria and one viral fever patients. In order to further evaluate its antigenicity using bioinformatics, we used the B cell epitope of EF1-α, predicted and synthesized earlier for urine-based diagnosis [15]. This epitope has been identified as a potent peptide reactive with urine antibodies of VL patients. Using EF1-α epitope herein we found that the synthetic B cell epitope is as effective as the native 51 kDa antigen in terms of specificity (93.33%). Nevertheless, the sensitivity of the peptide is better (100%) than its native protein. PKDL, a sequel of human VL, is another complication of the skin in which the skin acts as a reservoir of the parasite and is a major hurdle in elimination of VL in the Indian subcontinent [1]. The serological methods available for PKDL diagnosis vary in terms of performance. Previously our lab has reported the reactivity of 51 kDa antigen of LAg with VL urine. Here we evaluated the immunoreactivity of 51 kDa antigen with PKDL patients’ urine. Remarkably, we found 100% sensitivity for PKDL patients with 100% specificity when compared with endemic and nonendemic healthy controls and other diseases. In summary our present study further strengthens the diagnostic potential of EF1-α as 51 kDa antigen with serum and urine samples. However, the use of this antigen in future as rapid immunochromatographic test for VL diagnosis and as test of cure remains to be explored.
Acknowledgements
Authors would like to thank Ms. Naqiya Ambareen for editing and proof reading of the manuscript.
Conflict of Interest
The authors declare no conflict of interests.
Authors’ Contribution
MK, SAE and NA contributed in conceptualizing the study, designing of experiments and interpretation of data. MK, SAE and AB performed the experiments. Manuscript was written by MK and SAE. ND contributed in generation of anti-EF1-α in mice.
Funding
This work has in part, received funding in part from UK Research and Innovation via the Global Challenges Research Fund under the grant agreement ‘A Global Network for Neglected Tropical Diseases’ grant number MR/P027989/1, Sir J. C. Bose Fellowship, India, Indian Council of Medical Research and Council of Scientific and Industrial Research, India.
References
2. WHO. Control of the leishmaniases. Geneva: World Health Organization, 2010.
3. Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clinical and Diagnostic Laboratory Immunology. 2002 Sep 1;9(5):951-8.
4. Salotra P, Singh R. Challenges in the diagnosis of post kala-azar dermal leishmaniasis. Indian Journal of Medical Research. 2006 Mar 1;123(3):295.
5. Adams ER, Versteeg I, Leeflang MM. Systematic review into diagnostics for post-kala-azar dermal leishmaniasis (PKDL). Journal of Tropical Medicine. 2013 Jan 1;2013.
6. Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clinical and Diagnostic Laboratory Immunology. 2002 Sep 1;9(5):951-8.
7. Boelaert M, Verdonck K, Menten J, Sunyoto T, van Griensven J, Chappuis F, et al. Rapid tests for the diagnosis of visceral leishmaniasis in patients with suspected disease. Cochrane Database of Systematic Reviews. 2014(6).
8. Bhattacharyya T, Bowes DE, El-Safi S, Sundar S, Falconar AK, Singh OP, et al. Significantly lower anti- Leishmania IgG responses in Sudanese versus Indian visceral leishmaniasis. PLoS Negl Trop Dis. 2014 Feb 20;8(2):e2675.
9. Sivakumar R, Dey A, Sharma P, Singh S. Expression and characterization of a recombinant kinesin antigen from an old Indian strain (DD8) of Leishmania donovani and comparing it with a commercially available antigen from a newly isolated (KE16) strain of L. donovani. Infection, genetics and evolution. 2008 May 1;8(3):313-22.
10. Abass E, Bollig N, Reinhard K, Camara B, Mansour D, Visekruna A, et al. rKLO8, a novel Leishmania donovani– derived recombinant immunodominant protein for sensitive detection of visceral leishmaniasis in Sudan. PLoS Negl Trop Dis. 2013 Jul 18;7(7):e2322.
11. Pattabhi S, Whittle J, Mohamath R, El-Safi S, Moulton GG, Guderian JA, et al. Design, development and evaluation of rK28-based point-of-care tests for improving rapid diagnosis of visceral leishmaniasis. PLoS Negl Trop Dis. 2010 Sep 14;4(9):e822.
12. Mukhtar M, Abdoun A, Ahmed AE, Ghalib H, Reed SG, Boelaert M, et al. Diagnostic accuracy of rK28-based immunochromatographic rapid diagnostic tests for visceral leishmaniasis: a prospective clinical cohort study in Sudan. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2015 Sep 1;109(9):594-600.
13. Ejazi SA, Bhattacharya P, Bakhteyar MA, Mumtaz AA, Pandey K, Das VN, et al. Noninvasive diagnosis of visceral leishmaniasis: development and evaluation of two urinebased immunoassays for detection of leishmania donovani infection in India. PLoS Neglected Tropical Diseases. 2016 Oct 14;10(10):e0005035.
14. Saha S, Goswami R, Pramanik N, Guha SK, Saha B, Rahman M, et al. Easy Test for Visceral Leishmaniasis and Post–Kala-azar Dermal Leishmaniasis. Emerging infectious diseases. 2011 Jul;17(7):1304.
15. Ejazi SA, Bhattacharyya A, Choudhury ST, Ghosh S, Sabur A, Pandey K, et al. Immunoproteomic identification and characterization of Leishmania membrane proteins as non-invasive diagnostic candidates for clinical visceral leishmaniasis. Scientific reports. 2018 Aug 14;8(1):1-1.
16. Ejazi SA, Ghosh S, Saha S, Choudhury ST, Bhattacharyya A, Chatterjee M, et al. A multicentric evaluation of dipstick test for serodiagnosis of visceral leishmaniasis in India, Nepal, Sri Lanka, Brazil, Ethiopia and Spain. Scientific reports. 2019 Jul 9;9(1):1-7.
17. Ejazi SA, Ghosh S, Bhattacharyya A, Kamran M, Das S, Bhowmick S, et al. Investigation of the antigenicity and protective efficacy of Leishmania promastigote membrane antigens in search of potential diagnostic and vaccine candidates against visceral leishmaniasis. Parasites & Vectors. 2020 Dec;13(1):1-2.
18. Ravindran R, Anam K, Bairagi BC, Saha B, Pramanik N, Guha SK, et al. Characterization of immunoglobulin G and its subclass response to Indian kala-azar infection before and after chemotherapy. Infection and immunity. 2004 Feb 1;72(2):863-70.
19. Kühne V, Rezaei Z, Pitzinger P, Büscher P. Systematic review on antigens for serodiagnosis of visceral leishmaniasis, with a focus on East Africa. PLoS Neglected Tropical Diseases. 2019 Aug 15;13(8):e0007658.
20. Lopez M, Cherkasov A, Nandan D. Molecular architecture of Leishmania EF-1α reveals a novel site that may modulate protein translation: A possible target for drug development. Biochemical and Biophysical Research Communications. 2007 May 18;356(4):886-92.
21. Nandan D, Cherkasov A, Sabouti R, Yi T, Reiner NE. Molecular cloning, biochemical and structural analysis of elongation factor-1α from Leishmania donovani: comparison with the mammalian homologue. Biochemical and Biophysical Research Communications. 2003 Mar 21;302(4):646-52.
22. Sabur A, Bhowmick S, Chhajer R, Ejazi SA, Didwania N, Asad M, et al. Liposomal elongation factor-1α triggers effector CD4 and CD8 T cells for induction of long-lasting protective immunity against visceral leishmaniasis. Frontiers in immunology. 2018 Jan 30;9:18.