Commentary
In 1989, researchers proposed an intricate strategy in the field of adoptive cell therapy (ACT) [1]. Using the T-cell receptor (TCR) as a template, they replaced the coding sequence for the Vα and Vβ chains with the antigen- recognition domains from an antibody (VH and VL chains) [1]. While each format allows T cells to recognize unique antigens, the later supports T-cell activation in a major histocompatibility complex (MHC)- independent manner, such chimeric entities become known as “T-bodies” [1]. In a streamlined version of this approach, they reduced the antigen recognition moiety to a single-chain variable fragment domain (scFv) and fused it to the ζ chain of the TCR/CD3 complex [2]. Within this modular recombinant, the intracellular CD3ζ chain is sufficient to support T-cell activation following antigen engagement. From a design perspective, it explains the origin of the chimeric antigen receptors (CARs) used clinically to treat cancer. Several iterations of this approach have been developed, including very recent efforts that replace CD3ζ with CD3ε or growth factor receptor-bound protein 2 (GRB2) to permit optimal structural reconfigurations in the receptor complex and accentuate signal transduction [3]. Second-generation CARs included including “built-in” coreceptor stimulatory domains from CD28, 4-1BB and the Inducible T-cell costimulator (ICOS) to achieve complete functional competence and potency [4]. To generate CARs, scFvs are usually isolated from antibody libraries originating from an immunized host (non-human) [4-7]. There are inherent limitations to this approach as adoptive transfer can trigger an immune response characterized by the production of neutralizing antibodies against the foreign scFvs [5,6]. This limits “durable efficacy” as the administered cells are targeted for destruction and eliminated from the circulation [4-6]. Recent advances have addressed this with the aim of increasing long-term persistence and immune-surveillance following T-cell transfer.
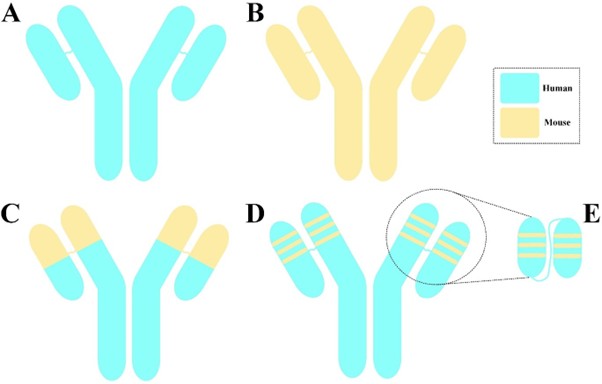
scFv humanization is increasingly recognized as an important design feature to optimize CAR-T cell longevity following infusion (Figure 1) [4,6]. An scFv is composed of four framework regions and three complementarity- determining regions (CDRs), which are responsible for antigen recognition [8]. CDR grafting describes a process where amino acids in the scFv framework of a murine-based CAR are substituted with those of its human counterpart [4,9]. This method is one of the most widely used approaches for the humanization of antibody fragments [9]. Given the dedicated effort to maintain high residue identification during this process, the humanized antibody fragment is expected to have similar characteristics with respect to affinity, sensitivity, and specificity as those of its native counterpart [9]. Another strategy to overcome the immunogenicity issue of animal-derived targeting moieties is to incorporate fully human antibody fragments into CAR constructs [6,10,11]; however, a limited number have been developed thus far. On this basis, scFv humanization remains the preferred option, and their therapeutic promise is currently being tested in preclinical as well as clinical settings (summarized in Table 1) within the CAR arena [12-18].
| Clinical trial identifier | Target antigen | Indication(s) | Number of patients | Start date | Completion date | Phase | Ref. |
|---|---|---|---|---|---|---|---|
| NCT02782351 | CD19 | R/R B-cell malignancies | 50 | May 2016 | December 2018 | I / II | [29] |
| NCT02349698 | CD19 | B-cell leukemia and lymphoma | 45 | December 2014 | December 2023 | I / II | [30] |
| NCT02374333 | CD19 | B-ALL and DLBCL | 85 | March 2014 | November 2022 | I | [38] |
| NCT04532268 | CD19 | B-ALL andB-cell NHL | 72 | August2020 | August2026 | Early Phase I | - |
Herein, we mention some CAR-T cell products that have humanized scFvs as their targeting domains and highlight humanized monoclonal antibodies (mAbs) that achieved success in the clinics.
As of August 4, 2021, five CAR-T cell products have been granted permission by the United States food and drug administration (FDA) for medical use [19-25]. Within this therapeutic group, four CARs are designed to recognize CD19 as their target antigen (namely tisagenlecleucel, axicabtagene ciloleucel, brexucabtagene autoleucel, and lisocabtagene maraleucel) and one (namely idecabtagene vicleucel) targets B-cell maturation antigen (BCMA) [19-25]. However, all these CAR-T products rely on murine scFvs to redirect their specificity against CD19 (FMC63 scFv) as well as BCMA [19-25]. Since there have been reports regarding the immunogenicity of animal-derived targeting domains, there is room for optimization [4-6].
In 2006, Kershaw and co-investigators conducted a Phase I clinical trial to investigate the safety of folate receptorredirected CAR-T cells in patients with metastatic ovarian cancer [26]. As reported, the administered CAR-T cells failed to react with folate receptor-expressing tumor cells in 3 out of 6 subjects (50%) which was attributed to the development of inhibitory factors in their sera [26]. Moreover, in 2011, Lamers and colleagues generated CAR-T cells against carbonic anhydrase IX (CAIX) and investigated their ability to control tumor burden in metastatic renal cell carcinoma patients [27]. Persistence issues were observed following infusion which resulted from immune reactions against the CDRs and framework regions of the CAR targeting domain. This compromised CAR-T cell-mediated antitumor responses [27]. Of note, CAR gene delivery was achieved by retroviral infection in this trial. Immune reactions against the γ-retroviral vectorencoded epitopes were also observed in 2 of the patients further exemplifying immunogenicity issues surrounding CAR transgenes and vectors used for gene transfer [27]. Finally, in a clinical trial (NCT01865617) which investigated the effectiveness of CD19-redirected CAR-T cells against B-cell acute lymphoblastic leukemia (B-ALL), Turtle et al. noted a CD8+ T-cell-mediated immune response against adoptively transferred cells expressing the synthetic receptor. This limited persistence of the administered CAR-T cells and increased the risk of disease relapse [28].
In 2018, Cao et al. reported the results from a clinical trial (NCT02782351) investigating the effectiveness of humanized version of the murine FMC63 antibody, included as the targeting domain of CAR-T cells in patients with R/R B-ALL (Table 1) [29]. 18 patients were enrolled in this study from which 14 did not have previous CAR-T cell therapy [29]. Among patients without previous CAR-T cell treatment, 13 (92.9%) achieved complete remission (CR) with incomplete count recovery (CRi) on day 30 [29]. Of note, CRi is defined as <5% bone marrow blasts, absence of extramedullary disease, and no recovery of peripheral blood counts independent of transfusion. Moreover, 17 patients (94.4%) experienced cytokine release syndrome (CRS) and 1 (5.5%) developed reversible neurotoxicity [29]. Of 4 patients with previous CAR-T cell therapy, 1 died on day 14 due to intracranial hemorrhage [29]. Moreover, 2 patients died after undergoing salvage therapy (one on day 145 and the other on day 169) [29]. The remaining patient was reported to be MRD-negative until day 168 [29]. These findings show that CD19-redirected CAR-T cells equipped with humanized scFvs can effectively mediate disease remission in R/R B-ALL patients even in those who have had multiple previous conventional CAR-T cell treatment [29].
In 2020, Heng and co-workers reported the results of another clinical trial (NCT02349698) investigating CAR-Ts with a humanized scFv against CD19 for the treatment of R/R B-ALL patients (Table 1) [30]. Ten patients with R/R B-ALL were enrolled in this study, all of which (100%) achieved CR, 8 patients (80%) remained CR (report published in 2020) and 6 patients (60%) had CR for more than one year and a half [30]. The researchers also reported CRS and neurotoxicity in 4 patients which was mitigated using tocilizumab, glucocorticoid, and plasma exchange [30]. They concluded that CAR-T cells equipped with humanized targeting domains demonstrate prolonged persistence leading to low rates of disease relapse [30].
In a recent clinical trial (NCT02374333), Myers et al., evaluated the antitumor response, persistence, and toxicity of CD19- redirected CAR-T cells with humanized scFvs as the targeting domain in children and young adults with B- ALL (72 patients) and B-lymphoblastic lymphoma (2 patients) (Table 1) [31]. Among these patients, 33 had previous CAR-T cell treatment with a CAR construct containing a murine scFv (FMC63) [31]. 62 patients (84%) experienced CRS and neurotoxicity was observed in 29 patients (39%) [31]. The overall response rate one month after CAR-T cell administration was 98% among the patients with no CAR-T cell treatment history and 64% among the patients with prior CAR-T cell treatment [31]. The researchers also indicated that the relapse-free survival rate at 24 months was 58% and 74% among patients with and without previous CAR-T cell treatment, respectively [31]. Collectively, these findings show that CAR-T cells with humanized targeting domains are capable of mediating durable disease remission with prolonged persistence in children and young adults with R/R B-ALL, even in patients that underwent unsuccessful treatments with CAR-T cells [31].
Several parameters are widely acclaimed to influence the effectiveness of CAR-T cell therapies including CAR-T cell quality, differentiation status, metabolic profile, and importantly CAR design [32-37]. Given the limited persistence and immunogenicity issues surrounding CAR-T cell products designed with murine-based scFvs, efforts to develop humanized versions without impairing affinity, specificity, and sensitivity might further enhance the therapeutic promise of CARs redirected against tumor antigens (Table 2). Exemplifying their therapeutic promise, several iterations of humanized CD19-specific CAR T cells are being tested in clinical trials.
| mAb name | Trade name | Structure | Target | Indication(s) | FDA approval date |
References |
|---|---|---|---|---|---|---|
| Loncastuximabtesirine | Zynlonta | Humanized IgG1 ADC | CD19 | DLBCL | 2021 | [39] |
| Belantamabmafodotin | BLENREP | HumanizedIgG1 ADC | BCMA | MM | 2020 | [40] |
| Tafasitamab | Monjuvi | Humanized IgG1 | CD19 | DLBCL | 2020 | [40] |
| Polatuzumab vedotin | Polivy | Humanized IgG1 ADC | CD79b | DLBCL | 2019 | [41] |
| Mogamulizumab | Poteligeo | Humanized IgG1 | CCR4 | CTCL | 2018 | [42] |
| Inotuzumab ozogamicin | BESPONSA | Humanized IgG4 ADC | CD22 | Hematological malignancies | 2017 | [43] |
| Elotuzumab | Empliciti | Humanized IgG1 | SLAMF7 | MM | 2015 | [44] |
| Obinutuzumab | Gazyva | HumanizedIgG1; Glycoengineered | CD20 | CLL | 2013 | [45] |
| Alemtuzumab | MabCampath, Campath-1H; Lemtrada | Humanized IgG1 | CD52 | CML | 2001 | [46] |
| Gemtuzumab ozogamicin | Mylotarg | Humanized IgG4 ADC | CD33 | AML | 2000 | [47] |
| Abbreviations: ADC: Antibody-Drug Conjugate; US FDA: United States Food and Drug Administration; DLBCL: Diffuse Large B-Cell Lymphoma; BCMA: B-Cell Maturation Antigen; MM: Multiple Myeloma; CCR4: C-C Motif Chemokine Receptor 4; CTCL: Cutaneous T-Cell Lymphoma; CLL: Chronic Lymphocytic Leukemia; CML: Chronic Myeloid Leukemia; AML: Acute Myeloid Leukemia. | ||||||
Conflict of interests
R.S. O’Connor reports a patent for CAR T-cell therapies licensed to Novartis AG. No disclosures were reported by the other authors.
Acknowledgments
R.S. O’Connor is supported by an NIH grant RO1CA226983–03.
References
2. Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proceedings of the National Academy of Sciences. 1993 Jan 15; 90(2):720-4.
3. Salter AI, Rajan A, Kennedy JJ, Ivey RG, Shelby SA, Leung I, et al. Comparative analysis of TCR and CAR signaling informs CAR designs with superior antigen sensitivity and in vivo function. Science Signaling. 2021 Aug 24; 14(697):eabe2606.
4. Kozani PS, Kozani PS, O’Connor RS. In Like a Lamb; Out Like a Lion: Marching CAR T Cells Toward Enhanced Efficacy in B-ALL. Molecular Cancer Therapeutics. 2021 Apr 26.
5. Wagner DL, Fritsche E, Pulsipher MA, Ahmed N, Hamieh M, Hegde M, et al. Immunogenicity of CAR T cells in cancer therapy. Nature Reviews Clinical Oncology. 2021 Jun; 18(6):379-93.
6. Sommermeyer D, Hill T, Shamah SM, Salter AI, Chen Y, Mohler KM, et al. Fully human CD19-specific chimeric antigen receptors for T-cell therapy. Leukemia. 2017 Oct; 31(10):2191-9.
7. Kozani PS, Kozani PS, Rahbarizadeh F. Novel antigens of CAR T cell therapy: New roads; old destination. Translational Oncology. 2021 Jul 1; 14(7):101079.
8. Ahmad ZA, Yeap SK, Ali AM, Ho WY, Alitheen NB, Hamid M.scFv antibody: principles and clinical application. Clinical and developmental immunology. 2012 Oct; 2012.
9. Kim JH, Hong HJ. Humanization by CDR grafting and specificitydetermining residue grafting. InAntibody Engineering 2012 (pp. 237- 245). Humana Press, Totowa, NJ.
10. Tan Y, Cai H, Li C, Deng B, Song W, Ling Z, et al. A novel full-human CD22-CAR T cell therapy with potent activity against CD22 low B-ALL. Blood Cancer Journal. 2021 Apr 10; 11(4):1-6.
11. Wang D, Wang J, Hu G, Wang W, Xiao Y, Cai H, et al. A phase 1 study of a novel fully human BCMA-targeting CAR (CT103A) in patients with relapsed/refractory multiple myeloma. Blood, The Journal of the American Society of Hematology. 2021 May 27; 137(21):2890-901.
12. Myers RM, Li Y, Barz Leahy A, Barrett DM, Teachey DT, Callahan C, et al. Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-Exposed Children and Young Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia. Journal of Clinical Oncology. 2021 Jun: JCO-20.
13. Wang S, Wang X, Ye C, Cheng H, Shi M, Chen W, et al. Humanized CD19-targeted chimeric antigen receptor T (CAR-T) cells for relapsed/ refractory pediatric acute lymphoblastic leukemia. American Journal of Hematology. 2021 May 1; 96(5):E162-5.
14. Heng G, Jia J, Li S, Fu G, Wang M, Qin D, et al. Sustained therapeutic efficacy of humanized anti-CD19 chimeric antigen receptor T cells in relapsed/refractory acute lymphoblastic leukemia. Clinical Cancer Research. 2020 Apr 1; 26(7):1606-15.
15. Li MY, Lin ZH, Hu MM, Kang LQ, Wu XX, Chen QW, et al. Secondary donor-derived humanized CD19-modified CAR-T cells induce remission in relapsed/refractory mixed phenotype acute leukemia after allogeneic hematopoietic stem cell transplantation: a case report. Biomarker Research. 2020 Dec;8(1):1-6.
16. Liu P, Liu M, Lyu C, Lu W, Cui R, Wang J, et al. Acute graft-versushost disease after humanized anti-CD19-CAR T therapy in relapsed B-ALL patients after allogeneic hematopoietic stem cell transplant. Frontiers in Oncology. 2020 Sep 29; 10:1975.
17. Perez-Amill L, Suñe G, Antoñana-Vildosola A, Castella M, Najjar A, Bonet J, et al. Preclinical development of a humanized chimeric antigen receptor against B-cell maturation antigen for multiple myeloma. Haematologica. 2021 Jan 1; 106(1).
18. Hucks GE, Barrett D, Rheingold SR, Aplenc R, Teachey DT, Callahan C, et al. Humanized chimeric antigen receptor (CAR)-modified T cells targeting CD19 induce remissions in children and young adults with relapsed/refractory lymphoblastic leukemia/lymphoma. Cytotherapy. 2017 May 1; 19(5):S9-10.
19. Bouchkouj N, Kasamon YL, de Claro RA, George B, Lin X, Lee S, et al. FDA approval summary: axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma. Clinical Cancer Research. 2019 Mar 15; 25(6):1702-8.
20. Prasad V. Tisagenlecleucel—the first approved CAR-T-cell therapy: implications for payers and policy makers. Nature Reviews Clinical Oncology. 2018 Jan; 15(1):11-2.
21. Mullard A. FDA approves first BCMA-targeted CAR-T cell therapy. Nature Reviews Drug Discovery. 2021 Mar 31.
22. Voelker R. CAR-T Therapy Is Approved for Mantle Cell Lymphoma. Jama. 2020 Sep 1; 324(9):832-.
23. Mullard A. FDA approves first CAR T therapy. Nature reviews Drug discovery. 2017 Oct 1; 16(10):669-70.
24. Mullard A. FDA approves fourth CAR-T cell therapy. Nature reviews. Drug Discovery. 2021 Feb 11; 20(3):166.
25. T-cell FA. Therapy. Cancer Discov. 2018 Jan;8(1):5-6.
26. Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clinical Cancer Research. 2006 Oct 15; 12(20):6106-15.
27. Lamers CH, Willemsen R, van Elzakker P, van Steenbergen- Langeveld S, Broertjes M, Oosterwijk-Wakka J, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo–engineered T cells. Blood, The Journal of the American Society of Hematology. 2011 Jan 6; 117(1):72-82.
28. Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR–T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. The Journal of Clinical Investigation. 2016 Jun 1; 126(6):2123-38.
29. Cao J, Wang G, Cheng H, Wei C, Qi K, Sang W, et al. Potent anti‐ leukemia activities of humanized CD19‐targeted Chimeric antigen receptor T (CAR‐T) cells in patients with relapsed/refractory acute lymphoblastic leukemia. American Journal of Hematology. 2018 Jul; 93(7):851-8
30. Heng G, Jia J, Li S, Fu G, Wang M, Qin D, et al. Sustained therapeutic efficacy of humanized anti-CD19 chimeric antigen receptor T cells in relapsed/refractory acute lymphoblastic leukemia. Clinical Cancer Research. 2020 Apr 1; 26(7):1606-15.
31. Myers RM, Li Y, Barz Leahy A, Barrett DM, Teachey DT, Callahan C, et al. Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-Exposed Children and Young Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia. Journal of Clinical Oncology. 2021 Jun: JCO-20.
32. Garcia-Canaveras JC, Heo D, Trefely S, Leferovich J, Xu C, Philipson BI, et al. CAR T-Cells Depend on the Coupling of NADH Oxidation with ATP Production. Cells. 2021 Sep; 10(9):2334.
33. Pellegrino M, Del Bufalo F, De Angelis B, Quintarelli C, Caruana I, de Billy E. Manipulating the Metabolism to Improve the Efficacy of CAR T-Cell Immunotherapy. Cells. 2021 Jan; 10(1):14.
34. Schwab RD, Bedoya DM, King TR, Levine BL, Posey AD. Approaches of T Cell Activation and Differentiation for CAR-T Cell Therapies. InChimeric Antigen Receptor T Cells 2020 (pp. 203-211). Humana, New York, NY.
35. Safarzadeh Kozani P, Safarzadeh Kozani P, Rahbarizadeh F. Optimizing the Clinical Impact of CAR-T Cell Therapy in B-cell Acute Lymphoblastic Leukemia: Looking Back While Moving Forward. Frontiers in Immunology.4453.
36. Safarzadeh Kozani P, Safarzadeh Kozani P, Rahbarizadeh F, Khoshtinat Nikkhoi S. Strategies for dodging the obstacles in CAR T cell therapy. Frontiers in Oncology. 2021 Apr 1; 11:924.
37. Boroojerdi MH, Rahbarizadeh F, Kozani PS, Kamali E, Kozani PS. Strategies for having a more effective and less toxic CAR T-cell therapy for acute lymphoblastic leukemia. Medical Oncology. 2020 Nov; 37(11):1-5.
38. Myers RM, Li Y, Barz Leahy A, Barrett DM, Teachey DT, Callahan C, et al. Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-Exposed Children and Young Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia. Journal of Clinical Oncology. 2021 Jun: JCO-20.
39. Jain N, Stock W, Zeidan A, Atallah E, McCloskey J, Heffner L, et al. Loncastuximab tesirine, an anti-CD19 antibody-drug conjugate, in relapsed/refractory B-cell acute lymphoblastic leukemia. Blood Advances. 2020 Feb 11;4(3):449-57.
40. Kaplon H, Reichert JM. Antibodies to watch in 2021. InMAbs 2021 Jan 1; 13(1): p. 1860476.
41. Kaplon H, Muralidharan M, Schneider Z, Reichert JM. Antibodies to watch in 2020. InMAbs 2020 Jan 1 (Vol. 12, No. 1, p. 1703531). Taylor & Francis.
42. Kaplon H, Reichert JM. Antibodies to watch in 2019. InMAbs 2019 Feb 17 (Vol. 11, No. 2, pp. 219-238). Taylor & Francis.
43. Kaplon H, Reichert JM. Antibodies to watch in 2018. InMAbs 2018 Feb 17 (Vol. 10, No. 2, pp. 183-203). Taylor & Francis.
44. Reichert JM. Antibodies to watch in 2016. InMAbs 2016 Feb 17 (Vol. 8, No. 2, pp. 197-204). Taylor & Francis.
45. Reichert JM. Antibodies to watch in 2014. InMAbs 2014 Jan 1 (Vol. 6, No. 1, pp. 5-14). Taylor & Francis.
46. Fenton C, Perry CM. Gemtuzumab Ozogamicin. Drugs. 2005 Nov;65(16):2405-27.
47. Fenton C, Perry CM. Gemtuzumab Ozogamicin. Drugs. 2005 Nov;65(16):2405-27.