Abstract
Chronic wound closure is the inability for a wound to progress through the standard wound healing stages and timeframe, often stalling during the inflammatory stage. This paper presents a two-year open wound endured by a Native American patient of geriatric age and uncontrolled type II diabetes based on elevated A1c levels. Multiple therapeutic modalities had been attempted to close the wound, without improvement. Yet, when a minimally processed human amniotic allograft was applied on a weekly basis, wound closure was demonstrated, based on surface area and volume measurement calculations. This case study shows that weekly applications of human amniotic membrane can impact delayed or stalled wound closure by facilitating healing. Thereby, demonstrating the efficacy of human allografts in difficult to heal chronic wounds, in diabetic patients.
Keywords
Chronic wounds, Hemoglobin A1c, Type II diabetes, Ulcer, Wound healing
Introduction
According to the updated 2022 compendium of wound estimates, chronic wounds will end up impacting 10.5 million Medicare recipients and effect over 8 million U.S. residents on a yearly basis [1]. These numbers represent an increasing trend in wound care cases from the previously published compendium in 2014. Many of these wounds will be chronic wounds, or wounds that have been open for more than four weeks and have not progressed through the wound healing phases: Hemostasis, Inflammation, Proliferation and Remodeling [1-3]. Based on these numbers almost all wound care clinicians and specialists will be periodically faced with the challenge of an intransigent wound not reaching its healing endpoint. Wound healing is a complex coordinated effort of various overlapping phases involving differing cell types and varying biochemical molecules such as cytokines, growth factors (GFs), matrix proteins, and chemokines [2,4]. See Figure 1 for summary of the wound healing phases.
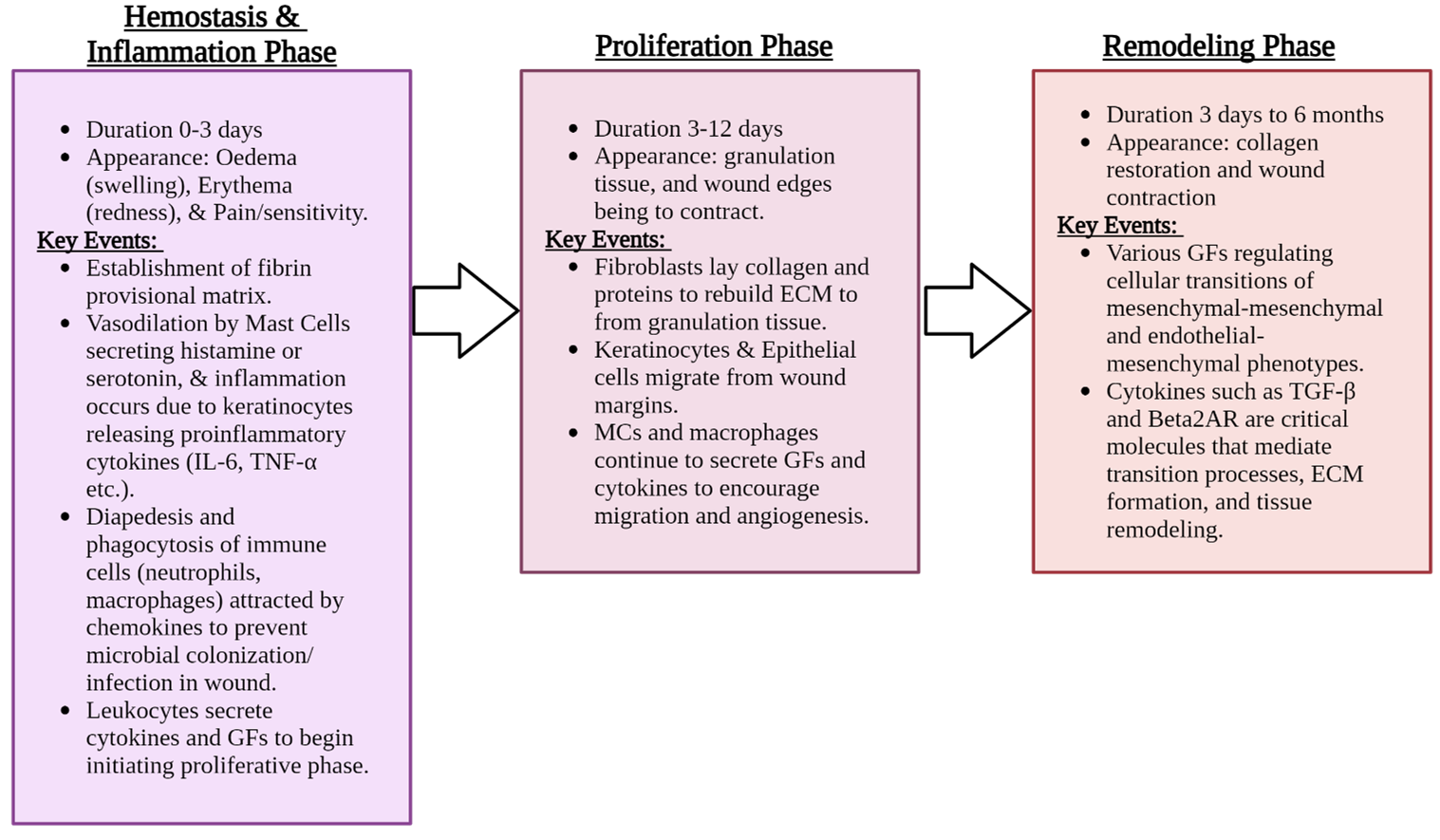
Figure 1. Summation of the Wound Healing Phases. Crafted in www.biorender.com (accessed 06, March 6, 2024). [Sources 2-5].
Most wounds, regardless of etiology, heal without difficulty. Many of these wounds can be considered acute wounds, as they occur suddenly and progress through the different phases of healing as expected [2,5]. In contrast, chronic wounds fail to progress through the wound phases and often remain in the inflammation stage and may never heal without proper intervention [3,6]. A wound is considered chronic or stalled when it enters a nonhealing or intransigent phase – this occurrence is related to several variable factors that can collectively halt an orderly healing process. This is often due to underlying etiologic causes such as pathogenic infections, malnutrition such as vitamin C and protein/amino acid deficiencies, autoimmune diseases, impaired or poor circulation, mismanaged diabetes and continuous pressure or immobility issues. The acute and chronic wound bed is described in detail in Figure 2 (Acute and Chronic Wound Biology).
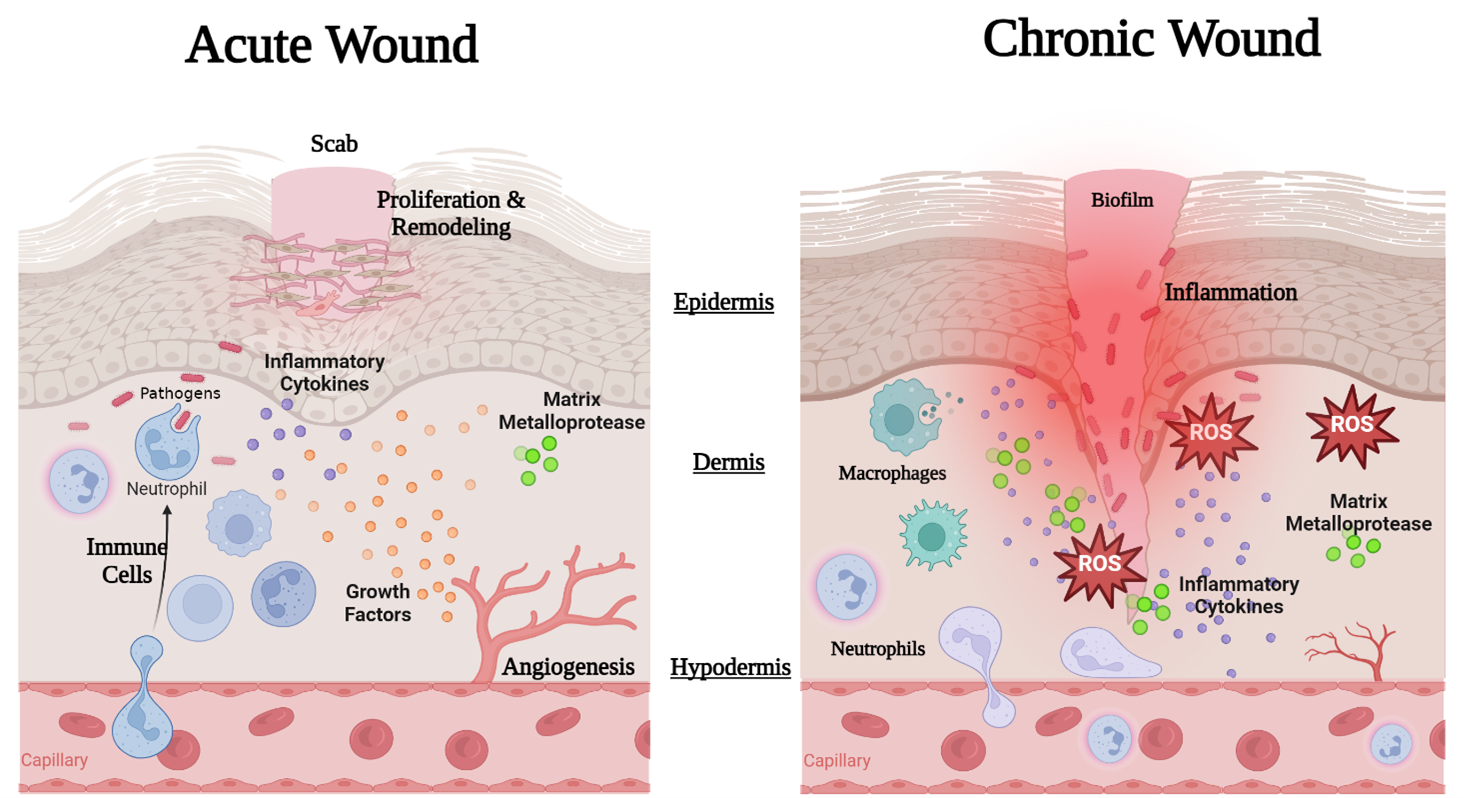
Figure 2: Example of Acute and Chronic Wound Biology: The healing of acute wound involves the coordinated healing phases with both cellular and molecular responses. In acute wounds a controlled immune cell response is maintained with neutrophils and macrophages signaled via chemokines to facilitate wound cleansing, inflammation is controlled, angiogenesis occurs, growth factors are secreted and encourage ECM deposition for cellular proliferation, tissue remodeling and subsequent tissue healing. A chronic wound is often infected and exhibits aberrant inflammation, poor cellular proliferation, and deprived angiogenesis limiting the oxygen output through the wound leaving increased levels of reactive oxygen species (ROS) and degrading matrix metalloproteases (MMPs). High quantities of pro-inflammatory mediators and macrophages remain present, and a microbial biofilm may form [1-6]. Image is created with www.biorender.com (accessed 05, March 2024).
The treatment of chronic wounds takes a systematic approach that includes reviewing a patient’s medical history for co-morbidities (background in a chronic disease such as diabetes mellitus, heart disease, cancer, etc.), upkeeping supportive interventions (nutrition support, pain control), and controlling local wound components (bioburden, moisture content, etc.). Numerous studies have been conducted on the treatment and management of chronic wound healing, with multiple immunomodulatory strategies and biological interventions being considered and explored.
Diabetes mellitus interferes with all four stages of wound healing, with abnormalities in cellular function among neutrophils, macrophages, fibroblasts, & keratinocytes, neuropathic issues, immune function alterations, biochemical changes, hormonal abnormalities, and micro or macrovascular impairments [7,8]. Both hypoglycemia (low blood glucose) and hyperglycemia (high blood glucose) are short term complications of diabetes and diabetic treatment plans. Hyperglycemia correlates with slower circulation and microvascular dysfunction, causing reduced tissue oxygenation and diminished healing potential in patients and an increase rate of surgical site infections. The glycemic pathophysiological relationship between diabetes and impaired healing is complex and multifaceted. The underlying mechanisms and effects of diabetes mellitus are continuously being studied as complete understanding continues to elude the scientific and clinical community.
As wound healing comprehension has progressed, several wound closure predictors have been researched, including the hemoglobin A1c levels in diabetic patients with wounds, as well as patients who have pressure wounds and diabetes [9,10]. The A1c test is a primary tool for assessing glycemic status in both clinical practice and clinical trials and is strongly linked to diabetic complications [8]. Hemoglobin A1c is a laboratory assay using a standard blood collection technique and is used to determine the statistical percent average of blood glucose over a set time period, often reflecting average glycemia over approximately 2-3 months [7-10]. Normal A1c level is below 5.7%, levels between 5.7-6.5% are indicative of prediabetes and a level of 6.5% or more signify diabetes (See Table 1) [3,11-12]. Routine monitoring of blood glucose levels is a hallmark of diabetic patient care, with a common goal to maintain an A1c of ≤ 6.5% or below (normoglycemic or near-normoglycemic) to help prevent diabetic complications that predispose patients to neuropathy, ischemia, and infection [3,10,11]. This healthy A1c level can be achieved in a safe and affordable manner, but a higher target may be appropriate for certain individuals and may change for a given individual over time [8]. Some observational studies have shown a direct association between baseline hemoglobin A1c and rate of wound healing. A study by Nau et al. found that those with a single blood glucose measurement of more than 220 mg/dL on the first postoperative day had an increased risk for sepsis, pneumonia, and wound infection [13]. Diabetic patients with lower A1c levels experienced decreased healing times, and a decrease in patients’ risk of infection [7,11]. Multiple factors must be considered for chronic wound management in type II diabetic mellitus patients, such as the previous wound healing strategies, A1c management, age, dietary intake including protein consumption and the location/type of wound e.g. venous leg ulcer (VLU), diabetic foot ulcer (DFU) or pressure ulcers. Today, pressure ulcers are just one type of chronic wound that remain a significant health care concern. A variety of specific patient populations have been discussed as being at an increased risk for pressure ulcer formation including older adults (post-65 years old) and mobility limited or immobile surgical patients [10,11]. Compound these factors with predisposed populations (Native Americans and African Americans) and additional diseases that impact wound healing (i.e. diabetes mellitus), a complicated wound case can result.
|
Diagnosis |
Hemoglobin A1c Values |
Mean Blood Glucose (mg/dL) |
|
Normal |
Below 5.7% |
<90 |
|
Prediabetes *Risk of developing type 2 diabetes |
5.7 to 6.4% |
<120 |
|
Diabetes Elevated blood glucose levels (hyperglycemia) |
6.5% or above |
>150 |
In this case report, a 78-year-old Native American female with a medical history that includes significant comorbidities, including type II diabetes mellitus, gastroesophageal reflux disease (GERD), hypertension, and legal blindness is presented. Patient history reports as a nonsmoker, no alcohol use and is not prescribed to any chemotherapeutic or steroid medications. The patient previously underwent surgical intervention for drainage of a right soft mass in the buttock, believed to be an inflamed ischia-gluteal bursa in posterior region. The mass had presented complications as stated by the patient (pain and irritation). The surgical correction resulted in an open ulcer that failed to close and has been present for two years. With weekly medical interventions, continual treatment aimed at closing this chronic wound commenced for months. Previous applications and interventions included wound VAC, silver nitrate, collagen with saline, an attempted suture closure, and several other biological product interventions or applications. The open wound size fluctuated from treatment to treatment, but significant closure was never observed. Following the explanation of treatment change the patient consented to a new care plan using a dual-layered amniotic membrane product beginning in November, of 2023.
Materials and Methods
Utilization of amnion-amnion membrane
Standard wound care principles are similar across the different etiologies and includes systemic modalities such as debridement, moisture balance, off-loading for pressure relief, measures to optimize blood and oxygen supply and regular wound care support [14,15]. If a chronic wound, such as an ulcer, fails to heal with standard treatment, ‘advanced wound care’ therapies are implemented to progress the wound healing cascade. Several strategies have been proposed to improve healing of chronic wounds and are generally classified into biologic agents, biomaterials, and cell-based strategies [16]. For this clinical case, Axolotl DualGraftTM, a bi-layered dehydrated human amnion membrane allograft, derived from placental tissue layers was utilized. Occasionally, by simply changing the dressing routine or introducing new elements to a wound milieu, the wound healing process can be prompted and progress past the stalled phase. In this case study, the Axolotl DualGraftTM acted as a therapeutic stimulus and promoted a biologic response stimulating the wound closure.
Traditionally, amniotic membrane grafts have been used for treating burns, but have expanded into therapeutic care for a variety of wounds and tissue types. These biomaterials are known for being a successful non-immunogenic barrier with antibacterial properties that provide necessary cytokines and growth factors with a matrix that facilitates migration and proliferation of cells. Numerous studies have documented how the use of these biological materials has helped in reducing inflammation and the formation of scar tissue formation, making this material appealing for chronic or stalled wounds that are anchored in the inflammatory phase of healing [14-16].
Method of application
Each clinical visit, the clinician began with an inspection of the wound, condition of wound dressing, and any notable patient observations or updates from the previous visit. Following the initial review, the wound and the peri-wound is cleaned with a hypochlorous wash (Vashe®) wound solution-soaked gauze. Then the wound area is measured following standard wound measuring practice: the length and width are measured with a head-to-toe orientation, with the widest width perpendicular to the length. The depth of the wound is obtained by inserting a sterile soft-tipped tool into the wound bed at the greatest depth, marking applicator when reaches skin level, then measuring with a metric ruler. While measuring the depth, the clinician checks for tunneling, sinus formation or undermining of tissue.
All recorded wound measurements are taken by the lead clinician for consistency and to aid in the clinician’s understanding of the wound. After measurements, the clinician executes wound margin debridement by removal of epibolic and callus tissue using sharp instruments, to induce a controlled hemorrhage effect. This debridement only occurs when epibolic tissue exists at the wound margins. Additional cleaning is performed before applying the biologic membrane (Axolotl DualGraftTM) by folding with forceps and using a packing technique, inserting membrane fully into the wound bed. The wound is then covered with Cultimed® Sorbact WCL mesh, adhered with Steri-Strips to hold in place and finally covered with an absorbent foam.
Following each weekly clinical visit and wound dressing the patient and caretakers were advised to change the outer absorbent dressing daily, leaving the mesh and membrane beneath in place. Clear instructions for changing the dressing and how to properly apply and keep dressing dry were also provided to the patient’s family caregivers. The patient was also advised to monitor for signs of infection including increased edema, erythema, pain, or purulent drainage. Geriatric patients are generally considered to have an impaired ability to heal wounds; this impairment is further exacerbated with any underlying serious illnesses (e.g. type II diabetes, hypertension, etc.), further decreasing the capability of handling complicated chronic wounds. For this reason, it is crucial for patients’ treatment therapies to be initiated promptly to prevent further exacerbation.
Results
In this clinical case study, a geriatric diabetic patient with a stalled sacral pressure ulcer is presented. The treatment contained weekly wound inspections, debridement, nutritional education, and applications of Axolotl DualGraftTM, a bi-layered dehydrated human amnion membrane allograft. Weekly wound measurements were performed by the clinician and recorded (See Table 2).
Numerous potential reliability problems exist when measuring a wound. The irregularity of the wound perimeter makes it difficult to determine the best position of the wound surface to obtain measurements consistently. Defining the edge of a wound in the sacral region is difficult and inconsistencies of dimension occur. However, serial measurements repeated over time can at a minimum reveal a trend in the presence or absence of healing within the wound. From the recorded wound size measurements, the reduction trend was tracked, and the calculated percentage surface area reduction rate overtime was 89%. The wound depth also displayed a decreasing trend (See Table 2 Graph and Figure 3) until after week 12, with peri-wound discoloration decreasing.
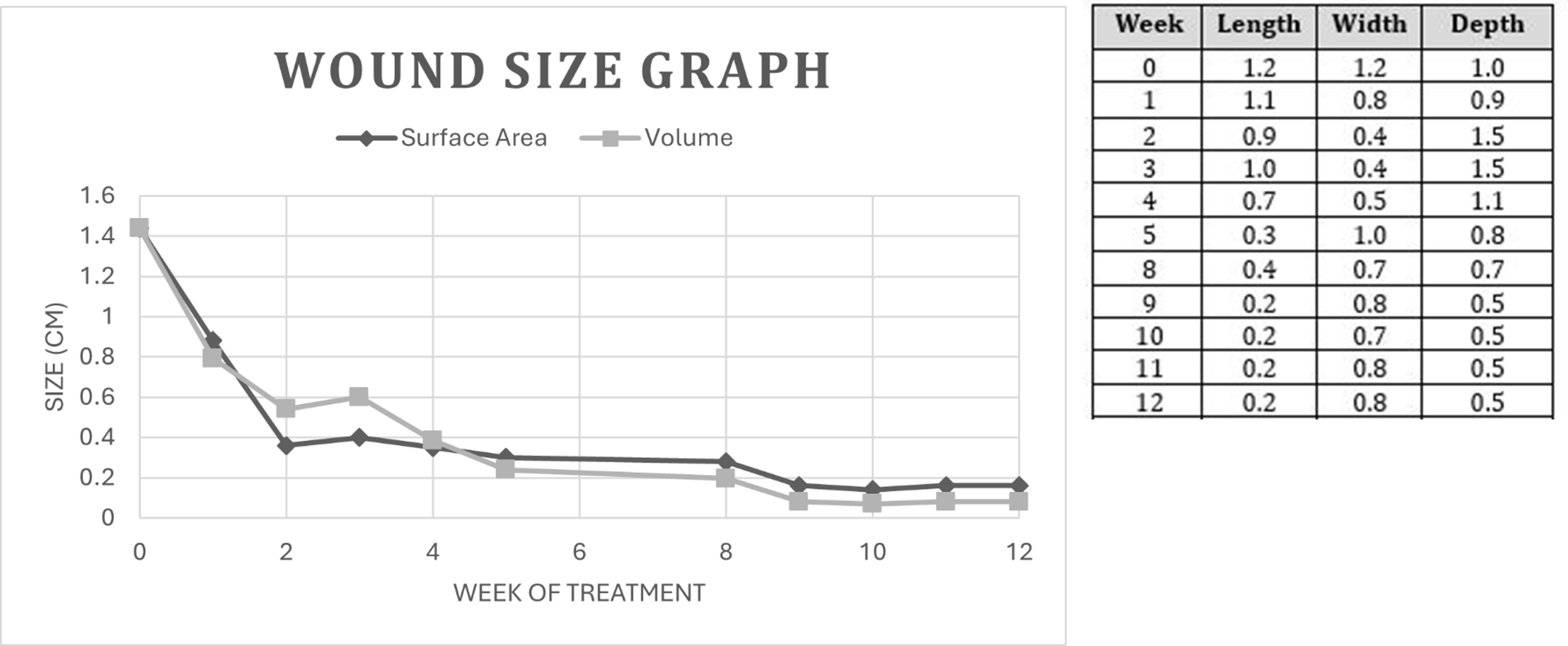
Table 2. Weekly wound area measurements, performed by leading clinician following proper wound area measuring techniques. All measurements are in centimeters (cm). Graph compares wounds changing surface area and volume measured following each week of treatment.
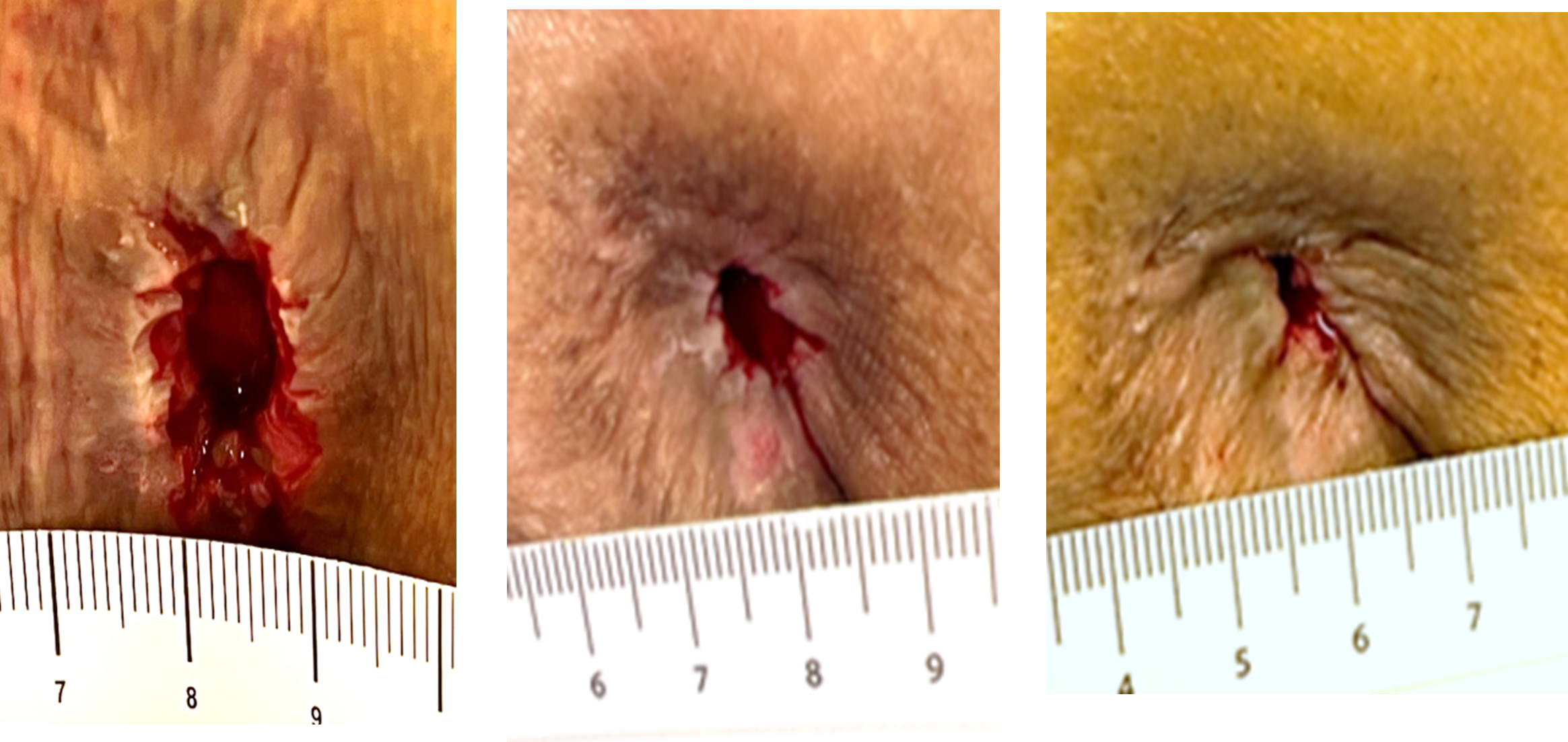
Figure 3. (A) Initial Wound Exam (Week 0) before new treatment plan: Open stalled sacral ischial tuberosity region pressure wound with epibolic margins, area measured 1.2 x 1.2 x 1.0 cm, 1.44 cm volume. Mild serous exudate, no undermining or tunneling present, peri wound is intact with mild maceration. (B) 1 Month Follow-up: (Week 4) One month of treatment with membrane, area measured 0.7 x 0.5 x 1.1 cm, decreased area with increased depth, no odor, or signs of undermining/tunneling, 100% wound bed granulation, mild serous exudate & epiboly edges pre-debridement. (C) 2 Month Follow-up: (Week 10) Second month with membrane treatment, wound area measured 0.2 x 0.7 x 0.5 cm, decreased length and depth with an increase in width. Mild serous present with epiboly edges. Peri-wound intact with mild maceration.
Overall, the initial application of Axolotl DualGraft TM resulted in a decrease in wound size and stimulated wound healing. The first 6-weeks of application showed a downward trend in wound size while the patients reported A1c level was 9.3%. This display of decreased inflammation, reconstruction of ECM, and the beginnings of reepithelization can be accredited to the incorporation of the amniotic allograft into the wound. There are many physiologic factors that contribute to poor wound healing in diabetic individuals, including impaired cell migration and proliferation, cytokine, and growth factor function, poor angiogenic response, and hyperglycemia [10]. The wound site's location should also be considered as a difficult area to control for wound healing. Managing a sacral pressure ulcer in an elderly patient involves protection of the peri-ulcer skin, prevention of fecal/urinary contamination, and reducing patient discomfort [17]. The patients skin integrity was maintained with no moisture lesions, and dressing contamination was prevented. Most importantly, the patient experienced little to no discomfort or distress during the treatment series.
Discussion
The management of chronic wounds is an ongoing issue due to underlying conditions and the difficulty in controlling the continuous inflammation phase. Treating these wounds is challenging and requires a multidisciplinary approach. There are many physiologic factors that are thought to contribute to poor wound healing in diabetic individuals, including decreased or impaired keratinocyte and fibroblast migration and proliferation, cytokine, and growth factor function, and angiogenic response and response to infection among others [10-12].
By using biological allograft membrane products, the wound healing cycle is restarted, and wound closure does occur. Amniotic membranes are an effective biologic dressing that promotes re-epithelialization in pressure ulcers [18]. The amnion contains many growth factors involved in wound healing, such as, platelet derived growth factor (PDGF), transforming growth factor beta 1 (TGF-β1), epidermal growth factor (EGF), as well as a rich cytokine content like interleukins (IL-4, IL-10) and Tissue Inhibitors of Metalloproteinase (TIMP-1, TIMP-2, TIMP-4) that are involved in regulating MMPs [16,18].
The patient reported that the application of graft membrane was painless and simple compared to previous interventions. The membrane treatment demonstrated a rapid wound closure rate when compared to previous intervention in this patient. However, in this case study, the patients’ underlying conditions reminded both practitioners and researchers alike that the human body is multifaceted and both internal and external factors can impact patient healing. By comparing the patients’ A1c level with the wound surface reduction trend, several stagnant zones of healing can be observed. The patient failed to get their A1c levels below 9%, missed an appointment, and failed to receive at home care in rebandaging the wound site. These factors likely resulted in slowing the healing process and encouraging both bandage adherence and infection.
Patient self-care practice and compliance with wound care protocol is key to successful wound closure. Close oversight by clinicians is also a major factor in a successful outcome. In rural areas, these facets of healthcare are difficult to access and have a major impact on how well a wound can be treated. People living in rural areas are at a greater risk of poor health conditions due to facing significant barriers in accessing care [19]. Strategies that can help improve and address care in rural America include improving access to specialized care, strengthening the rural provider safety net and provide supportive training to both the rural providers and patients. Patient education may increase self-care practices in the diabetic population regarding better glucose control. Overall, building positive health behaviors and maintaining psychological well-being are foundational for achieving diabetes management goals and maximizing quality of life [8]. Besides being a burden for patients, chronic wounds are among the most important cost drivers of today’s healthcare systems. With biological membranes, patient treatment time can be shortened, reducing travel time and healthcare costs but ultimately assisting the patients with reduced wound healing time. The option of utilizing amniotic membrane allograft in the management of chronic wounds is a positive development in wound care therapies.
The data presented in this case suggests future studies including a broader patient pool to include an increased number of patients with underlying chronic conditions, such as diabetes. Monitoring more wound healing indications such as molecular markers before and after treatment sessions. Encouraging the use of more advanced documentation and evaluation tools, such as the Pressure Ulcer Scale for Healing (PUSH) or utilizing Optical Coherence Tomography (OCT) as a viable non-destructive biopsy for monitoring wound healing [20]. Implementing more glycemic status assessments by A1C measurements, blood glucose monitoring (BGM) by capillary (finger stick) devices, and continuous glucose monitoring (CGM) using time in range or mean CGM glucose for improved glucose level correlation with wound closure rates would also be beneficial tools.
Acknowledgments
The authorship group would like to recognize the tribal nations within the Northern Arizona region. These lands are sacred to Native Americans throughout the region. We honor their past, present, and future generations, who have lived here for millennia and will forever call this region home.
References
2. Fernández-Guarino M, Hernández-Bule ML, Bacci S. Cellular and molecular processes in wound healing. Biomedicines. 2023;11(9):2526.
3. Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of Acute and Chronic Wound Healing. Biomolecules. 2021 May 8;11(5):700.
4. Diller RB, Tabor AJ. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics (Basel). 2022 Jul 1;7(3):87.
5. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015 Aug;173(2):370-8.
6. Widgerow AD. Deconstructing the stalled wound. Wounds. 2012 Mar;24(3):58-66.
7. Xu KP, Li Y, Ljubimov AV, Yu FS. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes. 2009 May;58(5):1077-85.
8. American Diabetes Association Professional Practice Committee. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes-2024. Diabetes Care. 2024 Jan 1;47(Suppl 1):S111-S125.
9. Center for Disease Control and Prevention; All About Your A1C, https://www.cdc.gov/diabetes/managing/managing-blood-sugar/a1c.html#:~:text=A%20normal%20A1C%20level%20is,6.5%25%20or%20more%20indicates%20diabetes. Accessed 03, March 6, 2024.
10. Christman AL, Selvin E, Margolis DJ, Lazarus GS, Garza LA. Hemoglobin A1c predicts healing rate in diabetic wounds. J Invest Dermatol. 2011 Oct;131(10):2121-7.
11. Fesseha BK, Abularrage CJ, Hines KF, Sherman R, Frost P, Langan S, et al. Association of Hemoglobin A1c and Wound Healing in Diabetic Foot Ulcers. Diabetes Care. 2018 Jul;41(7):1478-85.
12. Markuson M, Hanson D, Anderson J, Langemo D, Hunter S, Thompson P, et al. The relationship between hemoglobin A(1c) values and healing time for lower extremity ulcers in individuals with diabetes. Adv Skin Wound Care. 2009 Aug;22(8):365-72.
13. Nau KC, Lorenzetti RC, Cucuzzella M, Devine T, Kline J. Glycemic control in hospitalized patients not in intensive care: beyond sliding-scale insulin. Am Fam Physician. 2010 May 1;81(9):1130-5.
14. Marcus B. Treatment of large, complex, non-healing wounds with cryopreserved amniotic suspension allograft: a case series. J Wound Care. 2016 Oct 1;25(Sup10):S18-S24.
15. Brem H, Lyder C. Protocol for the successful treatment of pressure ulcers. Am J Surg. 2004 Jul;188(1A Suppl):9-17.
16. Ingraldi AL, Audet RG, Tabor AJ. The Preparation and Clinical Efficacy of Amnion-Derived Membranes: A Review. J Funct Biomater. 2023;14:531.
17. Beldon P. Problems encountered managing pressure ulceration of the sacrum. Br J Community Nurs. 2008 Dec;13(12):S6, S8, 10 passim.
18. Dehghani M, Azarpira N, Mohammad Karimi V, Mossayebi H, Esfandiari E. Grafting with Cryopreserved Amniotic Membrane versus Conservative Wound Care in Treatment of Pressure Ulcers: A Randomized Clinical Trial. Bull Emerg Trauma. 2017 Oct;5(4):249-58.
19. Maganty A, Byrnes ME, Hamm M, Wasilko R, Sabik LM, Davies BJ, et al. Barriers to rural health care from the provider perspective. Rural and Remote Health. 2023;23(2):1-11.
20. Glinos GD, Verne SH, Aldahan AS, Liang L, Nouri K, Elliot S, et al. Optical coherence tomography for assessment of epithelialization in a human ex vivo wound model. Wound Repair Regen. 2017 Nov;25(6):1017-26.
