Abstract
Venous ulcers are notorious for non-healing and plastic surgeons are faced with difficulty in wound management. In chronic wounds there will be deficiency of growth factors. Insulin therapy is a method to induce wound healing by stimulation of growth factors. In this study we have used topical insulin for wound bed preparation of patient’s venous ulcer.
Keywords
Insulin therapy; Venous ulcer
Introduction
Wound healing is a dynamic process whereby cellular structures and the tissue layers are reconstructed. Adult wound healing can be categorized into three stages: inflammatory phase, proliferative phase, and remodelling phase. Blood cells like macrophages, neutrophils, extracellular matrix and mediators, various proteins, and various genes play an important role in these phases. The perfect execution of the three stages results in wound healing. Wound bed preparation is a new concept which is used to manage wounds that fail to heal and can be summarized as T.I.M.E with T for tissue: non-viable or deficient. I for infection/inflammation, M for moisture balance. E for epidermis which was changed to E for edge and allows the granulation tissue to come. Recently in literature, we happened to come across use of topical insulin for use in wound bed preparation.
Materials and Methods
This study was conducted in the department of Plastic Surgery at tertiary care center after the departmental ethical committee approval was taken. Informed written consent was taken from the patient. The details of the patient are as follows:
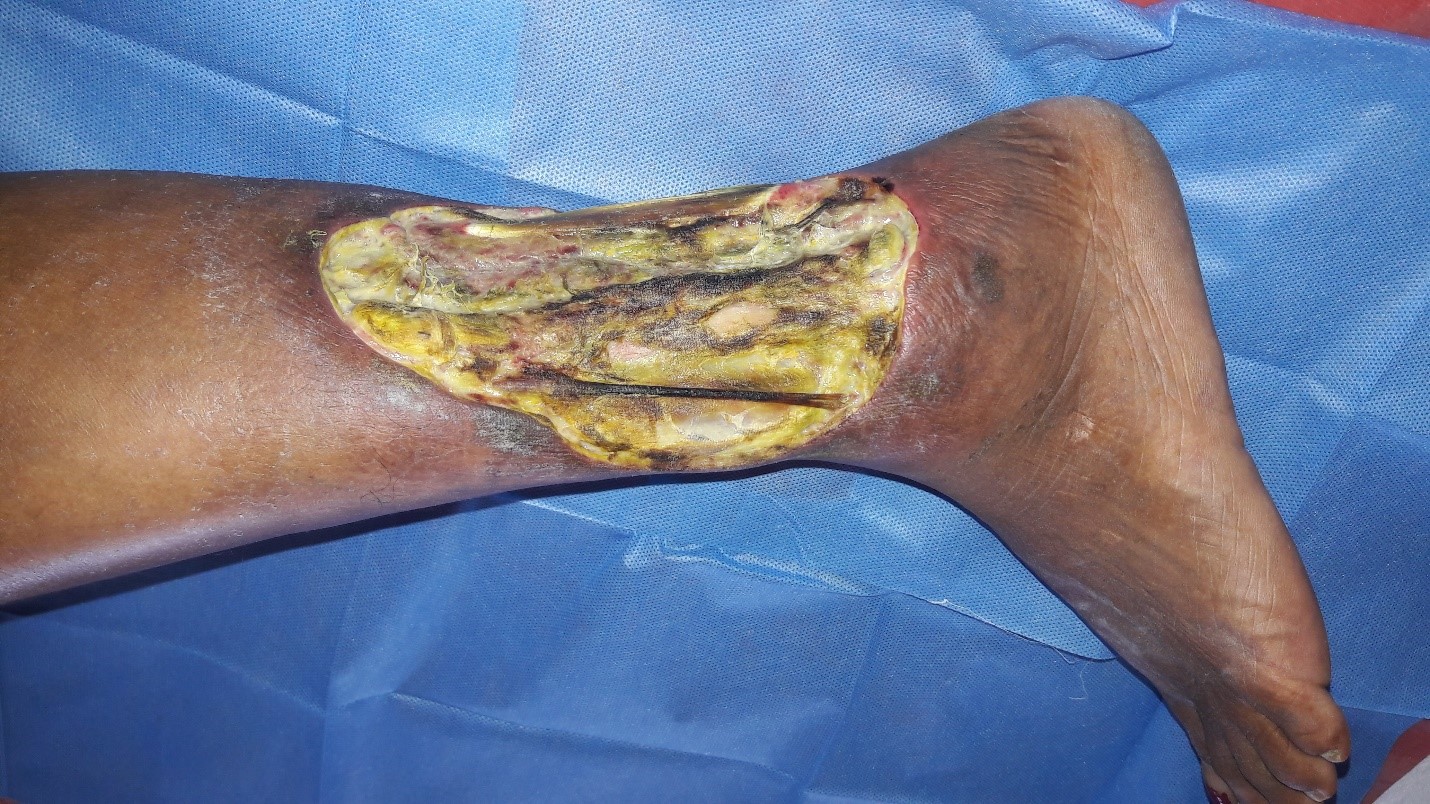
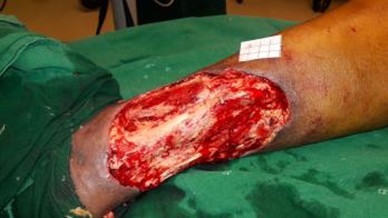
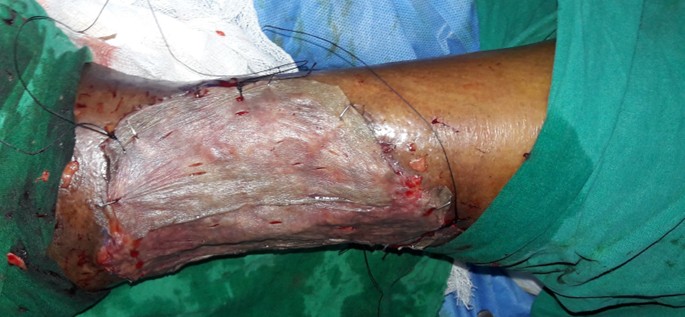
A 50 years old female patient without any known co-morbidities was admitted with infected raw area over the left leg. On evaluation she was found to have incompetent venous perforators of the left lower limb. The patient was treated with conventional dressing. Wound bed preparation was planned for the patient with topical insulin therapy as her ulcer did not show any evidence of healing and was infected (Figures 1a and 1b). 0.1 ml of regular insulin was either injected or sprayed onto 1 cm2 of the wound during the regular dressing of the wound (Figure 2). This was repeated once every 3-5 days when the dressing was changed depending on the soakage. The wound was assessed after 2 weeks.
Figure 1a: Infected venous ulcer left leg.
Figure 1b: Wound after debridement.
Figure 2: Application of topical insulin to raw area.
Results
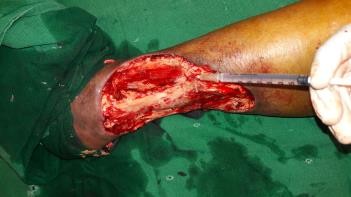
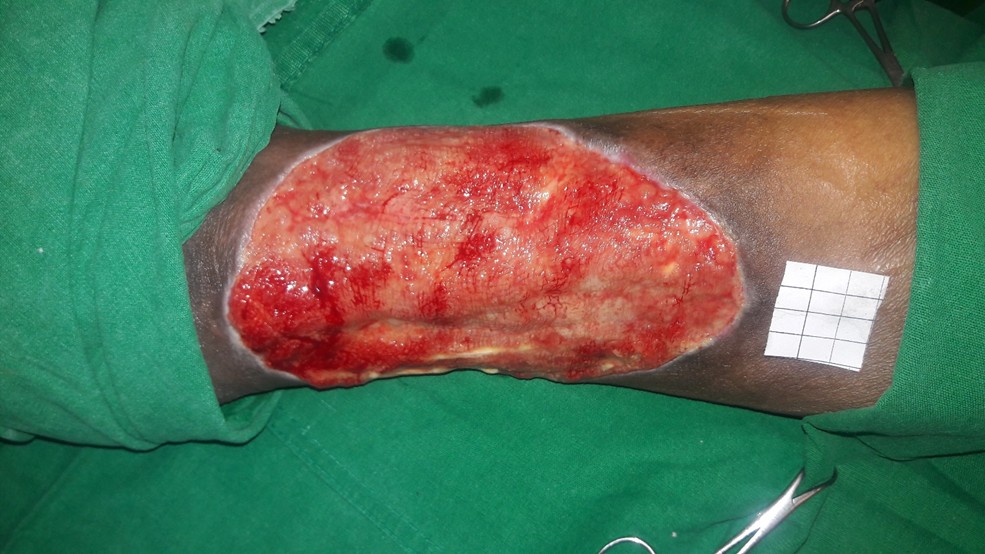
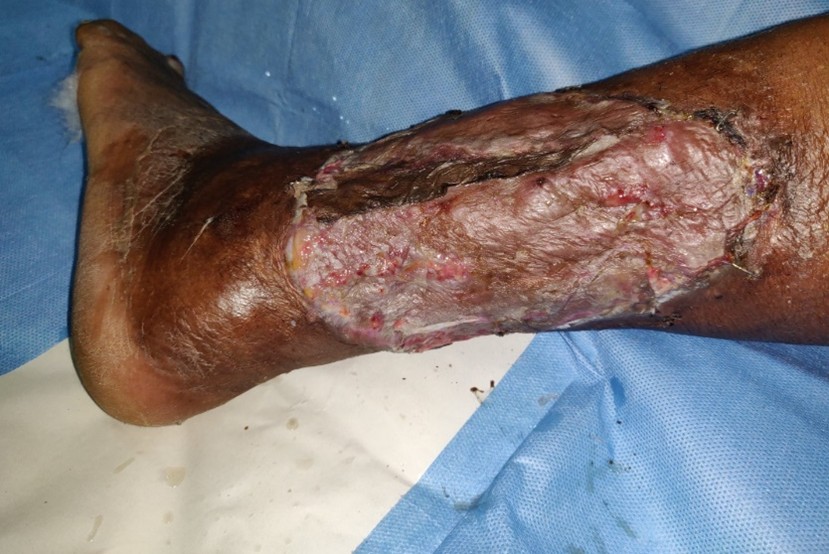
The wound bed showed good granulation tissue (Figure 3) and we were able to do skin grafting at a later date (Figure 4) with good take of the graft (Figure 5).
Figure 3. Healing wound bed.
Figure 4. Skin grafting after wound bed preparation.
Figure 5. Skin graft show good take after wound bed preparation with topical insulin.
Discussion
The venous ulcer is an entity which is known to be non-healing. Convalescent period adds to the cost of treatment and puts immense stress on to the patient and bystanders, health care providers. A chronic wound, especially non-healing type, is a common surgical condition a surgeon comes across. The peculiarity of a chronic wound is that, whatever management is tied, it refuses to heal, especially the pressure ulcers or bed sores. Many therapeutic methods are available to affect the wound healing such as the topical application of insulin, growth factors, negative pressure assisted wound closure, oxidized regenerated cellulose/collagen, hyaluronic acid conjugated with glycidyl methacrylate or gelatin dressings. A less clinically and economically complicated approach to healing chronic wounds seems necessary.
Role of topical insulin in wound healing has been in literature since 1970s [1]. Studies done in animal models and humans have found that topical Insulin therapy exerts its effects through the IGF 1 receptor. Insulin is known to stimulate keratinocytes and also the rate of endothelial proliferation leading to a faster neovascularization and also formation of granulation tissue [2]. Topical insulin application rapidly induces upregulation of the insulin signalling related proteins on wound areas following injury.
IRS-1 is a central player in the insulin effect, whose functions include regulation of glucose transportation. Increased phosphorylation/activation of IRS-1 tyrosine kinase has been observed since day 3 after wounding. IRS-1 binds the PI3-kinase, one of the SH2 proteins, through the multiple tyrosine phosphorylated sites [3,4]. Being a downstream effector of the PI3-kinase, AKT phosphorylation has also been found increased after insulin treatment. Although the insulin stimulation rapidly increased the IR expression, insulin does not have any evident effect on IGF-1 receptor; without any significant upregulation of the IGF-1 receptor which could be seen after the insulin treatment since day 3 after injury. This is in accordance with a recent report which claimed that in proliferating keratinocytes both insulin and IGF-1 induces phosphorylation of both the receptors [5], whereas insulin stimulation of the terminally differentiated cells results in phosphorylation of IR alone. Hence, insulin-stimulated growth promotion of the supraphysiologic concentrations is mediated via IGF-I receptor and insulin-stimulated growth promotion at the physiologic concentrations is mediated by the insulin receptor.
Insulin triggers the keratinocyte migration depending on the dose and time in the chronic wounds. Insulin demonstrates its effect through an insulin receptor dependent but EGF/EGF-R non-dependent way. The fact that it increases the keratinocyte migration on the PI3K-Akt-Rac1 pathway and cause stimulation of the keratinocytes by enabling the production of α3 and LN332 molecules has been proven by the in vitro studies [6-9]. Insulin is a hormone that also affects collagen production. Insulin selectively and strongly stimulates the collagen production in dermal fibroblasts [10,11]. The cost of insulin ranges from 130-500 INR per vial. There are no systemic side effects from topical insulin. The main advantage is that it is more cost-effective and allows for OPD basis management for the non-healing wound.
Limitations
The study was done on a single patient and needs large population-based study to apply in practice
Authors’ Contributions
All authors made contributions to the article
References
2. Scimeca CL, Bharara M, Fisher TK, Giovinco N, Armstrong DG. Novel use of doxycycline in continuousinstillation negative pressure wound therapy as” wound chemotherapy”. Foot & ankle specialist. 2010 Aug; 3(4):190-3.
3. Hrynyk M, Neufeld RJ. Insulin and wound healing. Burns. 2014 Dec 1;40(8):1433-46.
4. Takahashi Y, Tobe K, Kadowaki H, Katsumata D, Fukushima Y, Yazaki Y, Akanuma Y, Kadowaki T. Roles of insulin receptor substrate-1 and Shc on insulin-like growth factor I receptor signaling in early passages of cultured human fibroblasts. Endocrinology. 1997 Feb 1;138(2):741- 50.
5. Garza-Garcia A, Patel DS, Gems D, Driscoll PC. RILM: a web-based resource to aid comparative and functional analysis of the insulin and IGF-1 receptor family. Human mutation. 2007 Jul;28(7):660-8.
6. Schilling JA. Wound healing. Surg Clin North Am 1976; 56:859-74.
7. Coulombe PA. Wound epithelialization: accelerationg the pace of discovery. Journal of Investigative Dermatology. 2003 Aug 1;121(2):219-30.
8. Madibally SV, Solomon V, Mitchell RN, Van De Water L, Yarmush ML, Toner M. Influence of insulin therapy on burn wound healing in rats. Journal of surgical research. 2003 Feb 1;109(2):92-100.
9. Benoliel AM, Kahn-Peries B, Imbert J, Verrando P. Insulin stimulates haptotactic migration of human epidermal keratinocytes through activation of NF-kappa B transcription factor. Journal of cell science. 1997 Sep 1;110(17):2089-97.
10. Chaiken RL, Moses AC, Usher P, Flier JS. Insulin stimulation of aminoisobutyric acid transport in human skin fibroblasts is mediated through both insulin and type I insulin-like growth factor receptors. The Journal of Clinical Endocrinology & Metabolism. 1986 Nov 1;63(5):1181-5.
11. Flier JS, Usher P, Moses AC. Monoclonal antibody to the type I insulin-like growth factor (IGF-I) receptor blocks IGF-I receptor-mediated DNA synthesis: clarification of the mitogenic mechanisms of IGF-I and insulin in human skin fibroblasts. Proceedings of the National Academy of Sciences. 1986 Feb 1;83(3):664-8.