Abstract
Lactulose, either as crystals or liquid syrup, is a frequently prescribed osmotic laxative agent in patients with diabetes mellitus (DM). During the manufacturing process, carbohydrate impurities can arise which could increase blood glucose levels with a possible impact on glycemic control. This important issue has now been addressed for the first time in individuals with type 2 DM. A recent RCT confirmed that there is no need to consider carbohydrate impurities in oral lactulose products administered at the recommended doses of 20 g/day and 30 g/day for blood glucose management.
The clinical efficacy of lactulose in treating constipation in adults has been established during the last 5 decades. Lactulose compares well in terms of improved stool consistency and frequency as compared to stimulant and/or fibre-based laxatives while being equally well tolerated. Laxative properties of lactulose appear to be somewhat weaker compared to polyethylene glycol, yet, evidence indicates that it may be associated with a lower risk of diarrhea. This advantage can be explained by the prebiotic properties of lactulose leading to a beneficial modulation of the gut microbiome. It should therefore be generally acknowledged that, due to its proven prebiotic effects, the benefits of lactulose in constipated individuals go well beyond a simple osmotic laxative effect.
Lactulose has been safely used for more than 60 years in the treatment of constipation. Gastrointestinal side effects are generally mild and often transient. At doses in the range of 10-30 g/day, as indicated for the treatment of constipation in adults, the associated risk of diarrhea is rather low. Lactulose was well tolerated in individuals with type 2 DM, and no unexpected safety issues were identified. Consequently, lactulose preparations represent a safe and effective strategy to alleviate symptoms of constipation, also in individuals with impaired glucose tolerance or diabetes.
Keywords
Lactulose, Constipation, Blood glucose, Type 2 diabetes mellitus, Laxative, Sugar substitute, Prebiotic
Introduction
Gastrointestinal (GI) disorders are common, also in persons with diabetes mellitus (DM), and can substantially impact their quality of life [1,2]. In a representative sample of adults living in the United States, 25.8% of individuals with DM also reported disordered bowel habits and 14.6% experienced chronic constipation [3]. Others have reported an even higher prevalence of constipation of around 30% among patients with DM [2,4]. While, in some patients, symptoms can be relieved with lifestyle and dietary modifications, many individuals with DM rely on the regular use of laxatives for the management of constipation. Osmotic laxatives are commonly prescribed when bulk-forming fiber-based agents do not provide satisfactory outcomes. One of the most frequently prescribed osmotic laxative agents in patients with DM is lactulose [1].
Lactulose is a synthetic disaccharide composed of galactose and fructose, linked by a β-1,4-glycosidic bond. It is an isomerization product of the natural milk sugar, lactose, which is used as the starting substance for lactulose production. Since lactulose cannot be broken down enzymatically in the small intestine, the intact molecule reaches the large bowel where it is metabolized by colonic bacteria to the corresponding monosaccharides and then to short-chain fatty acids (SCFAs), hydrogen, and methane [5-7]. The natural laxative action of lactulose primarily results from its osmotic abilities, causing retention of water leading to softer stools, and peristalsis-activating effects. Moreover, the colonic metabolism of indigestible disaccharides gives rise to an increase in intraluminal gas formation and osmolality while lowering intraluminal pH, thereby reducing intestinal transit time [1,8]. Lactulose is also effective in reducing intestinal ammonia production and is thus used in preventing and treating hepatic encephalopathy (HE) [5,6]. The metabolic effects of lactulose appear to be dose-dependent [6]. While already lower doses (from 2 g/day upwards) produce a prebiotic effect and enhance the absorption of several minerals, e.g., calcium and magnesium, medium doses of 10-30 g/day elicit the laxative effect used for the treatment of constipation, and high doses of 60-100 g/day have a detoxifying effect exploited in the treatment of HE [5,6,9].
Lactulose given via the oral route, either as crystals, marketed as a powder to be dissolved in water, or liquid syrup containing 10 g lactulose per 15 ml solution represents the standard mode of administration for the past decades [5,8]. During the manufacturing process of these commercial lactulose preparations, some impurities, including epilactose, lactose, galactose, fructose, tagatose and small amounts of unspecified or unknown sugars can arise [7], accounting for up to 3% of total carbohydrates in crystal lactulose and approximately 30% in liquid lactulose [10]. Theoretically, these impurities could lead to an increase in blood glucose levels with a possible impact on glycemic control in individuals with DM [8,10]. On the other hand, the oral intake of unabsorbable disaccharides may affect carbohydrate metabolism by reducing transit time and, possibly, slowing down glucose absorption [11]. For lactulose consumers with DM, reliable information on the actual impact of the described carbohydrate impurities on blood glucose levels after lactulose ingestion is thus of high relevance since these effects would need to be considered in their blood glucose management.
Yet, available evidence on the effects of lactulose ingestion at doses commonly used in the treatment of constipation on blood glucose levels is scarce, limited to a case report in a patient with diabetes [12] and a few older studies conducted in lactose maldigesters [13] and in healthy volunteers [14]. A recent study in healthy subjects confirmed that, blood glucose levels remained unchanged after oral lactulose intake at doses of 10 and 20 g/day, suggesting that lactulose can also be safely used in subjects with impaired glucose tolerance [8]. However, to date, the impact of lactulose intake on blood glucose levels still remained to be investigated in individuals with diabetes.
In the 2021 June issue of the World Journal of Diabetes, Pieber et al. [10] have addressed this important issue, reporting results from a double-blind, randomized, controlled trial (RCT) conducted at the Medical University of Graz, Austria. Briefly, in this trial 24 adult mildly constipated, non-insulin-dependent subjects with type 2 (T2) DM received single doses of 20 g or 30 g lactulose, either as crystal or liquid formulation, water as negative control, or 30 g glucose as a positive control, in a crossover design. Capillary blood glucose concentrations were then measured over a follow-up period of 180 minutes. No differences in the primary endpoint, blood glucose response expressed as baseline-corrected area under the curve from 0 to 180 minutes, were observed between the different lactulose formulations and water as a control. Lactulose increased the number of bowel movements and was generally well tolerated with only mild to moderate GI symptoms. The findings, for the first time, confirm that there is no need for mildly constipated, non-insulin dependent lactulose consumers with T2DM to consider carbohydrate impurities in commercial crystal or liquid lactulose products, administered at the recommended doses of 20 g/d and 30 g/d, for their blood glucose management.
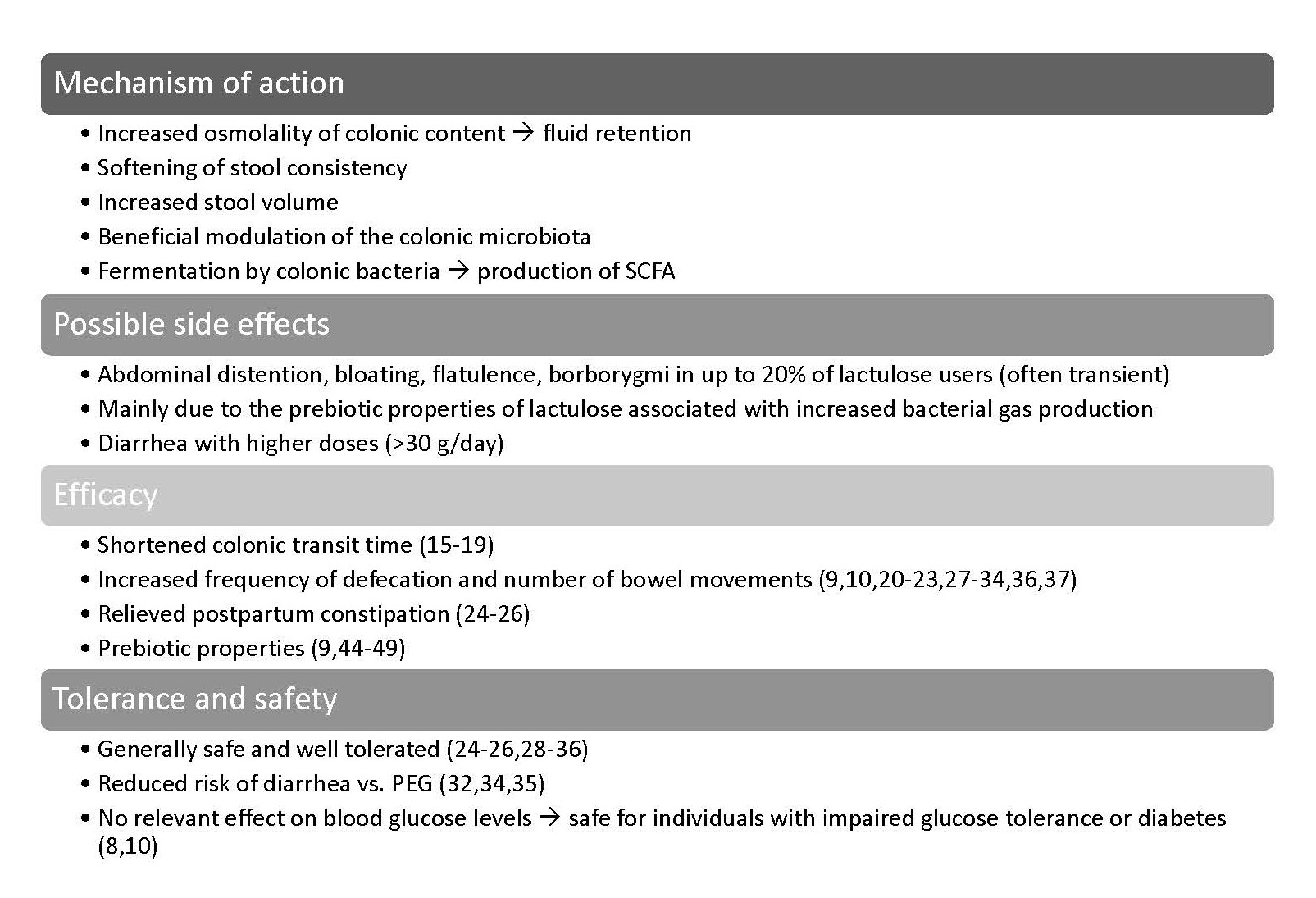
In the present commentary, we provide an updated summary of the body of evidence with lactulose in the treatment of constipation (see Figure 1 for an overview). These data underpin the safety and efficacy of commercial crystal and liquid lactulose preparations for use as laxative agents which, in the light of the current findings by Pieber et al., also applies to consumers with impaired glucose tolerance and diabetes.
Figure 1. Lactulose effects in the management of constipation.
Lactulose Accelerates Intestinal Transit Time
Lactulose has been repeatedly shown to enhance intestinal transit time. Among hospitalized patients with hepatic or GI disorders and normal bowel function before admission, daily consumption of 10 g/day liquid or crystal lactulose significantly shortened mean colonic transit times, mainly in the proximal part of the colon compared with controls [15]. Two studies compared the effects of lactulose versus polyethylene glycol (PEG) on the colonic transit time in healthy volunteers. High-dose (67 or 134 g/day) lactulose resulted in a dose-dependent acceleration of colonic transit, both in the proximal and distal colon [16]. In a subsequent RCT, lactulose administered at 10 g/day for 5 days significantly expedited ascending colon emptying compared to PEG 4000, whereas gastric emptying and small bowel transit time were not different between treatment groups [17]. When effects of the different dosage forms of lactulose on orocaecal transit time (OCTT) were compared in healthy volunteers, 12 g lactulose syrup administered with a test meal had the most pronounced effect versus identical doses of lactulose powder and inulin, respectively. The shortest OCTT of approximately 85 minutes was seen with lactulose syrup; OCTT with lactulose crystals was approximately doubled (P=0.014) and with inulin almost 3.5 times higher than with lactulose crystals (P=0.008) [18]. These findings are reflected by a health claim approved in 2010 by the EFSA, stating that consumption of 10 g of lactulose per day contributes to an acceleration of intestinal transit time in the general population, which is considered a beneficial physiological effect [19].
Benefits of Lactulose for Patients with Constipation
The clinical efficacy and safety of lactulose in treating constipation have been established in several randomized, placebo-controlled studies conducted during the last 5 decades. The first publication identified, dating from 1968, reported results from a double-blinded RCT conducted in older patients regularly taking laxatives for the treatment of constipation. Lactulose (5-20 g/day) was superior to placebo (glucose syrup administered in equal volumes as lactulose syrup) in improving frequency of defecation in truly constipated patients [20]. In a double-blinded RCT comparing 50% lactulose syrup (lactulose dose 7.5-20 g/day) with placebo (50% glucose syrup, administered in equal volumes as lactulose syrup), lactulose was more effective in increasing the number of bowel movements, and decreasing the severity of GI symptoms such as cramping, griping, flatulence, tenesmus, and bloating in older nursing home residents with constipation [21]. Another double-blind RCT comparing lactulose (20 and 40 g/day) with sucrose in normal and constipated subjects showed that lactulose syrup had significant, dose-related laxative effects in terms of greater mean stool frequency, mean stool volume, mean stool wet and dry weights, mean moisture content, as well as a softer consistency score versus placebo. In particular with the higher dosage, slight abdominal discomfort, nausea, diarrhea and flatulence were reported by the participants. These GI symptoms, however, were tolerable and considered extensions of the pharmacologic effects of the drug [22].
More recently, a multicenter RCT investigating escalating doses of lactulose in 250 patients with chronic constipation receiving 13, 26, or 39 g/day of crystal lactulose, respectively, for two weeks found that the 26 and 39 g doses induced significantly and dose-dependently greater increases in spontaneous bowel movements after 1 (primary endpoint) and 2 weeks, respectively, compared to placebo. With these doses, also significant improvements in stool consistency and constipation severity were seen. There were no significant differences in the incidence of adverse drug reactions between the placebo and lactulose groups [23]. Another recent crossover RCT assessed the effects of low-dose intervention with lactulose powder (2 g/day for 2 weeks) compared to placebo (glucose) on defecation parameters in healthy women with 2-4 reported defecations per week. Frequency of defecation, stool consistency using the Bristol Stool Scale, as well as bifidobacteria in feces were significantly higher during lactulose than placebo treatment. No significant differences were observed between lactulose and placebo with regard to flatulence and severe adverse effects did not occur [9]. Overall, the available evidence uniformly confirms that lactulose is an effective and generally well-tolerated laxative in the treatment of constipation in adults. Moreover, lactulose has also proven safety and efficacy in relieving postpartum constipation [24-26].
Several investigators have evaluated the efficacy and tolerance of lactulose preparations in comparison to other types of laxatives, e.g., stimulants or fibre as active ingredients (see Table 1). After 1-week of laxative treatment, consistently more patients continued to produce normal stools with a commercial lactulose syrup (30 ml/15 g per day) than when they had received stimulant laxatives containing senna, anthraquinone derivatives or bisacodyl [27]. Lactulose also showed better efficacy compared to fibre, the recommended “first-choice treatment” for the treatment of constipation in outpatient setting. In a crossover RCT, lactulose syrup (20-60 ml/13.3-40 g per day) ingestion resulted in significantly higher mean bowel frequency and stool consistency score while being equally well tolerated as fibre in terms of adverse GI effects [28]. Compared to a fibre-based product containing ispaghula, a commercial lactulose syrup (up to 30 ml/20 g per day lactulose) was equally well tolerated and effective in increasing stool frequency and improving stool consistency. Notably, lactulose was rated significantly better in terms of palatability [29]. Among long-stay older patients in hospital or nursing home care with constipation or a need for regular laxatives, both lactulose syrup (up to 60 ml/40 g per day) and a combination of senna and bulk forming fibre (psyllium) were well tolerated and improved stool frequency and consistency. In this study, however, the senna-fibre combination was even more effective than lactulose alone, resulting in a cost-benefit [30]. Likewise, among geriatric long-term hospitalized patients randomized to receive either lactulose (30-60 ml/20-40 g per day) or psyllium-senna, the latter combination produced significantly more frequent bowel habits than lactulose alone. Both treatments were safe and well tolerated [31]. Even though more recent evidence is lacking, the findings from these studies, published between 1974 and 2006, indicate that lactulose compares well to stimulant and/or fibre-based laxatives in terms of efficacy and tolerance in adults with constipation, even though it may be somewhat less effective in older long-stay patients.
| Agents | Efficacy | Tolerance and safety |
| Polyethylene glycol (PEG) |
|
|
| Fibre |
|
|
| Ispaghula |
|
|
| Senna-psyllium combination |
|
|
Polyethylene glycol (PEG) is another osmotic laxative agent with proven efficacy commonly used in the treatment of constipation. In a crossover RCT in patients with opioid-induced constipation, tolerance and efficacy of lactulose syrup (30 ml/20 g per day) compared to PEG 3350 solution with electrolytes were investigated. Both interventions produced significantly more "non-hard" stools versus placebo and the numbers of “non-hard” stools observed in the run-in phase (control), respectively; with the PEG/electrolyte solution being most effective in producing loose stool. Of note, PEG/electrolyte solution also resulted in the loosest (diarrheal) stool. There were no drug-related adverse events leading to withdrawal from the study [32]. Similarly, in a multicentre RCT including general and geriatric in- and outpatients with idiopathic constipation, PEG was superior to crystal lactulose (20 g/day) in terms of improving number of stools and ease of defecation. There was no significant difference between groups with regard to liquid stools, abdominal pain, flatus, and rumbling [33]. In another RCT, however, conducted in in a similar patient setting, lactulose (10-30 g/day) proved to be equally effective as PEG 4000 in improving stool and constipation characteristics. Again, reported GI symptoms (bloating, borborygmi, abdominal pain, excess flatus) were similar for both treatments. Notably, in the last week of treatment, patients in the PEG group presented mostly soft/liquid stools, whereas those in the lactulose group reported principally normal stools [34]. From these comparisons it can be derived that the laxative properties of lactulose appear to be somewhat weaker compared to PEG. Nonetheless, lactulose benefits in terms of improved stool consistency and/or frequency were obvious in all of the described studies and lactulose was equally well tolerated as PEG. Moreover, there is some evidence indicating that lactulose may be associated with a lower risk of diarrhea as compared to PEG [32,34,35]. This benefit could be related to the prebiotic properties of lactulose (discussed in the following section), leading to a beneficial modulation of the gut microbiome and thereby reducing the risk of diarrhea.
While the previously described studies investigated the short-term application of osmotic laxatives with treatment durations in the range of 2-4 weeks, a more recent multicenter RCT was aimed to assess the long-term efficacy of crystal lactulose application (10-30 g/day) for a mean duration of 5.5 months in comparison to PEG 4000 in older patients with constipation. Stool frequency and consistency improved in both treatment groups, yet, significantly more in the PEG 4000 versus the lactulose group. No differences in the proportion of patients with abnormal blood levels of nutrients, electrolytes, vitamins, ferritin, and transferrin were observed between the groups at the end of the study period and the reported incidence of adverse events was comparable. Adverse events considered potentially treatment-related, principally diarrhea and abdominal pain, were documented in less than 20% of patients [36]. Another recent RCT including 363 outpatients with functional constipation diagnosed according to Rome III criteria was powered to detect differences in the presence and severity of constipation-related symptoms (assessed by the PAC-SYM score) between a combination of lactulose and paraffin (3.5 g lactulose/day) and PEG 3350. After 28 days of intervention, the mean PAC-SYM score decreased significantly versus baseline with both treatments. Non-inferiority of lactulose plus paraffin versus PEG was established. At least one adverse event occurred in 11.2% of patients in the lactulose plus paraffin group and in 14.2% of patients in the PEG group, most of which were of mild or moderate severity and unrelated to study drugs. This study, for the first time, established the non-inferiority of lactulose (in combination with paraffin) compared to PEG in the alleviation of symptoms of functional constipation in adults [37].
Prebiotic Properties of Lactulose
Lactulose as a “bifidus factor” for intestinal regulation was first described in the 1950s by Friedrich Petuely in Austria [6,8,38]. In the colon, lactulose stimulates the growth or activity of a number of health-promoting bacteria, mainly Bifidobacteria and Lactobacilli, while inhibiting the growth of pathogenic bacteria, e.g., certain Clostridia, and increasing the production of beneficial metabolites, such as SCFAs [6]. These properties are referred to as “bifidogenic effects” and lactulose is consequently rated as a prebiotic compound [6,39]. Prebiotic intervention to beneficially modulate the gut microbiota is recognized as a valuable option in the management of GI disorders [40]. Underlying mechanism include the inhibition of pathogens, strengthening of the gut epithelial barrier, and modulation of the mucosal immune system [41]. Moreover, via the generation of SCFAs, Bifidobacteria contribute to osmotic regulation and the modulation of gut motility, thereby potentially reducing the risk of diarrhea associated with the application of higher doses of osmotic laxatives [40,42,43].
A solid body of evidence from human studies underpins the prebiotic properties of lactulose. In healthy volunteers, lactulose administered twice daily at a total dose of 20 g/day for 4 weeks has been shown to increase beneficial bacteria while significantly reducing putrefactive bacteria and potential pathogens. Moreover, activities of pro-carcinogenic enzymes and concentrations of aromatic compounds were reduced, while contents of SCFA in feces increased and fecal pH decreased [44]. Numerous RCTs conducted in healthy volunteers or patients with constipation have reported significant increases in fecal Bifidobacteria with lactulose administered at doses between 10 and 30 g/day over 4-22 weeks [34,44-49]. Notably, lactulose was even effective when given at doses as low as 2 g/day for 2 weeks in healthy women [9]. It should therefore be generally acknowledged that the benefits of lactulose in constipated individuals go well beyond a simple osmotic laxative effect, but also comprise the benefits of a prebiotic functional food ingredient.
Tolerance and Safety
Lactulose has been safely used for more than 60 years in the treatment of constipation, also in long-term administration [50-52]. Since only negligible amounts of lactulose (<1% of the administered dose) are absorbed from the intestine and rapidly excreted by the kidneys, any potential side effects mainly affect the GI system [5,39]. The most frequently reported side effects are abdominal distention, bloating, flatulence, and increased bowel sounds (borborygmi) which are often transient. These symptoms have been reported to occur in up to 20% of lactulose users and are mainly due to the prebiotic properties of lactulose associated with increased bacterial gas production [51-54].
Along with its indication as laxative, lactulose ingestion also carries the risk of causing diarrhea, depending on the dose administered and the individual capacity of the colonic microbiota to metabolize the disaccharide and its degradation products [5,35]. Since severe diarrhea may lead to electrolyte disturbances (hypokalemia, hypernatremia), careful monitoring of electrolyte levels and signs of dehydration is mandatory in patients presenting with this symptom [55]. Whereas diarrhea occurring with lower doses of lactulose is mainly due to unabsorbed organic acids and associated cations, with larger doses, unmetabolized carbohydrates are the major underlying cause [35]. In healthy humans, the maximum capacity of colonic bacteria to metabolize lactulose is around 80 g/day [35]. Proper dosing is thus of utmost importance to minimize the risk of lactulose-induced diarrhea [55]. Indeed, doses in the range of 10-30 g/day, as indicated for the treatment of constipation in adults, carry a rather low risk: in the RCT by Kasugai et al. 2019, evaluation escalating doses of a commercial preparation, lactulose was generally well tolerated up to the highest dose of 39 g/day. Diarrhea developed in 9.7% of patients treated with the highest dose, yet its severity was mild, and it resolved after discontinuation of the study treatment, dose reduction, or dose suspension [23].
In the study by Pieber et al., lactulose was well tolerated in individuals with type 2 DM, and no unexpected safety issues were identified. Abdominal distention and flatulence of mild to severe intensity occurring 3-24 hours after lactulose intake were reported by 36% and 52% of patients with T2DM, respectively. Reported adverse events were of mild to moderate intensity and mainly affected the GI tract. Overall, none of the reported events was rated as serious or necessitated study discontinuation or modification of product dosage [10].
Overall Conclusions
A solid body of evidence supports the application of lactulose as a safe, reliable, and efficient laxative to relieve the symptoms of constipation by accelerating intestinal transit, softening stool consistency and increasing the number of bowel movements and frequency of defecation. Moreover, lactulose users may also benefit from the prebiotic properties associated with a beneficial modulation of the colonic microbiota, while associated GI side effects are generally transient and of mild intensity. Nonetheless, some health practitioners have been reluctant to prescribe lactulose preparations for constipation management in persons with impaired glucose tolerance or diabetes, due to the uncertainty about the potential effects of carbohydrate impurities on blood glucose levels. With the recent publication by Pieber et al. 2021 [10], addressing this important issue for the first time in individuals with T2DM taking lactulose as a laxative, it has now been confirmed that there is no need to consider carbohydrate impurities in oral lactulose products administered at the recommended doses of 20 g/day and 30 g/day for blood glucose management. Consequently, lactulose preparations represent a safe and effective strategy to alleviate symptoms of constipation, also in individuals with impaired glucose tolerance or diabetes.
Acknowledgements
Supported by Fresenius Kabi Deutschland GmbH, Germany.
Author Contribution Statement
Faerber V and Kuhn KS wrote the manuscript; all authors reviewed, edited, and approved the manuscript for submission.
References
2. Du YT, Rayner CK, Jones KL, Talley NJ, Horowitz M. Gastrointestinal symptoms in diabetes: prevalence, assessment, pathogenesis, and management. Diabetes Care. 2018 Mar 1;41(3):627-37.
3. Sommers T, Mitsuhashi S, Singh P, Hirsch W, Katon J, Ballou S, et al. Prevalence of chronic constipation and chronic diarrhea in diabetic individuals in the United States. Official journal of the American College of Gastroenterology| ACG. 2019 Jan 1;114(1):135-42.
4. Lins MÁ, Moreno KA, Graça RC, Lima SM. Constipation prevalence in diabetic patients?. Journal of Coloproctology (Rio de Janeiro). 2014 Apr;34:83-6.
5. Mukherjee S, John S. Lactulose. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
6. Karakan T, Tuohy KM, Janssen-van Solingen G. Low-dose lactulose as a prebiotic for improved gut health and enhanced mineral absorption. Frontiers in Nutrition. 2021;8:672925.
7. Panesar PS, Kumari S. Lactulose: production, purification and potential applications. Biotechnology Advances. 2011 Nov 1;29(6):940-8.
8. Steudle J, Schön C, Wargenau M, Pauly L, Schwejda-Güttes S, Gaigg B, et al. Blood glucose response after oral intake of lactulose in healthy volunteers: A randomized, controlled, cross-over study. World Journal of Gastrointestinal Pharmacology and Therapeutics. 2018 Aug 7;9(3):22-30.
9. Sakai Y, Seki N, Hamano K, Ochi H, Abe F, Masuda K, Iino H. Prebiotic effect of two grams of lactulose in healthy Japanese women: a randomised, double-blind, placebo-controlled crossover trial. Beneficial Microbes. 2019 Jul 10;10(6):629-39.
10. Pieber TR, Svehlikova E, Mursic I, Esterl T, Wargenau M, Sartorius T, et al. Blood glucose response after oral lactulose intake in type 2 diabetic individuals. World Journal of Diabetes. 2021 Jun 15;12(6):893-907.
11. Bianchi G, Ronchi M, Marchesini G. Effect of lactulose on carbohydrate metabolism and diabetes mellitus. Scandinavian Journal of Gastroenterology. 1997 Jan 1;32(sup222):62-4.
12. Kirkman MS, Zimmerman DR, Filippini SA. Marked deterioration in glycemic control with change in brand of lactulose syrup. Southern Medical Journal. 1995 Apr 1;88(4):492-3.
13. Teuri U, Vapaatalo H, Korpela R. Fructooligosaccharides and lactulose cause more symptoms in lactose maldigesters and subjects with pseudohypolactasia than in control lactose. The American Journal of Clinical Nutrition. 1999 May 1;69(5):973-9.
14. VAN DE KAMER JH. Studies on the mechanism of action of lactulose (beta-galactoside action of lactulose (beta-galactosido-fructose) in the intestine. Klinische Wochenschrift. 1964 Feb 1;42:126-30.
15. Pontes FA, Silva AT, Cruz AC. Colonic transit times and the effect of lactulose or lactitol in hospitalized patients. European Journal of Gastroenterology & Hepatology. 1995 May 1;7(5):441-6.
16. Fritz E, Hammer HF, Lipp RW, Högenauer C, Stauber R, Hammer J. Effects of lactulose and polyethylene glycol on colonic transit. Alimentary Pharmacology & Therapeutics. 2005 Feb;21(3):259-68.
17. Jouet P, SABATE JM, Flourie B, Cuillerier E, Gambini D, Lemann M, et al. Effects of therapeutic doses of lactulose vs. polyethylene glycol on isotopic colonic transit. Alimentary Pharmacology & Therapeutics. 2008 May;27(10):988-93.
18. Clegg M, Shafat A. Gastric emptying and orocaecal transit time of meals containing lactulose or inulin in men. British Journal of Nutrition. 2010 Aug;104(4):554-9.
19. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to lactulose and decreasing potentially pathogenic gastro-intestinal microorganisms (ID 806) and reduction in intestinal transit time (ID 807) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010 Oct;8(10):1806.
20. Wesselius-De Casparis A, Braadbaart S, Bergh-Bohlken G, Mimica M. Treatment of chronic constipation with lactulose syrup: results of a double-blind study. Gut. 1968 Feb;9(1):84-6.
21. Sanders JF. Lactulose syrup assessed in a double-blind study of elderly constipated patients. Journal of the American Geriatrics Society. 1978 May;26(5):236-9.
22. Bass P, Dennis S. The laxative effects of lactulose in normal and constipated subjects. Journal of Clinical Gastroenterology. 1981 Jan 1;3:23-8.
23. Kasugai K, Iwai H, Kuboyama N, Yoshikawa A, Fukudo S. Efficacy and safety of a crystalline lactulose preparation (SK-1202) in Japanese patients with chronic constipation: a randomized, double-blind, placebo-controlled, dose-finding study. Journal of Gastroenterology. 2019 Jun;54(6):530-40.
24. Meng S, Pan Y, Deng Q, Wang L, Chang Q. Efficacy and safety of lactulose on the treatment of puerperal constipation. Zhonghua yi xue za zhi. 2015 Jul 1;95(28):2288-90.
25. Huang P, Gou WL, Wang XT, Ding YL, He J, Wang CH. Lactulose oral solution for the treatment of postpartum constipation. Journal of Biological Regulators and Homeostatic Agents. 2016 Apr 1;30(2):523-8.
26. Zhou Y, Yang X, Fan L, Zhu Y, Jiang Y, Li Z, et al. Observations on the curative effect of lactulose for postpartum constipation based on a large sample study. International Journal of Clinical and Experimental Medicine. 2015;8(10):19167-71.
27. Connolly P, Hughes IW, Ryan GW. Comparison of ‘Duphalac’and ‘irritant’laxatives during and after treatment of chronic constipation: a preliminary study. Current Medical Research and Opinion. 1975 Jan 1;2(10):620-5.
28. Quah HM, Ooi BS, Seow-Choen F, Sng KK, Ho KS. Prospective randomized crossover trial comparing fibre with lactulose in the treatment of idiopathic chronic constipation. Techniques in Coloproctology. 2006 Jun;10(2):111-4.
29. Rouse M, Chapman N, Mahapatra M, Grillage M, Atkinson SN, Prescott P. An open, randomised, parallel group study of lactulose versus ispaghula in the treatment of chronic constipation in adults. The British Journal of Clinical Practice. 1991 Jan 1;45(1):28-30.
30. Passmore AP, Wilson-Davies K, Stoker C, Scott ME. Chronic constipation in long stay elderly patients: a comparison of lactulose and a senna-fibre combination. British Medical Journal. 1993 Sep 25;307(6907):769-71.
31. Kinnunen O, Winblad I, Koistinen P, Salokannel J. Safety and efficacy of a bulk laxative containing senna versus lactulose in the treatment of chronic constipation in geriatric patients. Pharmacology. 1993;47(Suppl. 1):253-5.
32. Freedman MD, Schwartz HJ, Roby R, Fleisher S. Tolerance and efficacy of polyethylene glycol 3350/electrolyte solution versus lactulose in relieving opiate induced constipation: a double-blinded placebo-controlled trial. The Journal of Clinical Pharmacology. 1997 Oct;37(10):904-7.
33. Attar A, Lemann M, Ferguson A, Halphen M, Boutron MC, Flourie B, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut. 1999 Feb 1;44(2):226-30.
34. Bouhnik Y, Neut C, Raskine L, Michel C, Riottot M, Andrieux C, et al. Prospective, randomized, parallel-group trial to evaluate the effects of lactulose and polyethylene glycol-4000 on colonic flora in chronic idiopathic constipation. Alimentary Pharmacology & Therapeutics. 2004 Apr;19(8):889-99.
35. Hammer HF, Santa Ana CA, Schiller LR, Fordtran JS. Studies of osmotic diarrhea induced in normal subjects by ingestion of polyethylene glycol and lactulose. The Journal of Clinical Investigation. 1989 Oct 1;84(4):1056-62.
36. Chassagne P, Ducrotte P, Garnier P, Mathiex-Fortunet H. Tolerance and long-term efficacy of polyethylene glycol 4000 (Forlax®) compared to lactulose in elderly patients with chronic constipation. The Journal of Nutrition, Health & Aging. 2017 Apr 1;21(4):429-39.
37. Piche T, Dapoigny M. Comparative efficacy and safety of lactulose plus paraffin vs polyethylene glycol in functional constipation: a randomised clinical study. UEG Journal. 2020 Oct;8(8):923-32.
38. PETUELY F. Über den Bifidusfaktor Lactulose. Bifidobacteria and Microflora. 1986;5(1):3-11.
39. Ruszkowski J, Witkowski JM. Lactulose: patient-and dose-dependent prebiotic properties in humans. Anaerobe. 2019 Oct 1;59:100-6.
40. Kanauchi O, Andoh A, Mitsuyama K. Effects of the modulation of microbiota on the gastrointestinal immune system and bowel function. Journal of Agricultural and Food Chemistry. 2013 Oct 23;61(42):9977-83.
41. Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflammatory Bowel Diseases. 2009 Feb 1;15(2):300-10.
42. Russell DA, Ross RP, Fitzgerald GF, Stanton C. Metabolic activities and probiotic potential of bifidobacteria. International Journal of Food Microbiology. 2011 Sep 1;149(1):88-105.
43. Mondot S, de Wouters T, Doré J, Lepage P. The human gut microbiome and its dysfunctions. Digestive Diseases. 2013;31(3-4):278-85.
44. Ballongue J, Schumann C, Quignon P. Effects of lactulose and lactitol on colonic microflora and enzymatic activity. Scandinavian Journal of Gastroenterology. 1997 Jan 1;32(sup222):41-4.
45. Tuohy KM, Ziemer CJ, Klinder A, Knöbel Y, Pool-Zobel BL, Gibson GR. A human volunteer study to determine the prebiotic effects of lactulose powder on human colonic microbiota. Microbial Ecology in Health and Disease. 2002 Jan 1;14(3):165-73.
46. Mangin I, Bouhnik Y, Suau A, Rochet V, Raskine L, Crenn P, et al. Molecular analysis of intestinal microbiota composition to evaluate the effect of PEG and lactulose laxatives in humans. Microbial Ecology in Health and Disease. 2002 Jan 1;14(1):54-62.
47. Bouhnik Y, Attar A, Joly FA, Riottot M, Dyard F, Flourie B. Lactulose ingestion increases faecal bifidobacterial counts: a randomised double-blind study in healthy humans. European Journal of Clinical Nutrition. 2004 Mar;58(3):462-6.
48. De Preter V, Coopmans T, Rutgeerts P, Verbeke K. Influence of long-term administration of lactulose and Saccharomyces boulardii on the colonic generation of phenolic compounds in healthy human subjects. Journal of the American College of Nutrition. 2006 Dec 1;25(6):541-9.
49. Vanhoutte T, De Preter V, De Brandt E, Verbeke K, Swings J, Huys G. Molecular monitoring of the fecal microbiota of healthy human subjects during administration of lactulose and Saccharomyces boulardii. Applied and Environmental Microbiology. 2006 Sep;72(9):5990-7.
50. Mayerhofer F, Petuely F. Untersuchungenzur Regulation der Darmtrdgheit des ErwachsenenmitHilfe der Lactulose (Bifidus-Faktor). Wien. Klin. Wochenschr. 1959;71:865-9.
51. Schumann C. Medical, nutritional and technological properties of lactulose. An update. European Journal of Nutrition. 2002 Oct;41(1):i17-25.
52. Gattuso JM, Kamm MA. Adverse effects of drugs used in the management of constipation and diarrhoea. Drug safety. 1994 Jan;10(1):47-65.
53. Kot TV, Pettit-Young NA. Lactulose in the management of constipation: a current review. Annals of Pharmacotherapy. 1992 Oct;26(10):1277-82.
54. Schiller LR. The therapy of constipation. Alimentary pharmacology & therapeutics. 2001 Jun 12;15(6):749-63.
55. Lukens B, Nierman DM, Schiano TD. Lactulose: how many ways can one drug be prescribed?. The American Journal of Gastroenterology. 2011 Sep 1;106(9):1726-7.