Commentary
With the advent of the direct oral anticoagulants (DOACs), patients requiring anticoagulation for common conditions such as atrial fibrillation and venous thromboembolism no longer need to worry about dietary restrictions or regular monitoring of the international normalized ratio which complicated warfarin treatment. Switching from warfarin to apixaban, a DOAC, has been shown to improve patient satisfaction by reducing treatment burden [1]. The clotting factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban) and the direct thrombin inhibitors (argatroban and dabigatran) have shown non-inferiority in preventing thromboembolic events and a superior safety profile in terms of bleeding in several trials when compared to warfarin [2-6]. Furthermore, apixaban and dabigatran have even shown superiority in preventing stroke or systemic embolism [3,6].
Despite these promising outcomes, there remains an important subgroup of patients who have been excluded from the initial DOAC trials: advanced chronic kidney disease (CKD stage 4-5) and hemodialysis (HD) patients. Specifically, the ARISTOTLE trial evaluating the efficacy of apixaban versus warfarin in preventing stroke or systemic embolization in patients with atrial fibrillation excluded individuals with a serum creatinine greater than 2.5 mg/mL or an estimated GFR less than 25 mL/min [7]. The AVERROES trial similarly compared apixaban to warfarin and excluded patients with renal insufficiency utilizing identical criteria to the ARISTOTLE study [8]. This population, however, having a high burden of atrial fibrillation and cardiovascular disease [9,10], is at increased risk of stroke [11], and would theoretically benefit greatly from treatment with DOACs.
Concerns regarding the use of oral anticoagulation in general and with DOACs in particular emerge from two perspectives: that of efficacy and that of safety. Available retrospective studies both with warfarin [12-16] and with apixaban [17] have yielded conflicting results with regards to the benefit of anticoagulation in CKD patients. The differential effect of statins in CKD and non-CKD populations should serve as a warning against extrapolating the results of prospective trials that have not studied the population in question [18]. On the other side of the problem, the safety of anticoagulation in CKD and HD patients is an important one given their inherently higher propensity for bleeding (including with warfarin [15]) and the impaired clearance of renally-excreted DOACs in ESRD [19,20]. All DOACs are renally excreted to some degree and, in the setting of kidney insufficiency, even properly dosed medications may accumulate in the body which can increase the risk for major bleeding events [21]. With one of the lowest percentages of renal clearance at 27% [22], apixaban may represent one of the safest choices in CKD patients and two retrospective studies have found a significantly lower bleeding risk with apixaban compared to warfarin in the CKD population, which was not demonstrated with other DOACs [23,24]. Apixaban dosing in the setting of HD remains controversial. One pharmacokinetic study showed that a reduced dose of apixaban at 2.5 mg twice daily in HD patients was found to predictably result in equivalent drug exposure to a dose of 5 mg twice daily in patients with preserved renal function. While a full dose of 5 mg twice daily led to supratherapeutic doses in HD patients [25]. The timing of administration is also likely to influence drug levels [26]. Others, however, found no increase in drug levels in HD patients [27,28].
Clinical evidence on the safety and efficacy of apixaban in CKD and HD patients remains limited as only one randomized controlled trial has been completed, but was stopped early. Decisions on DOAC use in this cohort must rely primarily on retrospective studies, registry data, metanalyses, one post-hoc analysis of the ARISTOTLE trial and one underpowered randomized controlled trial. Available observational data has shown a consistent trend of lower bleeding with apixaban compared to warfarin [29-31], with one large study demonstrating higher efficacy as well for the prevention of thrombotic events at doses of 5 mg twice daily [31]. Although prospective in nature, the ARISTOTLE trial did not include renal function amongst its randomization variables and any results should be interpreted as a post-hoc analysis. The study by Stanifer and colleagues found a lower incidence of bleeding with apixaban compared to warfarin in patients with creatinine clearance between 25 and 30 ml/min, with a larger effect size compared to patients with better renal function [28]. RENAL-AF, the only completed randomized controlled trial investigating the use of apixaban in HD patients was unfortunately stopped early due to lack of funding and low accrual [32]. Out of a planned 760 patients only 154 were included in the final analysis and, as such, the study remained underpowered and any interpretation of their findings must be done cautiously. It is, however, reassuring that even with this limited sample, the frequency of clinically relevant non-major bleeding was lower in patients treated with apixaban 5 mg twice daily. No differences were noted in the rates of major bleeding, intracranial bleeding or ischemic stroke between the two groups.
The interpretation of retrospective evidence is difficult given the substantial risk for confounding. An additional complicating issue is the inability to investigate clinical variables that may impact the safety of apixaban due to lack of quality data. Chiefly, analyses of bleeding risk with DOACs in CKD and HD patients have largely ignored the interaction of antiplatelets and anticoagulants, which has recently gained attention in the cardiovascular disease literature. As previously discussed, CKD and HD patients are underrepresented in these studies, but the importance of this issue is underscored by a high percentage of antiplatelet use (40%-50.6%) in our cohort and that of others [32,33]. In the RENAL-AF trial, the impact of antiplatelet therapy on bleeding, despite a high rate of use (40%), was not explored.
In non-HD patients, one study found that the concurrent use of any antiplatelet and apixaban increased the risk of major bleeding (adjusted odds ratio of 2.01; 95% confidence interval [95% CI] 1.29-3.11) [34]. A large retrospective study demonstrated a similarly increased bleeding risk with addition of aspirin to DOAC (hazard ratio [HR] 1.30; 95% CI 1.11-1.52) [35]. Another retrospective analysis, however, found that add-on dual or single agent antiplatelet therapy to DOAC did not influence the risk in multivariate analysis [36]. Perhaps the strongest evidence for the increased bleeding risk with addition of antiplatelet medication comes from the AUGUSTUS study, a multicenter, randomized trial with a 2x2 factorial design which investigated the use of a P2Y12 inhibitor in combination with warfarin versus apixaban and aspirin versus placebo in patients with atrial fibrillation and acute coronary syndrome or percutaneous coronary intervention [37]. No patients on hemodialysis were included and the number of patients with a creatinine above 1.5 mg/dL was small (creatinine clearance was not reported). The analysis found an increased risk of bleeding with the use of a dual antiplatelet regimen in combination with anticoagulation compared to single antiplatelet agent combined with anticoagulation with no additional benefit. Although exploring primarily the risk profile of adding dual antiplatelet therapy compared to single antiplatelet therapy, AUGUSTUS provides compelling evidence for increased bleeding potential with concurrent use of interacting pharmacokinetic drugs.
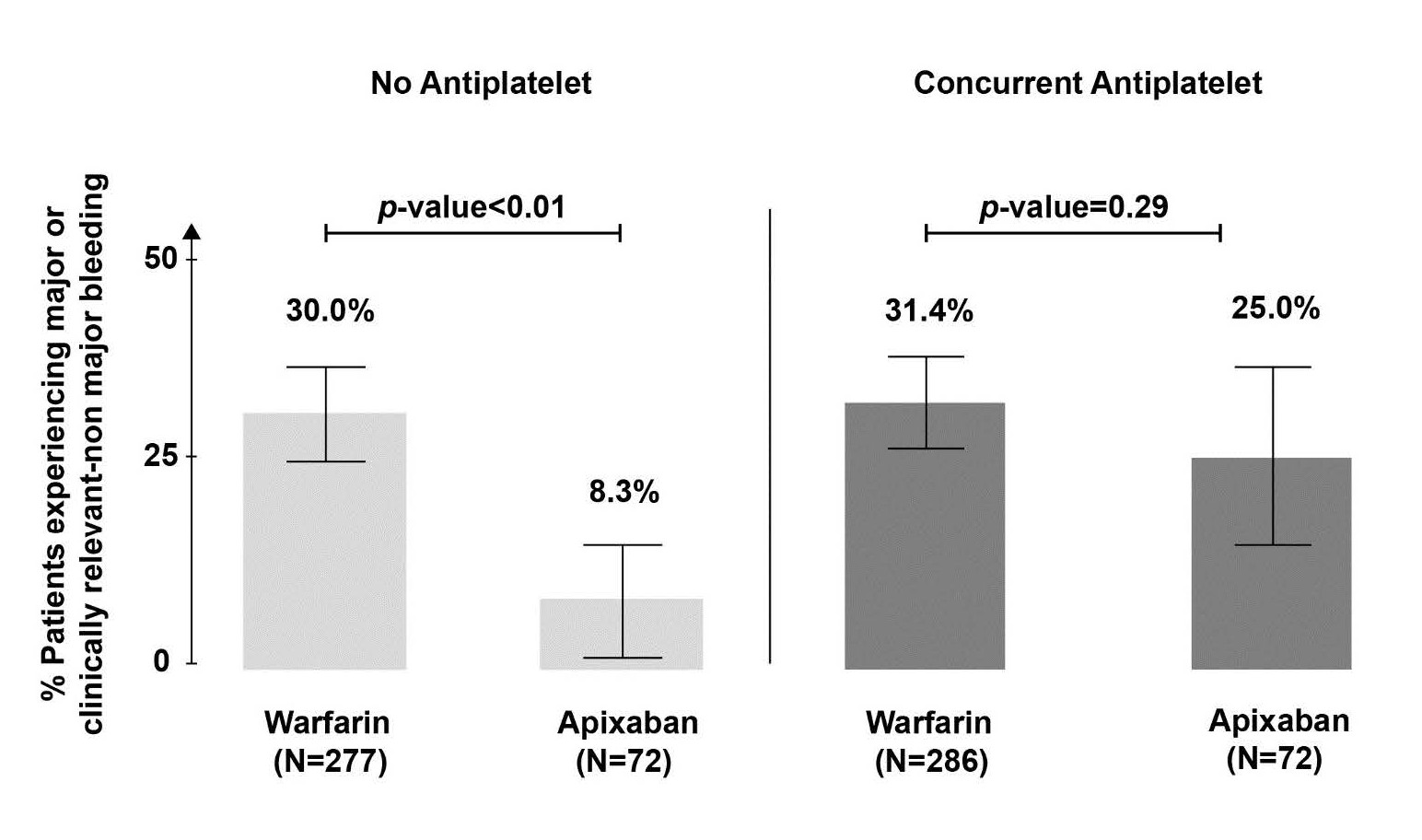
Our group recently reported findings from a retrospective analysis of bleeding rates in HD patients treated with either warfarin or apixaban [33]. We found a significantly lower bleeding propensity with apixaban use alone compared to warfarin alone, whereas concurrent antiplatelet therapy markedly reduced the difference (Figure 1). Additionally, in a multivariate competing risk model, the use of apixaban alone was again associated with less bleeding (HR 0.24, 95% CI 0.10-0.55), but this safety advantage was not observed with concurrent use of apixaban and any antiplatelet (HR 0.93, 95% CI 0.55-1.56). It is worthwhile to note that a large database study of non-HD patients found a lower risk of major bleeding with concomitant DOAC-antiplatelet use compared to warfarin-antiplatelet (HR 0.68; 95% CI 0.51-0.91), again raising the question of whether CKD and HD patients have a different bleeding risk profile with antithrombotic combinations compared to other populations [38]. The majority of bleeding in our cohort (nearly 80% in the apixaban group and 50% in the warfarin group) originated in the gastrointestinal tract raising the question of whether the ulcerogenic activity of antiplatelets underlies the observed difference. Notably, use of proton pump inhibitors or H2 blockers did not impact the bleeding risk in our analysis, but this remains an important avenue for future research.
Figure 1. Frequency of major or clinically relevant-non major bleeding with warfarin and apixaban stratified by antiplatelet use (Modified from [33]).
Important unknowns remain with regards to the use of anticoagulant and antiplatelet medication in the advanced CKD and HD populations. The fundamental question pertains to the efficacy of anticoagulation in preventing stroke or venous thromboembolism recurrence in these cohorts as available data for warfarin and DOACs is of poor quality and conflicting. One ongoing trial (NCT03987711) is actively addressing this question by inclusion of a placebo (no anticoagulation) comparison arm. Secondly, the safety of DOACs remains to be demonstrated in a large, prospective study and two are currently ongoing (NCT02933697, NCT03987711). Importantly, with the current trend in decreasing the intensity of antithrombotic regimens in patients with coronary artery disease, antiplatelet therapy should be included among the randomization criteria of prospective studies as preliminary findings suggest that their addition may minimize safety benefits without a concomitant decrease in thrombotic events. Lastly, with the advent of betrixaban, a newer DOAC with the lowest documented renal clearance (15%) [39], it remains to be determined if this new medication represents a more attractive option for advanced CKD and HD patients compared to apixaban.
References
2. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. New England Journal of Medicine. 2011 Sep 15;365(11):981-92.
3. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. New England Journal of Medicine. 2011 Mar 3;364(9):806-17.
4. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. New England Journal of Medicine. 2011 Sep 8;365(10):883-91.
5. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. New England Journal of Medicine. 2013 Nov 28;369(22):2093-2104.
6. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. New England Journal of Medicine. 2009 Sep 17;361(12):1139-51.
7. Lopes RD, Alexander JH, Al-Khatib SM, Ansell J, Diaz R, Easton JD, et al. Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. American Heart Journal. 2010 Mar 1;159(3):331-339.
8. Eikelboom JW, O’Donnell M, Yusuf S, Diaz R, Flaker G, Hart R, et al. Rationale and design of AVERROES: apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. American Heart Journal. 2010 Mar 1;159(3):348-53.
9. Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). American Heart Journal. 2010 Jun 1;159(6):1102- 1107.
10. Reinecke H, Brand E, Mesters R, Schäbitz WR, Fisher M, Pavenstädt H, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. Journal of the American Society of Nephrology. 2009 Apr 1;20(4):705- 711.
11. Olesen JB, Lip GY, Kamper AL, Hommel K, Køber L, Lane DA, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. New England Journal of Medicine. 2012 Aug 16;367(7):625-35.
12. Chan KE, Lazarus JM, Thadhani R, Hakim RM. Anticoagulant and antiplatelet usage associates with mortality among hemodialysis patients. Journal of the American Society of Nephrology. 2009 Apr 1;20(4):872-81.
13. Van Der Meersch H, De Bacquer D, De Vriese AS. Vitamin K antagonists for stroke prevention in hemodialysis patients with atrial fibrillation: A systematic review and meta-analysis. American Heart Journal. 2017 Feb 1;184:37-46.
14. Masson P, Kelly PJ, Craig JC, Lindley RI, Webster AC. Risk of stroke in patients with ESRD. Clinical Journal of the American Society of Nephrology. 2015 Sep 4;10(9):1585-92.
15. Dahal K, Kunwar S, Rijal J, Schulman P, Lee J. Stroke, major bleeding, and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta-analysis of observational studies. Chest. 2016 Apr 1;149(4):951-959.
16. Yang F, Chou D, Schweitzer P, Hanon S. Warfarin in haemodialysis patients with atrial fibrillation: what benefit?. Europace. 2010 Dec 1;12(12):1666-72.
17. Mavrakanas TA, Garlo K, Charytan DM. Apixaban versus no anticoagulation in patients undergoing longterm dialysis with incident atrial fibrillation. Clinical Journal of the American Society of Nephrology. 2020 Aug 7;15(8):1146-54.
18. Baigent C, Herrington WG, Coresh J, Landray MJ, Levin A, Perkovic V, et al . Challenges in conducting clinical trials in nephrology: conclusions from a Kidney Disease— Improving Global Outcomes (KDIGO) Controversies Conference. Kidney International. 2017 Aug 1;92(2):297-305.
19. Lutz J, Menke J, Sollinger D, Schinzel H, Thürmel K. Haemostasis in chronic kidney disease. Nephrology Dialysis Transplantation. 2014 Jan 1;29(1):29-40.
20. Wilke T, Wehling M, Amann S, Bauersachs RM, Böttger B. Renal impairment in patients with thromboembolic event: prevalence and clinical implications. A systematic review of the literature. Deutsche medizinische Wochenschrift (1946). 2015 Aug 25;140(17):e166-74.
21. Lutz J, Jurk K, Schinzel H. Direct oral anticoagulants in patients with chronic kidney disease: patient selection and special considerations. International Journal of Nephrology and Renovascular Disease. 2017;10:135.
22. Kumar S, Lim E, Covic A, Verhamme P, Gale CP, Camm AJ, et al. Anticoagulation in concomitant chronic kidney disease and atrial fibrillation: JACC review topic of the week. Journal of the American College of Cardiology. 2019 Oct 29;74(17):2204-15.
23. Cheung CY, Parikh J, Farrell A, Lefebvre M, Summa- Sorgini C, Battistella M. Direct Oral Anticoagulant Use in Chronic Kidney Disease and Dialysis Patients With Venous Thromboembolism: A Systematic Review of Thrombosis and Bleeding Outcomes. Annals of Pharmacotherapy. 2021 Jun;55(6):711-22.
24. Bowie M, Valencia V, Perez-Alvarez I, Tran MH. Safety analysis of apixaban versus warfarin in patients with advanced kidney disease. Journal of Thrombosis and Thrombolysis. 2018 Aug;46(2):246-52.
25. Mavrakanas TA, Samer CF, Nessim SJ, Frisch G, Lipman ML. Apixaban pharmacokinetics at steady state in hemodialysis patients. Journal of the American Society of Nephrology. 2017 Jul 1;28(7):2241-2248.
26. Van den Bosch I, Bouillon T, Verhamme P, Vanassche T, Jacquemin M, et al. Apixaban in patients on haemodialysis: a single-dose pharmacokinetics study. Nephrology Dialysis Transplantation. 2021 May;36(5):884-889.
27. Wang X, Tirucherai G, Marbury TC, Wang J, Chang M, Zhang D, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. The Journal of Clinical Pharmacology. 2016 May;56(5):628-636.
28. Stanifer JW, Pokorney SD, Chertow GM, Hohnloser SH, Wojdyla DM, Garonzik S, et al . Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation. 2020 Apr 28;141(17):1384-92.
29. Andò G, Capranzano P. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with chronic kidney disease: a systematic review and network meta-analysis. International Journal of Cardiology. 2017 Mar 15;231:162-169.
30. Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, Kaewput W, Pachariyanon P, Cheungpasitporn W. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: Meta-analysis. Pacing and Clinical Electrophysiology. 2018 Jun;41(6):627-34.
31. Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, et al . Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018 Oct 9;138(15):1519-29.
32. Pokorney SD, Kumbhani DJ, Bhatt DL. RENal hemodialysis patients ALlocated apixaban versus warfarin in Atrial Fibrillation-RENAL-AF. Presentation at the American Heart Association Annual Scientific Sessions (AHA 2019), Philadelphia, PA. 2019 Nov 16;16.
33. Ionescu F, Cooper C, Petrescu I, George J, Mansuri S. Safety of apixaban compared to warfarin in hemodialysis patients: Do antiplatelets make a difference?. European Journal of Haematology. 2021 May;106(5):689-96.
34. Zhang Y, Souverein PC, Gardarsdottir H, van den Ham HA, Maitland-van der Zee AH, de Boer A. Risk of major bleeding among users of direct oral anticoagulants combined with interacting drugs: A population-based nested case–control study. British Journal of Clinical Pharmacology. 2020 Jun;86(6):1150-64.
35. Said A, Keeney S, Matka M, Hafeez A, George J, Halalau A. Concomitant use of direct oral anticoagulants and aspirin versus direct oral anticoagulants alone in atrial fibrillation and flutter: a retrospective cohort. BMC Cardiovascular Disorders. 2020 Dec;20: 263.
36. Sotomi Y, Hirata A, Amiya R, Kobayashi T, Hirayama A, Sakata Y, et al . Bleeding risk of add-on anti-platelet agents to direct oral anticoagulants in patients with nonvalvular atrial fibrillation (from 2216 patients in the DIRECT Registry). The American Journal of Cardiology. 2019 Apr 15;123(8):1293-1300.
37. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al . Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. New England Journal of Medicine. 2019 Apr 18;380(16):1509- 1524.
38. Douros A, Renoux C, Yin H, Filion KB, Suissa S, Azoulay L. Concomitant use of direct oral anticoagulants with antiplatelet agents and the risk of major bleeding in patients with nonvalvular atrial fibrillation. The American Journal of Medicine. 2019 Feb 1;132(2):191-199.
39. Huisman MV, Klok FA. Pharmacological properties of betrixaban. European Heart Journal Supplements. 2018 May 1;20(suppl_E):E12-15.