Abbreviations
DACHPt/HANP: Dichloro (1, 2-diaminocyclohexane) platinum (II)-loaded and Hyaluronic acid polymer-coated nanodrug; EPR effect: Enhanced Permeability and Retention effect; MPS: Mononuclear Phagocytic System; MRI: Magnetic Resonance Imaging; PEG: Polyethylene Glycol; RBC, Red Blood Cell; RES: Reticuloendothelial System; TAM: Tumor-Associated Macrophages
Introduction
Encapsulation of therapeutic molecules (e.g., small molecule inhibitors, mRNA, siRNA, aptamers, etc.) into nanomaterials can improve the solubility and blood circulation of the drugs, alter their biodistribution, decrease their toxicities, overcome drug resistance, and facilitate their entry into target cells [1]. The development of anti-cancer nanodrugs has been the focus of intense study for decades. Several anti-cancer nanodrugs have been approved for clinical use all over the world [2]. These have contributed greatly to a lower death rate from some cancers, and thus are widely used. However, an extensive analysis of anti-cancer nanodrugs found an extremely low efficiency of delivery to the tumor, i.e., less than 1% [2]. This is obviously very wasteful and contributes greatly to the increasing cost of health care [1]. To date, most nanodrugs have been focused on cancer research, but the techniques have been translated for many other applications, e.g., vaccines, cardiovascular disease, and neuropathy disease [3-6].
In this review, we discuss the current challenges and possible progress for anti-cancer nanodrug delivery in the following three aspects: reticuloendothelial system (RES) clearance and toxicity; enhanced permeability and retention (EPR) effect; and immunotherapy. We show in each case how the use of Intralipid can improve anticancer activity.
The RES clears much of the injected nanodrugs, especially to the liver and spleen, resulting in organ toxicity. Lowering the clearance rate could decrease toxicity.
Our understanding of the accumulation of nanodrugs in solid tumors has generally been based on the EPR effect. Tumor blood flow is key to nanodrug delivery via the EPR effect [7,8]. Obstructed tumor blood flow as observed in advanced cancers is a major barrier to the therapeutic efficacy of anticancer nanodrugs.
The tumor immune micro-environment plays a critical role in the development, progression, and metastasis of several types of cancers [9]. Macrophages are important members of the tumor micro-environment [10]. In most cases, M1-like macrophages play a role in anti-tumor immunity, while M2-like macrophages play a role in immunosuppression and tumor immune escape. Thus, increasing the ratio of M1-like macrophages should improve anti-tumor immunity.
Intralipid is the brand name of the first safe fat emulsion for human use, approved in 1972. Intralipid 20.0% is composed of 20% soybean oil, 1.2% egg-yolk phospholipids, and 2.25% glycerol, and is manufactured by Fresenius Kabi (Uppsala, Sweden). The major fatty acid constituents are linoleic acid (44-62%), oleic acid (19-30%), palmitic acid (7-14%), linolenic acid (4-11%), and stearic acid (1.4-5.5%).
Intralipid Reduces the Off-Target Accumulation of Nanodrugs in the RES and Reduces Toxicities
The low tumor delivery efficiency (less than 1%) [11], has led to extensive discussion regarding the delivery effectiveness of anti-cancer nanodrugs. A major proportion of nanodrugs is taken by the mononuclear phagocytic system (MPS) or RES, especially by the liver and spleen, resulting in organ toxicity. In order to reduce the RES clearance, stealth and decoy strategies have been developed.
The first “stealth” nanoparticle can be dated back to 1977 [12]. “Stealth” coating of nanodrugs is one major achievement in the field of drug delivery. Nanoparticles may “escape” the RES when the particle’s surface is coated with hydrophilic polymers/surfactants, and/or the particle is formulated with biodegradable copolymers with hydrophilic segments [13,14]. Polyethylene glycol (PEG) is the most commonly used non-ionic hydrophilic polymer to make “stealth” nanoparticles in order to reduce the RES uptake and increase the blood circulation of the nanoparticles. The first approved PEGylated product, Doxil (doxorubicin HCl liposome injection), has already been in the clinic for ~25 years [15,16]. Recently approved Onivyde (irinotecan liposome injection) is also a PEGylated liposome [17]. Other modifications of the nanoparticle characteristics and surface properties, such as size, shape, charge, composition, and tumor targeting moiety might also decrease RES uptake and increase tumor delivery. However, with all the above efforts, the current status of using anti-cancer nanodrugs is that a very small fraction (0.7%, median) of the injected nanodrugs is delivered to solid tumors [11].
Lanza and Wickline in 2005 [18] tested decoy systems to decrease the RES uptake and increase the targeting. Their method comprises administering simultaneously a targeted nanoparticle (i.e., therapeutic agent) and an excess of untargeted carrier or decoy. This simultaneous administration enhances the delivery of the targeted nanoparticle to the desired location in a subject. The decoy must mimic the behavior of the targeted nanoparticle. The inactive carrier (decoy) and the biocompatible nanoparticle need to share a similar “non-active” part of the active nanoparticle or the compound of interest. According to this decoy strategy, each active nanoparticle has its own empty nanoparticle as the decoy. This system needs additional FDA approval for clinical use.
The major obstacle to long-term circulation and delivery of nanodrugs is clearance by the Kupffer cells. Clodronate liposome depletion of Kupffer cells has been carried out to investigate the effect of Kupffer cell depletion on nanodrug delivery [19,20]. Tumor delivery of the nanodrugs increased up to 150 times with clodronate liposomes to deplete the Kupffer cells. However, the maximum delivery efficiency was only 2%! Depletion of Kupffer cells can achieve long-term circulation of the nanodrugs, but 98% do not accumulate in the tumor [19]. This is an effective method to deplete Kupffer cells and to address their functions in nanodrug delivery, but we have concerns that clodronate liposomes will deplete all types of monocytes/ macrophages in the body, including the tumor-associated macrophages, which might affect some properties of the tumor. It is important to note that clodronate liposomes are not an FDA-approved agent. Later, it was shown that the removal of Kupffer cells increased fecal elimination of nanodrugs by >10 times [20].
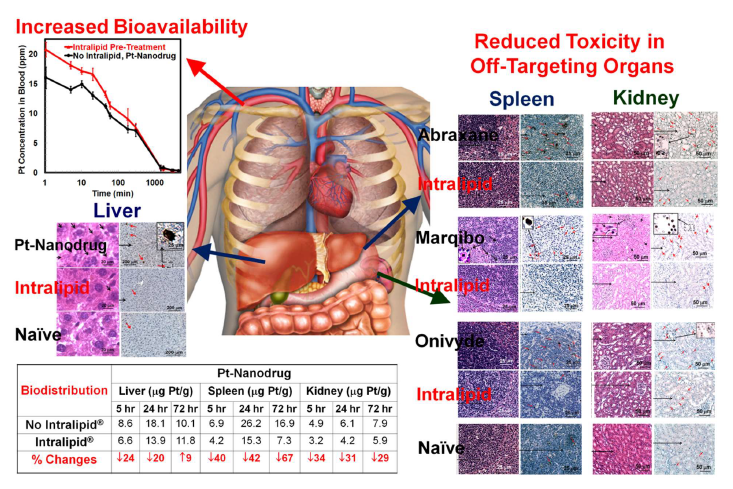
We have developed a strategy to temporarily blunt the RES uptake of nanoparticles, instead of chemically depleting Kupffer cells, by using an FDA-approved lipid emulsion, Intralipid. We have tested our strategy by using nano- and micron-sized MR imaging agents [21], an in-development experimental dichloro (1, 2-diaminocyclohexane) platinum (II)-loaded and hyaluronic acid polymer-coated nanodrug (DACHPt/HANP) [22,23], and FDA approved anti-cancer nanodrugs, e.g., Abraxane, Marqibo, and Onivyde [24], as shown in Figure 1. The animals (rats) were treated with Intralipid (2 g/kg, clinical dosage) intravenously (clinical route) 1-hr prior to and 24-hr post the injection of the nanodrugs. We have found that this method can be very useful for decreasing the RES uptake of the nanoparticles and increasing their bioavailability [21,22]. For example, Intralipid can reduce platinum accumulation in the liver, spleen, and, interestingly, kidney by 20.4%, 42.5%, and 31.2% at 24-hr post DACHPt/HANP administration, respectively. The bioavailability of DACHPt/HANP increases by 18.7% and 9.4% during the first 5 and 24 hr, respectively. We have also found that DACHPt/HANP, Abraxane, Marqibo, and Onivyde exhibit different toxicity profiles. Intralipid can reduce these drugs’ toxic side effects in the RES and kidney to different levels [22,24,25]. Recently, we have also found that Intralipid protects the viability of human monocytic cells, but not breast, lung, or pancreatic cancer cells in the presence of paclitaxel [26].
Figure 1. Intralipid reduces the toxicity and improves the bioavailability and biodistribution of an experimental anti-cancer nanodrug, DACHPt/HANP. Intralipid also reduced the toxic side effects of FDA approved anti-cancer nanodrugs, e.g., Abraxane, Marqibo, and Onivyde. Modified from Figures 1 and 7 of Liu et al. [22] and Table 1, Figures 3, 4, and 5 of Liu et al. [24].
Using a xenograft breast cancer mouse model, we have found that Intralipid pre-treatment significantly increases the amount of paclitaxel reaching the tumor and promotes tumor apoptosis [26]. The combination of Intralipid with half the standard clinical dose of Abraxane reduces the tumor growth rate as effectively as the standard clinical dose alone. A recent study from Maeda and colleagues showed that Intralipid 24-hr pre-treatment induced a 40% reduction in liver uptake of a polymeric nanoprobe used in photodynamic therapy [27]. As a consequence, there was a 1.5-fold-increased nanoparticle accumulation in tumors using a murine sarcoma S180 model. This increased accumulation led to significantly better therapeutic effects, as validated by using Doxil. Thus, Intralipid methodology could be a valuable complement to the above-mentioned “stealth” strategies, i.e., reducing the RES uptake of anticancer nanodrugs and increasing the delivery to the tumor.
Intralipid Improves Tumor Blood Flow Thus Enhancing EPR Effect-Based Nanodrug Delivery
Our understanding of the accumulation of nanodrugs in solid tumors has generally been based on the EPR effect. This concept, however, has received increasing concern in recent years. The heterogeneity of the EPR effect has provoked a debate about the real value of this effect, which varies depending on a patient’s pathological and physiological characteristics and clinical condition. The EPR effect becomes more pronounced when the systolic blood pressure is elevated (e.g., to 110-160 mmHg) via intravenous infusion of hypertensive agents, such as angiotensin II. When a patient’s systolic blood pressure is on the low side of about 90 mm Hg instead of 120–130 mm Hg, the hydrodynamic force pushing blood from the luminal side of a vessel into the tumor tissue becomes significantly low, which results in a low EPR [28]. Studies have confirmed that tumor EPR is high in both mouse xenograft tumors and human cancers, when tumors are compared with normal tissues. In human studies, nanodrugs’ tumor accumulation was significantly higher compared to the other low background areas of the body [7,29-31].
Imaging-guided personalized precision medicine can be very helpful for cancer treatment. To address EPR effect heterogeneity and to predict nano therapy outcomes, several studies have established companion nanodiagnostics and nanotheranostics [32,33]. Ferumoxytol is an FDAapproved 30-nm iron-oxide nanoparticle used to treat iron deficiency anemia. Ferumoxytol can be used off-label to characterize EPR heterogeneity via magnetic resonance imaging (MRI) [34]. Ferumoxytol-enhanced MRI correlated with therapeutic nanoparticle uptake in tumorassociated macrophages (TAM) and enabled prediction of tumor accumulation and anti-tumor efficacy [34]. In the clinic, ferumoxytol-enhanced MRI demonstrated that higher ferumoxytol accumulation levels in the tumors correlated with greater lesion-size reductions following treatment with Onivyde [35].
Tumor blood flow is key to nanodrug delivery via the EPR effect [7,8]. Obstructed tumor blood flow as observed in advanced cancers is a major barrier to the therapeutic efficacy of anticancer nanodrugs. Islam et al. [27] showed that Intralipid could improve tumor blood flow and lower blood viscosity. The therapeutic benefit of Intralipid pretreatment was shown by the use of Doxil in two different solid tumor models: S180 sarcoma cancer and a C26 cancer cachexia model. For example, in the S180 tumor model, Doxil alone at 1.25 mg/kg did not suppress tumor growth, whereas when this dose was combined with the Intralipid treatment (1 g/kg, 24 h pre-treatment) a significant suppression of tumor growth was achieved. This result was almost the same as that with 2.5 mg/kg Doxil, which indicated about a 2-fold increased therapeutic benefit. This result is very similar to the use of Intralipid to deliver Abraxane in a breast cancer model [26]. This improved efficacy is shown not only because of the reduction of RES clearance of the nanodrugs, but also because of the increase of tumor blood flow.
As the proposed mechanism, it has been reported that Intralipid infusion significantly reduced blood viscosity in neonates and children [36]. Intralipid can interrupt the binding of fibrinogen and other larger proteins on the surface of the red blood cell (RBC), thereby increasing the negative charge on the RBC surface. The negative surface charge of the vascular endothelial luminal surface repelled contact with the RBC surface, thus, the formation was disrupted and blood flow improved. Fibrinogen-mediated clot formation is partly responsible for the blood vessel occlusion and for the reduced tumor blood flow, which are major barriers to the delivery of nanodrugs to tumors [7]. Upregulation of fibrinogen has been reported in cancer patients [37]. Indeed, beneficial effects of Intralipid on ischemic disease have been observed [38,39].
Intralipid Promotes the Polarization of Macrophages to the Anti-cancer M1-like Phenotype
Immunotherapy is revolutionizing the treatment of cancer. The tumor immune micro-environment plays a critical role in the development, progression, and metastasis of several types of cancers [9]. Macrophages are important members of the tumor micro-environments infiltrating immune cell population [10]. In most cases, M1- like macrophages play a role in anti-tumor immunity, while M2-like macrophages play a role in immunosuppression and tumor immune escape. It has been known that lipid can affect the polarizations and functions of macrophages.
Saturated fatty acids enhance an M1-like differentiation, whereas the M2 sub-type is induced by longer, more unsaturated fatty acids [40]. The oxidations or metabolites of fatty acids can promote macrophages differentiating into the M2 subtype [41], whereas circulating levels of free fatty acids elevate the populations of M1 subtypes [42].
Intralipid promotes the polarization of macrophages to the anti-cancer M1-like phenotype [26]. Chemically induced M0/M1/M2 macrophages were incubated with Intralipid for 72 hrs. In M1 macrophages, the expression levels of the M1 markers (CD80 and CD215) are maintained, and those of the M2 markers (CD206 and CD163) are decreased. In M0 and M2 macrophages, the expression levels of M1 markers are elevated 1.5- to 2-fold with statistical significance. In-vivo experiments have further confirmed the effect of Intralipid on immune modulation. Treating macrophages in vivo with Intralipid does not affect phagocytosis, but promotes polarization into the M1-like phenotype as shown by the expression levels of CXCL10 and iNOS in tumor sections.
Conclusions and Future Perspective
The delivery of anti-cancer nanodrugs to tumors is much more complicated than we originally thought, as indicated by the studies mentioned above. We need to understand how nanodrugs are eliminated from the body. We also need an in-depth knowledge of the heterogeneity of cancers and biological factors that influence the behavior of a nanodrug towards a tumor. In addition to the EPR effect, the tumor targeting ligands are also critical to increasing the delivery of nano-drugs. An appropriate animal model and testing protocol are highly desired.
The understanding of cancer and nanomaterial as well as the methods to deliver safer and more effective chemotherapeutics are facing many challenges. Intralipid has the potential to decrease RES clearance and the toxicities of nanodrugs, increase tumor blood flow and delivery, and modulate the tumor immune microenvironment. We hope that our intralipid nano-drug delivery methodology can give physicians more options to treat cancer patients with powerful nanodrugs. A critical limitation in the current delivery of the anti-cancer drugs to patients is the amount of these cytotoxic drugs that a patient can tolerate. Since Intralipid can reduce the off-target toxicities in multiple organs, a physician could increase the dosage of a nanodrug to kill more cancer cells. Or, if the Intralipid treatment can improve the bioavailability of the drug as shown in DACHPt/HANP, thus, improving the delivery of the nanodrug, a physician could reduce the dosage of the drug, which is very expensive, without affecting the efficacy of the drug. Thus, our findings for the use of Intralipid for nanodrug delivery can lead to improving the quality of life for patients who undergo the therapeutic treatments as well as reducing the healthcare costs. This approach is a general one, i.e., applicable to any approved nanodrugs as well as those in-development, reducing RES clearance, and thus decreasing toxicity, improving tumor blood flow, and modulating the innate immune system, without additional modification of the nanoparticles and the drugs.
Acknowledgement
This research was supported (in part) by the Intramural Research Program of the NIH, NINDS. We thank Dr. E. Ann Pratt for her suggestions to improve our manuscript.
References
2. van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJ, Lammers T. Smart cancer nanomedicine. Nature Nanotechnology. 2019 Nov;14(11):1007-17.
3. Wang N, Chen M, Wang T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. Journal of Controlled Release. 2019 Jun 10;303:130-50.
4. Pala R, Anju VT, Dyavaiah M, Busi S, Nauli SM. Nanoparticle-mediated drug delivery for the treatment of cardiovascular diseases. International Journal of Nanomedicine. 2020;15:3741.
5. Kulkarni JA, Witzigmann D, Leung J, Tam YY, Cullis PR. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale. 2019;11(45):21733-9.
6. Saddik MS, Elsayed M, Abdelkader MS, El-Mokhtar MA, Abdel-Aleem JA, Abu-Dief AM, Al-Hakkani MF, et al. Novel green biosynthesis of 5-fluorouracil chromium nanoparticles using harpullia pendula extract for treatment of colorectal cancer. Pharmaceutics. 2021 Feb;13(2):226.
7. Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Advanced Drug Delivery Reviews. 2015 Aug 30;91:3-6.
8. Fang J, Islam W, Maeda H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Advanced Drug Delivery Reviews. 2020 Jan 1;157:142-60.
9. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine. 2018 May;24(5):541-50.
10. Cheng H, Wang Z, Fu L, Xu T. Macrophage polarization in the development and progression of ovarian cancers: an overview. Frontiers in Oncology. 2019 May 22;9:421.
11. Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WC. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials. 2016 Apr 26;1(5):1-2.
12. Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. Journal of Biological Chemistry. 1977 Jun 10;252(11):3582-6.
13. Guo S, Huang L. Nanoparticles escaping RES and endosome: challenges for siRNA delivery for cancer therapy. Journal of Nanomaterials. 2011 Oct;2011.
14. Salmaso S, Caliceti P. Stealth properties to improve therapeutic efficacy of drug nanocarriers. Journal of Drug Delivery. 2013;2013.
15. Woodle MC. Sterically stabilized liposome therapeutics. Advanced Drug Delivery Reviews. 1995 Sep 1;16(2-3):249-65.
16. Lasic DD, Needham D. The” stealth” liposome: a prototypical biomaterial. Chemical Reviews. 1995 Dec;95(8):2601-28.
17. Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017 Jun;9(2):12
18. Lanza GM, Wickline SA, inventors; Barnes Hospital, assignee. Efficacy and Safety of Targeted Particulate Agents with Decoy Systems. United States Patent Application US 10/588,572. 2008 Aug 14.
19. Tavares AJ, Poon W, Zhang YN, Dai Q, Besla R, Ding D, Ouyang B, et al. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proceedings of the National Academy of Sciences. 2017 Dec 19;114(51):E10871-80.
20. Poon W, Zhang YN, Ouyang B, Kingston BR, Wu JL, Wilhelm S, Chan WC. Elimination pathways of nanoparticles. Acs Nano. 2019 Apr 16;13(5):5785-98.
21. Liu L, Hitchens TK, Ye Q, Wu Y, Barbe B, Prior DE, Li WF, et al. Decreased reticuloendothelial system clearance and increased blood half-life and immune cell labeling for nano-and micron-sized superparamagnetic iron-oxide particles upon pre-treatment with Intralipid. Biochimica et Biophysica Acta (BBA)-General Subjects. 2013 Jun 1;1830(6):3447-53.
22. Liu L, Ye Q, Lu M, Lo YC, Hsu YH, Wei MC, Chen YH, et al. A new approach to reduce toxicities and to improve bioavailabilities of platinum-containing anti-cancer nanodrugs. Scientific Reports. 2015 Jun 3;5(1):1-1.
23. Liu L, Ho C. A New Approach to Decrease the RES Uptake of Nanodrugs by Pre-administration with Intralipid® Resulting in a Reduction of Toxic Side Effects. InIntracellular Delivery III 2016 (pp. 125-146). Springer, Cham.
24. Liu L, Ye Q, Lu M, Chen ST, Tseng HW, Lo YC, Ho C. A new approach to deliver anti-cancer nanodrugs with reduced off-target toxicities and improved efficiency by temporarily blunting the reticuloendothelial system with intralipid. Scientific Reports. 2017 Nov 23;7(1):1-3.
25. Ho C, Liu L, Ye Q, inventors; Carnegie Mellon University, assignee. Methods to reduce toxicities and to improve bioavailabilities of nanodrugs. United States Patent US 10,792,366. 2020 Oct 6.
26. Chen YJ, Tsai CY, Cheng YM, Nieh SW, Yeh TK, Chen CP, Wang MH, et al. Impacts of intralipid on nanodrug abraxane therapy and on the innate immune system. Scientific Reports. 2020 Feb 18;10(1):1-1.
27. Islam R, Gao S, Islam W, Šubr V, Zhou JR, Yokomizo K, Etrych T, et al. Unraveling the role of Intralipid in suppressing off-target delivery and augmenting the therapeutic effects of anticancer nanomedicines. Acta Biomaterialia. 2021 May 1;126:372-83.
28. Maeda H. The link between infection and cancer: tumor vasculature, free radicals, and drug delivery to tumors via the EPR effect. Cancer Science. 2013 Jul;104(7):779-89.
29. Arrieta O, Medina LA, Estrada-Lobato E, Hernández- Pedro N, Villanueva-Rodríguez G, Martínez-Barrera L, Macedo EO, et al. First-line chemotherapy with liposomal doxorubicin plus cisplatin for patients with advanced malignant pleural mesothelioma: phase II trial. British Journal of Cancer. 2012 Mar;106(6):1027-32.
30. Koukourakis MI, Koukouraki S, Fezoulidis IA, Kelekis N, Kyrias G, Archimandritis S, Karkavitsas N. High intratumoural accumulation of stealth® liposomal doxorubicin (Caelyx®) in glioblastomas and in metastatic brain tumours. British Journal of Cancer. 2000 Nov;83(10):1281-6.
31. Koukourakis Sofia Koukouraki, Alexandra Giatromanolaki, Stelios Kakolyris, Vassilis Georgoulias, Antigoni Velidaki, Spyridon Archimandritis and Nikolaos N. Karkavitsas MI. High intratumoral accumulation of stealth liposomal doxorubicin in sarcomas: rationale for combination with radiotherapy. Acta Oncologica. 2000 Jan 1;39(2):207-11.
32. Miller MA, Arlauckas S, Weissleder R. Prediction of anti-cancer nanotherapy efficacy by imaging. Nanotheranostics. 2017;1(3):296.
33. Chen H, Zhang W, Zhu G, Xie J, Chen X. Rethinking cancer nanotheranostics. Nature Reviews Materials. 2017 May 9;2(7):1-8.
34. Miller MA, Gadde S, Pfirschke C, Engblom C, Sprachman MM, Kohler RH, Yang KS, et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Science Translational Medicine. 2015 Nov 18;7(314):314ra183-.
35. Ramanathan RK, Korn RL, Raghunand N, Sachdev JC, Newbold RG, Jameson G, Fetterly GJ, et al. Correlation between ferumoxytol uptake in tumor lesions by MRI and response to nanoliposomal irinotecan in patients with advanced solid tumors: a pilot study. Clinical Cancer Research. 2017 Jul 15;23(14):3638-48.
36. Kessler U, Poeschl J, Raz D, Linderkamp O, Bauer J. Effects of intralipid infusion on blood viscosity and other haemorheological parameters in neonates and children. Acta Paediatrica. 2004 Aug;93(8):1058-62.
37. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Tagawa ST, Panageas KS, DeAngelis LM. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood, The Journal of the American Society of Hematology. 2019 Feb 21;133(8):781-9.
38. Wu YL, Liu L, Yeh FC, Rosario BL, Ho C. MRI Investigation of New Approach to Improve the Recovery of Myocardial Ischemia Reperfusion Injury by Treatment with Intralipid®. World Journal of Cardiovascular Diseases. 2016 Oct 10;6(10):352-71.
39. Ho C, Liu L, Wu Y, Hitchens TK, Ye Q, inventors; Carnegie Mellon University, assignee. Methods and materials for reducing organ transplant rejection or ischemic/reperfusion injury in a subject. United States patent US 9,795,621. 2017 Oct 24.
40. Prieur X, Mok CY, Velagapudi VR, Núñez V, Fuentes L, Montaner D, Ishikawa K, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011 Mar 1;60(3):797-809.
41. Ohue-Kitano R, Yasuoka Y, Goto T, Kitamura N, Park SB, Kishino S, Kimura I, et al. a-Linolenic acidderived metabolites from gut lactic acid bacteria induce differentiation of anti-inflammatory M2 macrophages through G protein-coupled receptor 40. The FASEB Journal. 2018 Jan;32(1):304-18.
42. Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, et al. Hematopoietic cellspecific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metabolism. 2009 Nov 4;10(5):419-29.