Abstract
Hypoxia-inducible factor 1-alpha (HIF-1α) plays a critical role in regulating cellular responses to low oxygen levels. In our study, we transfected HIF-1α mRNA into human dermal fibroblasts and assessed its biological activity by measuring the upregulation of downstream angiogenic genes, aiming to investigate the delivery mechanism of HIF-1α mRNA and its functional impact. In this short communication, we will describe our transfection methodology, present the results we obtained, and discuss the potential implications of these findings for future therapeutic applications.
Keywords
HIF-1a, mRNA transfection, Pedicle flap, Angiogenic genes
Introduction
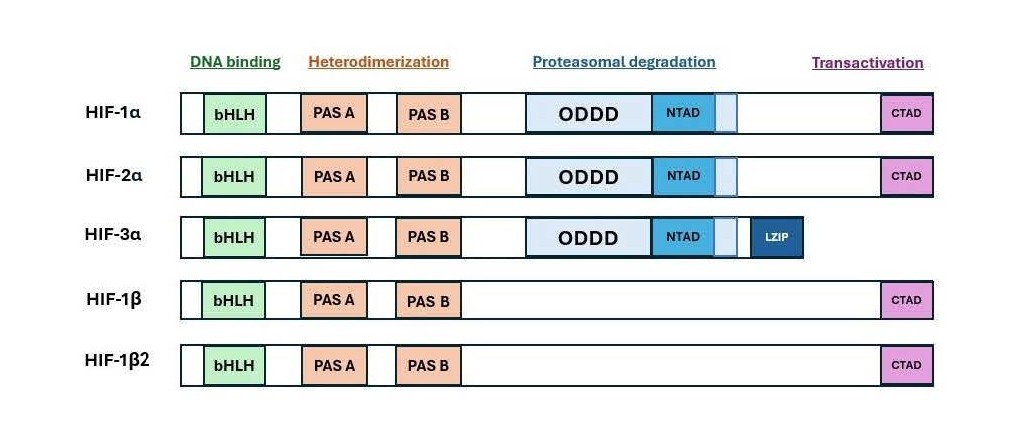
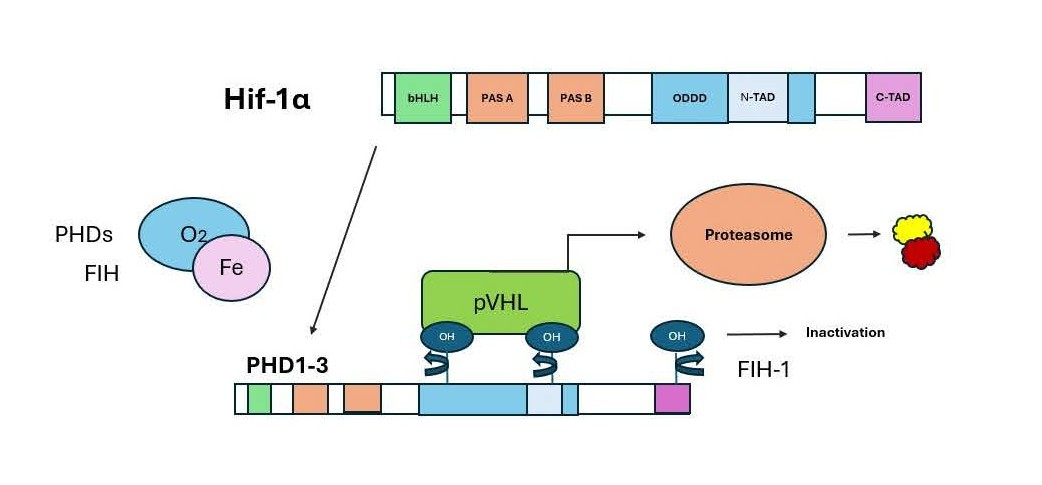
Hypoxia-inducible factor 1 (HIF-1α) is a subunit of a heterodimeric transcription factor (Figure 1), which coordinates the response of the cells to hypoxia and thereby promotes cell survival (Figures 2 and 3) [1,2].
Figure 1. Domain structures of HIF-1α protein in comparison with other HIF proteins and their potential functions.
Figure 2. Regulation of HIF-1α protein in normoxia.
Figure 3. Regulation of HIF-1α protein in hypoxia.
HIF-1α controls the expression of over 200 genes that encode angiogenic growth factors, such as platelet-derived growth factor B (PDGF-B), stromal cell-derived factor-1 (SDF-1), angiopoietins (ANGPT1, ANGPT2), vascular endothelial growth factor (VEGF), and placental growth factor (PGF). It has been shown that HIF-1α plays a role in the maintenance of natural killer cells as well as other cells of innate immunity. These factors lead to vascularization, angiogenesis, metabolism, and cell survival. The possible function of HIF-1α in wound healing and the ischemic preconditioning of surgical flaps has been investigated in a number of studies [3-5]. In a recent animal model study, we demonstrated that preconditioning surgical pedicle flaps with a DNA plasmid expressing hypoxia-inducible factor-1α (HIF-1α) promotes angiogenesis in the skin, thereby reducing ischemia and necrosis in the skin flaps [6].
Previous experiments with DNA plasmid delivery faced two major concerns. First, for successful genome modification, the plasmid must be transferred into the cell nucleus, where it can integrate into the genome. However, this process carries the risk of tumorigenicity or other potential adverse effects, as it could lead to unwanted genetic alterations or disruptions.
Second, DNA expression plasmids often use a constitutive promoter to continuously express the encoded gene, resulting in a permanent integration of the plasmid into the genome. This prolonged expression could lead to long-term, unintended effects due to the persistent genetic modification. Expressing HIF-1α using HIF-1α mRNA transcripts can prevent these two issues. First, the mRNA translates in the cytoplasm and does not enter the nucleus. Second, unlike DNA integration, mRNA transfection is transient and degrades in the cytoplasm through defined cellular mechanisms. This degradation process ensures that the effects are not permanent, unlike the lasting changes that can occur with genomic DNA modification.
This short communication explains how HIF-1α is expressed in primary human dermal fibroblast cells using mRNA transfection. We worked with predominant full-length HIF-1α form transcripts, variant isoforms, and mutated isoforms. Intracellular HIF-1α transcript levels for all transfected HIF-1α isoforms increased dramatically over the course of three hours and remained continuously high for at least ten hours. All downstream angiogenic genes reached their highest response six hours following the initial HIF-1α transfection. The application of mRNA transcripts may result in a treatment that may be made quickly and affordably. The production and administration of physiologically active HIF-1α may be a potent treatment to increase the longevity of surgical pedicle flaps.
Methods
DNA PCR primers correspond to the 5’ and 3’ ends of the HIF-1α transcript. Full-length HIF-1α form (P1) transcripts with a 3' poly-A tail and a 5' methyl cap were produced. We worked with predominant isoform (P1), the three variant isoforms (V1, V2, V3), and the two mutated isoforms of P1 (P2, P3). P1 is the full-length predominant version of HIF-1α. Two proline residues that are known to contribute to degradation were removed from the predominant isoform (P1) in order to raise the levels of the HIF-1α response gene. Oligo primers were created by using PCR amplification, and a circular plasmid was produced by in vitro DNA recombination.
The American Type Culture Collection (ATCC, Manassas, VA) provided the primary human dermal fibroblasts, which were then cultivated in a fibroblast growth medium.
The HIF-1α mRNA transcripts were transfected into fibroblasts. A comprehensive set of tests was conducted to ascertain the optimal lipofectamine reagent dosage and the different concentrations of HIF-1α mRNA needed for the greatest increase in response gene mRNA levels. Ten thousand primary dermal fibroblasts were seeded into each well of a 96-well cell culture plate. Using the relevant mRNA and lipofectamine in each well, the transfection was carried out in 24 hours. After transfection, the cells were taken three, six, and ten hours later, and total RNA was extracted.
Following cell lysis and RNA extraction, response gene expression levels were assessed using real-time quantitative RT-PCR with specified gene-specific primers. The responder genes were vascular endothelial growth factor (VEGF), platelet-derived growth factor B (PDGF-B), angiopoietin 1 (ANGPT1), FMS-related receptor tyrosine kinase 1 (FLT1), and endothelin 1 (EDN1). All particular gene quantitative values were standardized to the expression level of the housekeeping gene β-actin to guarantee reliable comparisons between samples.
The Student's t-test was employed to compare the variations in gene expression levels among distinct isoforms. For all statistical tests, the significance threshold (alpha) was set at 0.05, and the figures indicate p-values that are less than 0.05, 0.01, or 0.001.
Results
Transfection analysis
The expression levels of several angiogenesis-related genes changed when HIF-1α mRNA was transfected into primary dermal fibroblasts.
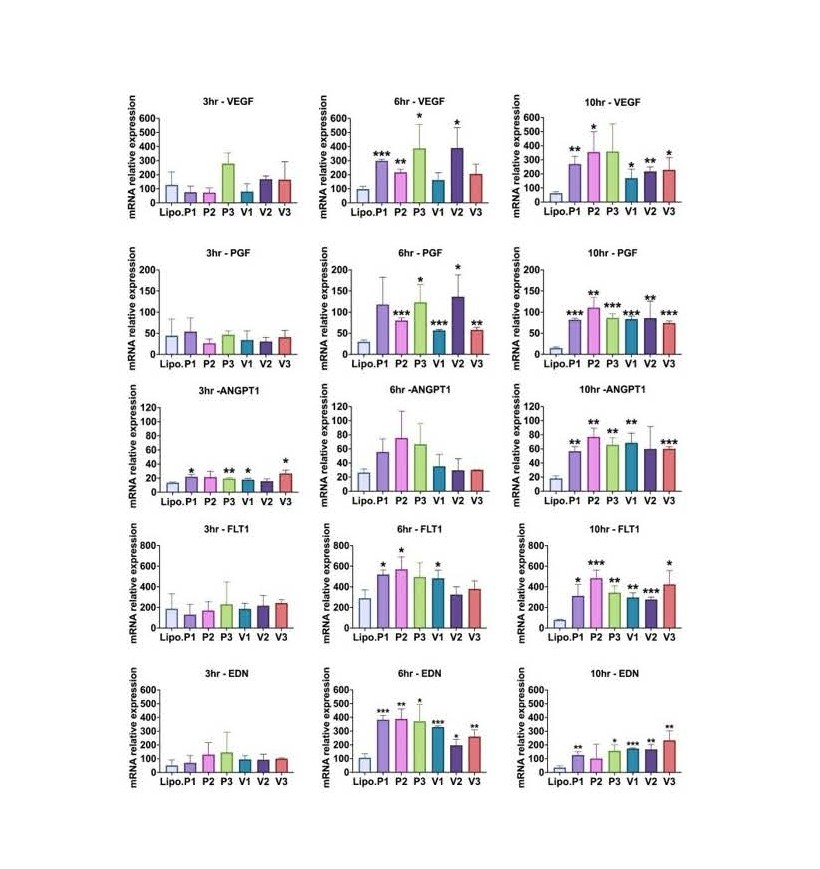
At every time point analyzed, HIF-1α mRNA levels significantly increased in comparison to the control group, which received Lipofectamine alone (all p<0.05). All groups treated with the isoforms and mutant isoforms exhibited a substantial increase in VEGF transcript levels at 6 and 10 h post-transfection as compared to the control group. For PGF mRNA levels, When compared to the control group, all isoform-treated groups exhibited a highly significant increase at 10 hours post-transfection (all p<0.05). All treatment groups, with the exception of isoform V2, had significantly higher ANGPT1 transcripts at 10 hours after transfection than the control group (all p<0.05). FLT1 mRNA was significantly higher in all treatment groups than in the control group at 10 hours after transfection (all p<0.05). When compared to the control group, all groups treated with the isoforms exhibited a significant rise in EDN1 mRNA levels 6 hours post-transfection (all p<0.05). For all transfected HIF-1α isoforms, intracellular HIF-1α transcript levels grew significantly over the course of three hours and stayed consistently high for at least ten hours. The response of all downstream angiogenic genes typically peaked six hours after the first HIF-1α transfection (Figure 4).
Figure 4. HIF-1α mRNAs (P1, P2, P3. V1, V2, V3) were transfected into primary dermal fibroblasts. At 3, 6, or 10 h, total RNA was extracted and assayed for VEGF, PGF, ANGPT1, FLT-1, or EDN1 expression levels by qRT-PCR. Values were normalized to beta-actin levels. “Lipo.” is lipofectamine alone control. The Student’s T-test was used to determine significance, and the mean and standard deviation of independent triplicate wells is shown. *p = 0.05 or lower, **p = 0.01 or lower, and ***p = 0.001 or lower [7].
Discussion
Researchers have been looking for a workable and efficient way to increase the longevity of surgical pedicle flaps for decades [7]. Numerous non-invasive options have been investigated, with varying degrees of success. Fibroblasts, EGF (Epidermal Growth Factor), PDGF (platelet-derived growth factor), TGF-β (Transforming Growth Factor-beta), ANGPT1 (Angiopoietin-1), and other growth factors transfected with modified mRNA have recently been applied individually to promote overall wound repair. These findings, however, lacked statistical significance. By using a master gene at the start of the angiogenesis cascade, we prefer a continuous generation of angiogenic products as opposed to those methods that only express one downstream gene. This may be a far more potent and successful strategy since it coordinates variations in gene expression in a sequence with kinetics that are biologically meaningful. A master regulator of the adaptive response to hypoxia is Hypoxia-Inducible Factor-1α (HIF-1α). HIF-1α has the potential to be a promising therapeutic for ischemic preconditioning of surgical flaps because it controls the expression of hundreds of growth factors, including VEGF (Vascular Endothelial Growth Factor), PGF (Placental Growth Factor), ANGPT1 (Angiopoietin-1), ANGPT2 (Angiopoietin-2), SDF-1 (Stromal Cell-derived Factor 1), PDGF-B (platelet-derived growth factor B), and others, as well as different types of cytokines and enzymes that support tissue viability under ischemic conditions [1,2]. Systemic administration of dimethyloxalylglycine (DMOG) increases HIF-1α levels, which in turn promotes tissue survival in a mouse model [5]. This lays the groundwork for subsequent research that examined the effectiveness of delivering HIF-1α using a DNA expression plasmid vector [6]. A steady and localized delivery, occasionally facilitated by plasmid transduction techniques like electroporation, would be feasible for the clinical implementation of the therapy, according to our own past experience with DNA plasmid delivery [4,5]. Our team demonstrated that electroporation after intradermal injection of the HIF-1a plasmid in injured diabetic mice led to elevated levels of VEGF, PGF, PDGF-B, and ANGPT2 mRNA on day 7 post-injection and elevated levels of HIF-1α mRNA at the injection site on day 3 [4]. While DNA expression plasmids address the short half-life of angiogenic proteins, they have the potential to permanently express the encoded gene by integrating into the nuclear DNA of the host cell, which could be detrimental. In order to get around this restriction, we developed a technique that might introduce physiologically active HIF-1α mRNA into the wound area by following therapeutic mRNA transfer methods now employed in COVID-19 and cancer vaccines. Transfected HIF-1a mRNA does not reach the nucleus, enabling rapid translation and a steady transcript reduction over time [8,9]. Many of mRNA's drawbacks, such as its intrinsic low stability and potential immunogenicity, have been addressed by the development of in vitro transcription (IVT) and mRNA chemical modification, all while maintaining translation efficiency. Our findings at six and ten hours after transfection revealed a quick, controlled, and temporary production of HIF-1a response genes when we introduced each of the six HIF-1a mRNA isoforms into dermal fibroblasts. Lipid nanoparticles have been effectively used to carry mRNA, siRNA medications, and small compounds. These lipid-based carriers have the ability to facilitate the delivery of synthesized mRNA to the target cells and improve its stability in vivo [8,9]. We transfected our therapeutic HIF-1a mRNA isoforms into primary human dermal fibroblast cells using lipofectamine, a liposomal cationic lipid-based carrier.
Conclusion
Our findings demonstrate that all six HIF-1α mRNA isoforms were successfully transfected into human primary fibroblast cells. Consequently, we saw the quick and controlled overexpression of all five downstream angiogenic targets that were examined. The HIF-1α mRNA might be locally injected into and around the reconstruction site in future surgical scenarios. Further research will contrast the transfection effectiveness of our lipofectamine-formulated therapeutic composition of HIF-1α mRNA, which has already been tested in vitro, with a novel delivery method that uses polypeptide lipid nanoparticles (PLNP).
Acknowledgements
Research gift account of Dr. John W. Harmon.
Funding
This experiment was funded by Johns Hopkins Surgical Gift Fund (JWH).
Author Contributions
Conception and design: J.M.A., J.W.H. Financial support: J.W.H. Provision of study materials: J.M.A. Collection and assembly of data: J.M.A., J.W.H., M.F.I.C, F.M, A.Y. Data analysis and interpretation: All Authors. Manuscript writing: M.F.I.C, F.M, A.Y. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
2. Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012 Feb 3;148(3):399-408.
3. Semenza GL. Regulation of tissue perfusion in mammals by hypoxia-inducible factor 1. Exp Physiol. 2007 Nov;92(6):988-91.
4. Liu L, Marti GP, Wei X, Zhang X, Zhang H, Liu YV, et al. Age-dependent impairment of HIF-1alpha expression in diabetic mice: Correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J Cell Physiol. 2008 Nov;217(2):319-27.
5. Du J, Liu L, Lay F, Wang Q, Dou C, Zhang X, et al. Combination of HIF-1α gene transfection and HIF-1-activated bone marrow-derived angiogenic cell infusion improves burn wound healing in aged mice. Gene Ther. 2013 Nov;20(11):1070-6.
6. Chang KH, Shoureshi P, Lay F, Sebastian R, Alikhassy Habibabady Z, Born LJ, et al. Preconditioning of surgical pedicle flaps with DNA plasmid expressing hypoxia-inducible factor-1α (HIF-1α) promotes tissue viability. Gene Ther. 2021 Jun;28(6):319-28.
7. Wlodarczyk J, Leng A, Abadchi SN, Shababi N, Mokhtari-Esbuie F, Gheshlaghi S, et al. Transfection of hypoxia-inducible factor-1α mRNA upregulates the expression of genes encoding angiogenic growth factors. Sci Rep. 2024 Mar 20;14(1):6738.
8. Karikó K. Modified uridines are the key to a successful message. Nat Rev Immunol. 2021 Oct;21(10):619.
9. Karikó K, Muramatsu H, Ludwig J, Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011 Nov;39(21):e142.