Abstract
Background: Endemic Burkitt lymphoma has been associated with the Epstein Barr Virus (EBV), particularly in malaria-endemic regions. Primary ovarian Burkitt is an infrequent entity of this disease, and its diagnosis still poses a challenge.
Summary: We present two cases. The first case is that of a 23-year-old female G1P1 who presented with a two-week history of abdominal pain and progressive distension. Her examination was remarkable for two pelvic masses and signs of ascites. An ultrasound scan showed bilateral solid adnexal masses, ascites, and mild splenomegaly. Tumor markers for ovarian and germ cell tumors were within the normal ranges. Cytology on ascitic fluid was negative for malignancy. Lactate dehydrogenase (LDH) level and further imaging were not done due to financial constraints. With the working diagnosis of an ovarian epithelial malignancy versus drop metastases, she underwent exploratory laparotomy, total abdominal hysterectomy, bilateral salpingo-oophorectomy, appendectomy, omentectomy, and debulking of peritoneal implants. Histopathology two weeks after surgery showed Burkitt lymphoma. The second case is that of a 12-year old female whose management averted surgery, following the recommendations made from our first case.
Conclusion: Lymphomas are amongst the most common tumors in the pediatric age group. Clinicians should maintain a high index of suspicion in the face of fast-growing tumors. Burkitt lymphomas respond well to chemotherapy, and surgery is seldom necessary except for life-threatening presentations. Management of oncologic cases that are not straightforward should follow a multidisciplinary approach.
Keywords
Ovary, Burkitt lymphoma, Diagnosis
Background
Burkitt lymphoma (BL) is a malignant neoplasm stemming from a chromosomal translocation. The MYC gene on chromosome8 is translocated (most commonly) to chromosome 14, [t(8;14)]. The result is promotion of tumorigenesis. Less common translocations include [t(2;8)] and [t(8;22)]. This rare B cell non-Hodgkin lymphoma is very aggressive, with a doubling time of 24 hours [1]. The described subtypes include: endemic, sporadic, and related to immunosuppression. The endemic subtype is associated with Ebstein-Barr virus (EBV), particularly in malaria-endemic areas. Jaw masses occur in 50-60% of cases. Extranodal spread usually involves the kidney, testis, ovary, breast, meninges, or mesentery. Abdominal involvement is less common. In the sporadic subtype, abdominal swellings are more common, usually presenting with massive disease and ascites involving the ileum, stomach, caecum, mesentery, kidneys, breast, bone marrow, and CNS. The immunodeficiency variant relates to HIV/AIDS, prolonged immunosuppression after organ transplantation, and other acquired or congenital immune deficiency conditions. It usually involves lymph nodes, CNS, and bone marrow [2].
Case 1 Presentation
We present a 23-year-old G1P1001, married, mother of a three-year-old. She has no significant past medical history or allergies and was not taking any routine medication. She presented with a 2-week history of progressive abdominal distension associated with tolerable generalized abdominal pain, early satiety, and bloating. She denied anorexia, nausea, or vomiting, weight loss, change in bowel habits, urinary symptoms, or fever. On admission, she looked acutely ill, with a temperature of 36.2°C (which went up to 37.9°C on the ward), a pulse of 54 bpm, BP of 127/78 mmHg, O2 saturation of 98% on room air, and a respiratory rate of 18 bpm. She was neither pale or jaundiced and had no identifiable lymphadenopathy. Her abdomen was uniformly distended, with bilateral lower abdominal scarifications from traditional medicine. She had two palpable smooth, mobile, non-tender pelvic masses and clinical signs of ascites. Both vaginal and rectal exams were unremarkable except for an extraluminal mass compressing the anterior walls.
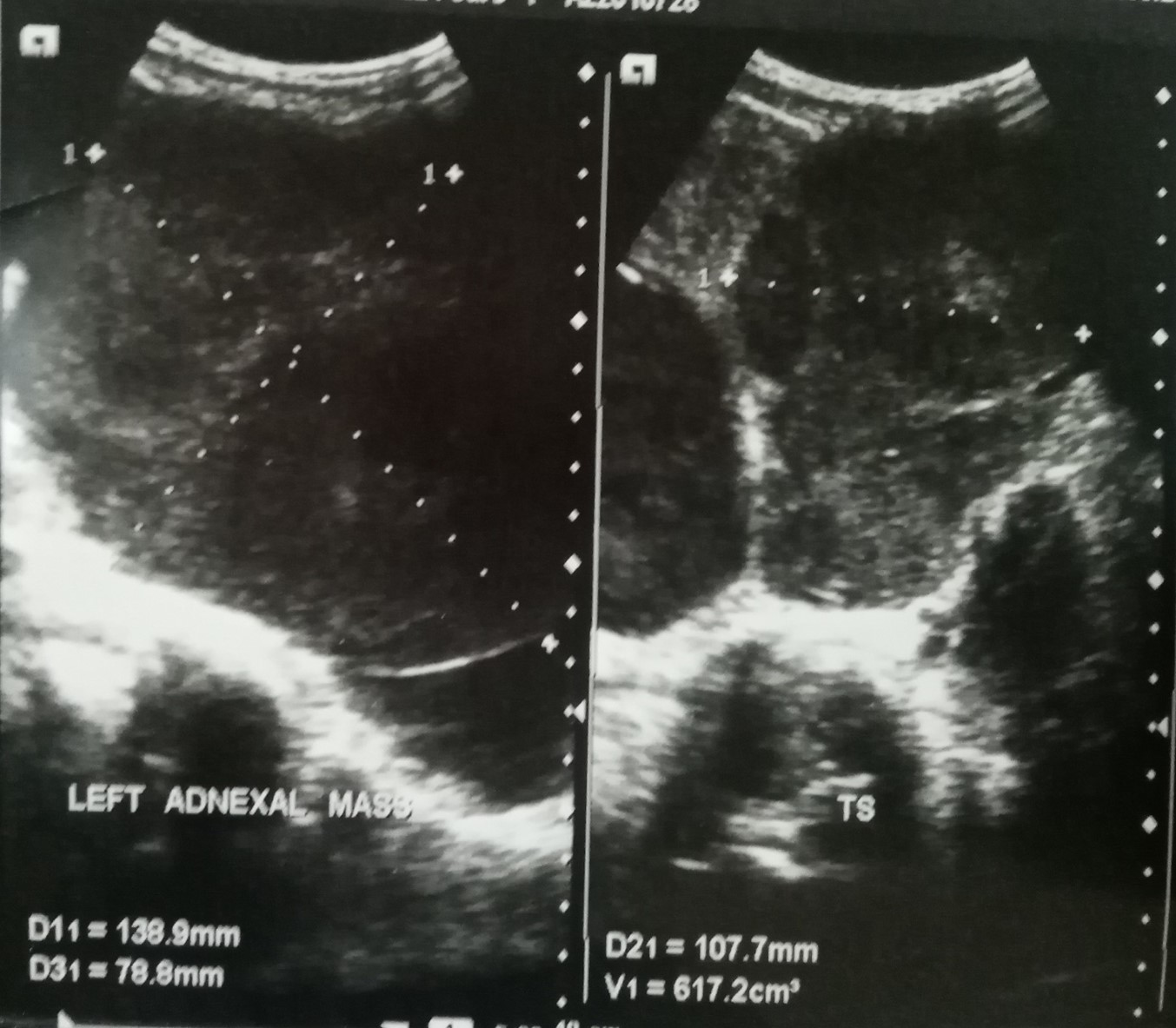
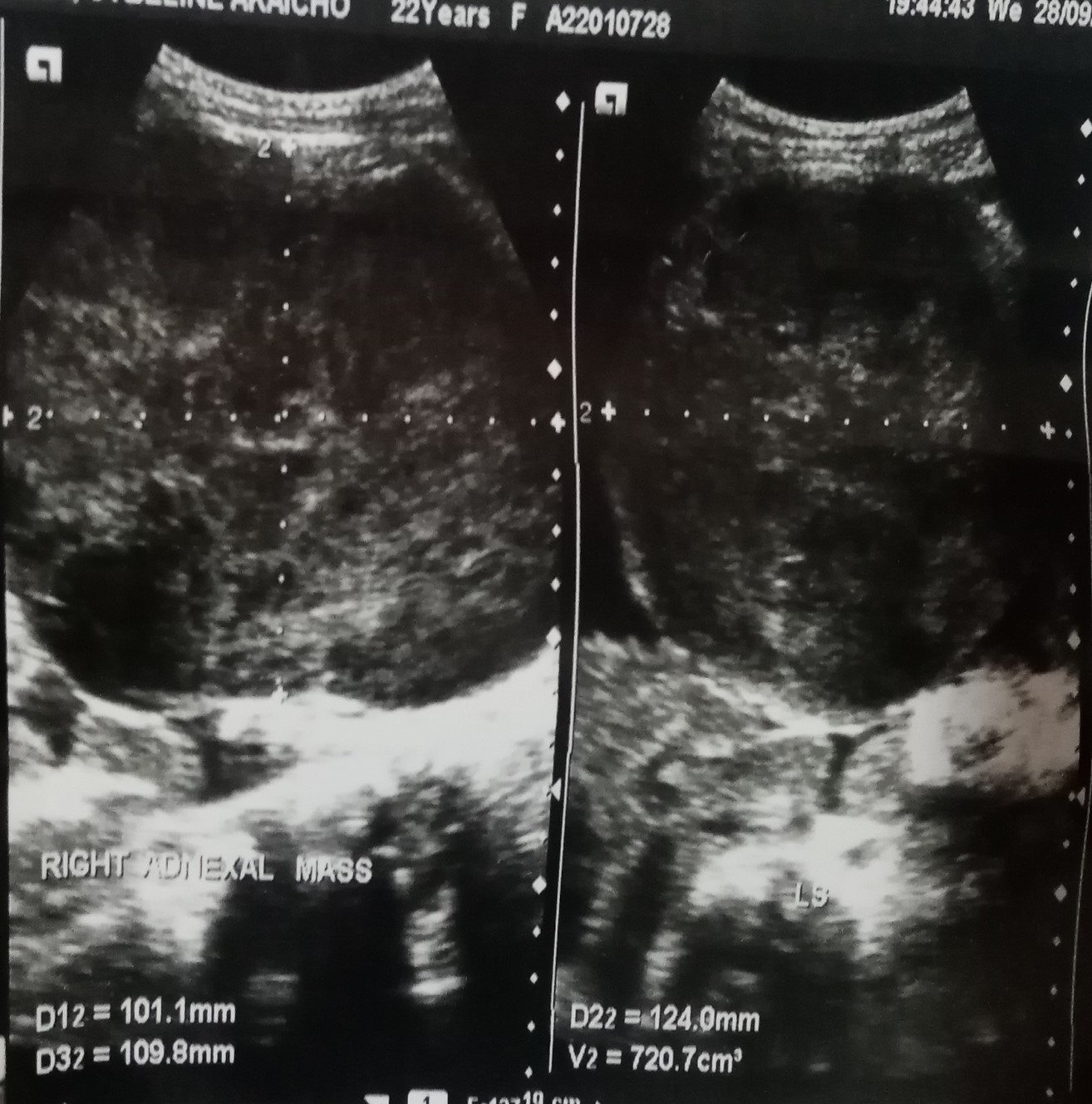
Her ultrasound scan showed huge bilateral adnexal solid masses, measuring 13.9 cm x 7.9 cm x 10.8 cm on the left (Figure 1a), 10.1 cm x 11.0 cm x 12.4 cm on the right (Figure 1b), moderate ascites, and mild splenomegaly. There was no evidence of liver metastases on the ultrasound scan. Tumor markers include βHcg; 0.100miu/ml, AFP; 0.5IU/ml(NV: <5.8IU/ml), CA-125: 6.13U/ml, all within the normal ranges for our lab. WBC 6,020/ml, Hb 12.9, Platelets 228000/ul. LFTs: ALP;37U/L, AST; 33.4U/L, ALT; 17.4U/L, Alb;3.65g/dl, Bilirubin; 0.8mg/dl. Urea/creat; 24.02/0.8(mg/dl). Na/K/Cl; 142/3.4/105/9.35(mmol/L), still within normal ranges. Cytology on ascitic fluid was negative for malignancy. Our working diagnosis was bilateral ovarian cancer with the possibility of metastases from a gastrointestinal origin. She could not afford further laboratory workup or other investigations (gastroscopy, colonoscopy, or CT scanning).
Figure 1a. Left ovarian solid mass. Source: Ultrasound Scan done at the Mbingo Baptist Hospital on 28/09/22.
Figure 1b. Right ovarian solid mass. Source: Ultrasound Scan done at the Mbingo Baptist Hospital on 28/09/22.
She underwent abdominal exploration through a midline incision with the findings of four liters of bloody ascites, bilateral ovarian solid masses adherent to the retroperitoneum, and direct extension into the uterus on the right. Implants were noted on the mesentery of the small bowel and a larger implant at the hepatic flexure of the colon with direct extension into segment 6 of the liver. No apparent gastrointestinal origin for the malignancy was identified. Therefore, believing the ovaries were the primary site of the tumor, we proceeded with bilateral salpingo-oophorectomy, total abdominal hysterectomy, appendectomy, omentectomy, and an R2 resection (debulking) of the tumor implant at the hepatic flexure. Postoperatively, she reaccumulated mild ascites but otherwise did well and was discharged home on postoperative day five to return in two weeks for her histopathology results.
Histopathology reported round cell stromal infiltrate in the cervix and no dysplasia. The endometrium was inactive, and the myometrium was unremarkable. Except for a small remnant of corpus luteum in one section, normal ovarian tissue was replaced by a monomorphic, discohesive, small round cell neoplasm with numerous tingible body macrophages giving a "starry sky" appearance at low power. The tumor cells had indiscernible cytoplasm, an immature chromatin pattern, and one or more nucleoli. The peritoneal implants were composed of the same tumor. The omentum had extensive chronic inflammation, but no malignancy was identified, and the appendix was free of tumor. In summary, the uterus, both ovaries, and the peritoneal implants revealed an aggressive non-Hodgkin lymphoma in favor of Burkitt lymphoma. The appendix and omentum were negative for malignancy.
On her review visit three weeks later, she was doing very well. She had no complaints, and her surgical site had healed, even though she had mild ascites. The implications of her histologic diagnosis were explained, including that chemotherapy would have been her primary mode of treatment had we known her diagnosis preoperatively. She was referred to the oncology service and received one cycle of a debulking COP regimen (cyclophosphamide, Vincristine, and prednisone) at the time of her first postoperative review. Unfortunately, the patient has defaulted on further treatment despite multiple attempts to have her follow up.
Case 2
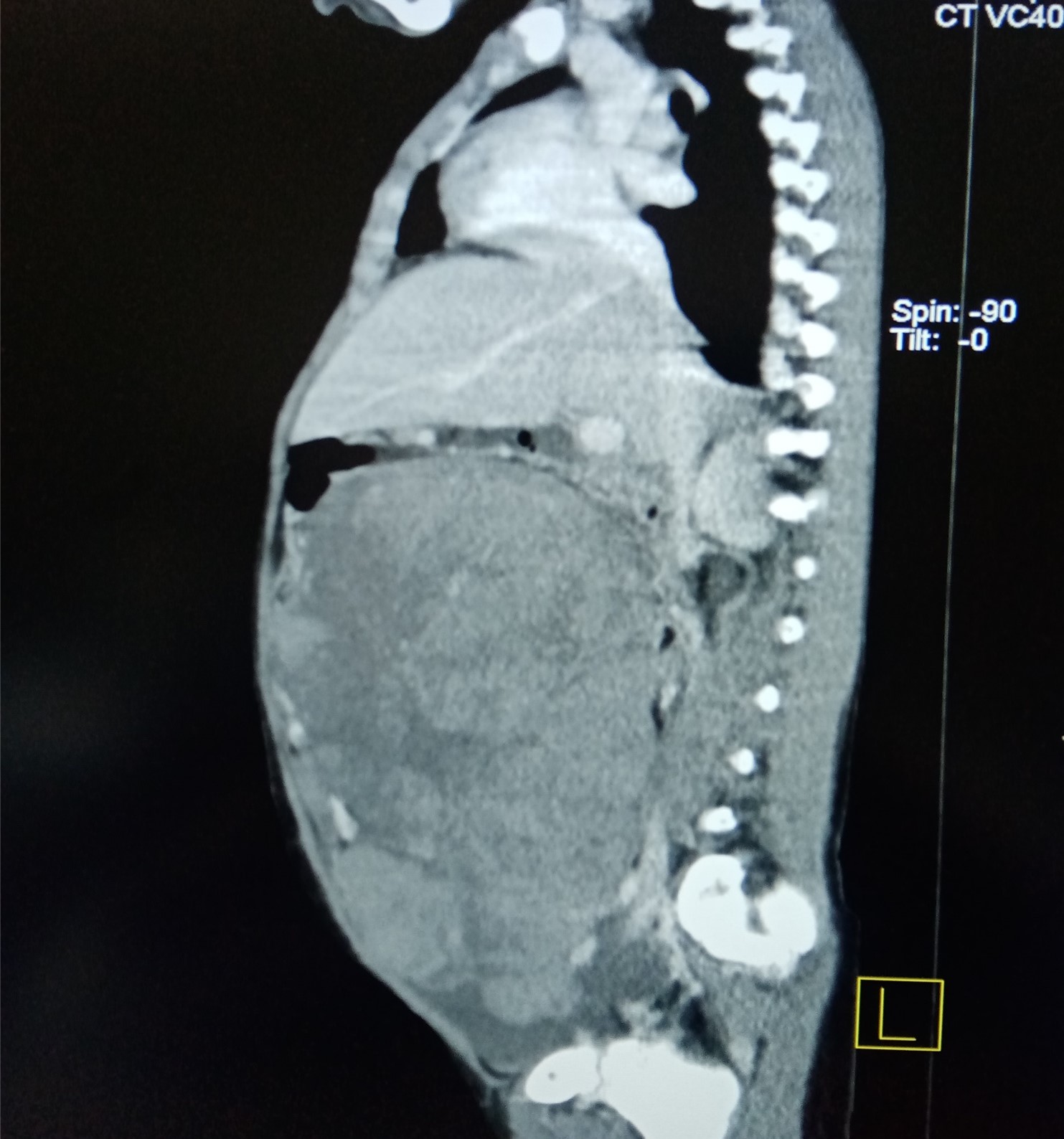
A 12 year old female with six months history of vague abdominal pain and three-week history of progressive abdominal distension associated with nausea, vomiting, early satiety, weight loss, and urinary frequency. On physical examination, she was chronic ill looking, tarchycardic at 124 bpm, tarchypneic at 40 bpm, saturating at 97% on room air and had a temperature of 37.8°C. She had multiple 1-1.5 cm non- tender cervical lymph nodes, <0.5 cm axillary nodes, and no palpable groin nodes. Her abdomen was grossly distended with a firm, smooth mildly tender pelvic mass extending into the left upper quadrant. A urine pregnancy test was negative, her AFP was 2.96 IU/ml and serum uric acid levels were within the normal limits. Her complete blood count was remarkable for a moderate microcytic hypochromic anaemia at 8.0 gm/dl. Her metabolic profile was remarkable for a mildly elevated AST at 72.7U/L(NV 0.1-37U/L). Her peripheral blood smear confirmed the anaemia as described above but was negative for evidence of malignancy. A CT scan showed a solid abdominopelvic mass arising from left ovary, measuring 16x12x24 cm (Figure 2) with necrotic components and mild enhancement. A fine needle aspiration (FNA) of the mass showed atypical lymphoid cells. The surgical service was consulted for a right oophorectomy. However, from our experience with case 1, a core biopsy was done before considering other invasive surgical options. Touch imprints on the cores revealed an aggressive non-Hodgkin lymphoma in favour of Burkitt Lymphoma. Chemotherapy was started as a primary mode of therapy without any further surgery. Histopathology result gotten 3 weeks after touch imprints and chemotherapy commencement was consistent with touch findings of BL.
Figure 2. Solid mass with areas of necrosis. Source: Computed Tomography CT Scan done at the Mbingo Baptist Hospital on 15/01/2023.
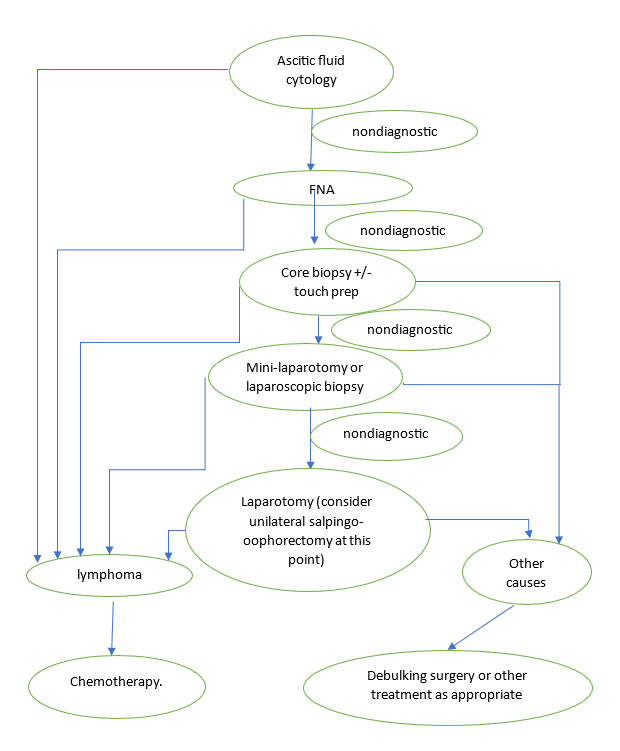
Figure 3. Algorithm for Diagnosis.
Discussion
Most cases of ovarian Burkitt lymphoma are believed to disseminate from another primary site. However, primary ovarian Burkitt lymphoma, defined as the absence of other organ system manifestations of the disease, is very rare, constituting 0.5% of non-Hodgkins lymphomas and 1.5% of all ovarian tumors [3]. As reported by Stepniak in the systemic review of 21 published cases of primary ovarian Burkitt lymphoma, 60% of these cases underwent surgery before a diagnosis was made. One could conclude that diagnosing primary ovarian Burkitt lymphoma still poses a big challenge. Some of these tumors mimic gynecologic malignancies and do not present with typical B symptoms. There has been some talk about using frozen sections intraoperatively to aid the diagnosis, but frozen sections of Burkitt tumors can be easily mistaken for sex cord tumors [4,5]. Lu et al. advocate fine needle aspiration in the diagnostic workup if Burkitt lymphoma is suspected [4]. Also, considering the rapid growth of Burkitt lymphoma, serum levels of LDH and uric acid are usually elevated [3,6], and Some patients present with tumor lysis syndrome [7-9].
Burkitt lymphoma is very chemosensitive, and debulking surgery, per se, is not indicated. However, surgery is indicated for life-threatening complications arising from the disease process, like pericardial effusion with tamponade, bowel obstruction, massive gastrointestinal bleeding, or airway obstruction [10,11]. Although Burkitt lymphoma is chemosensitive, intensive chemotherapy of ovarian Burkitt may result in infertility [3].
Conclusion
The clinician should maintain a high index of suspicion for Burkitt lymphoma when presented with a rapidly growing ovarian tumor in a child or young adult without elevated germ cell tumor markers. An elevated serum uric acid and LDH and an FNA of the mass may confirm the diagnosis preoperatively and avert unnecessary surgery. Frozen sections can be misleading and should be avoided. An important conclusion of our case is that the management of oncologic patients with no life-threatening emergencies should follow a multidisciplinary approach before considering surgery.
We propose the following algorithm in the investigation and management of rapidly growing tumor marker-negative solid ovarian mass in a female of childbearing age in an attempt to avoid unnecessary surgery and preserve fertility.
References
2. Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood. 2004 Nov 15;104(10):3009-20.
3. Stepniak A, Czuczwar P, Szkodziak P, Wozniakowska E, Wozniak S, Paszkowski T. Primary ovarian Burkitt's lymphoma: a rare oncological problem in gynaecology: a review of literature. Archives of Gynecology and Obstetrics. 2017 Oct;296:653-60.
4. Lu SC, Shen WL, Cheng YM, Chou CY, Kuo PL. Burkitt's lymphoma mimicking a primary gynecologic tumor. Taiwanese Journal of Obstetrics and Gynecology. 2006 Jun 1;45(2):162-6.
5. Shacham-Abulafia A, Nagar R, Eitan R, Levavi H, Sabah G, Vidal L, et al. Burkitt's lymphoma of the ovary: case report and review of the literature. Acta Haematologica. 2013;129(3):169-74.
6. Manganaro L, Bernardo S, Sergi ME, Sollazzo P, Vinci V, De Grazia A, et al. Burkitt's lymphoma presented as advanced ovarian cancer without evidence of lymphadenopathy: CT and MRI findings. Case Reports in Radiology. 2013 Mar 30;2013:940160.
7. Gentzler MD, Ryan D. A Case of Acute Spontaneous Tumor Lysis Syndrome and New Diagnosis of Burkitt's Lymphoma. The Medicine Forum. 2009;11(1):10.
8. Opyrchal M, Figanbaum T, Ghosh A, Rajkumar V, Caples S. Spontaneous tumor lysis syndrome in the setting of B-cell lymphoma. Case Reports in Medicine. 2010 Mar 10;2010:e610969.
9. Senbanjo IO. Tumor lysis and acute renal failure in Burkitt's lymphoma: A review on pathophysiology and management. Indian Journal of Nephrology. 2009 Jul;19(3):83-6.
10. Kemeny MM, Magrath IT, Brennan MF. The role of surgery in the management of American Burkitt's lymphoma and its treatment. Annals of Surgery. 1982 Jul;196(1):82-6.
11. Shamberger RC, Weinstein HJ. The role of surgery in abdominal Burkitt's lymphoma. J Pediatr Surg. 1992 Feb;27(2):236-40.