Abstract
Objectives: Multiple myeloma (MM), a neoplastic proliferation of plasma cells, is a common diagnosis among hematologic malignancies. Leukocytosis, specifically neutrophilia, is also very common, typically as a secondary abnormality. However, having both multiple myeloma and neutrophilia simultaneously is rare and could arise by two distinct pathways: either by separate, but concurrent, clonal neoplasm of neutrophils (e.g chronic neutrophilic leukemia) or by a paraneoplastic-type process thought to be secondary to plasma cell stimulation of granulocyte colony-stimulating factor (G-CSF). Distinguishing between the two determines treatment and prognosis.
Methods: A retrospective evaluation of two cases of MM with neutrophilia and comprehensive literature search for all MM with neutrophilia reported in the literature was completed.
Results: We report two cases in which persistent neutrophilia was related to underlying multiple myeloma. One case showed trending of neutrophilia concordant to the varying response of his MM to multiple pharmacotherapies, and the other case had steadily elevated neutrophils without worsening symptoms in the setting of observation alone.
Conclusions: After thorough evaluation of the literature and detailed comparison with our two new cases, a flow chart is presented to differentiate neutrophilia secondary to MM from other differential diagnoses, which could affect treatment options and potentially have prognostic implication.
Keywords
Multiple myeloma, Neutrophilia, Leukocytosis, Gammopathy, Chronic neutrophilic leukemia
Introduction
Multiple myeloma (MM) is a common hematologic malignancy, with 32,110 new cases diagnosed in the United States in 2019, resulting in 12,960 deaths [1]. While neutrophilia is also a common entity, it most often arises secondary to other etiologies, such as infection or inflammatory processes. Primary causes of neutrophilia, such as chronic myeloid leukemia (CML), are less common, with chronic neutrophilic leukemia (CNL) being an extremely rare cause of neutrophilia (~200 reported cases) [2-5].
While MM and neutrophilia are both common, it is uncommon for the two to present together. In a 2003 study of 1,027 patients newly diagnosed with MM, none reported a white blood cell count greater than 25 cells × 109 L that was not attributable to another cause [6]. Interestingly, in most cases, neutrophilia resolves during typical MM treatment, indicative of secondary neutrophilia related to the plasma cell clone. There are, however, rare incidences when neutrophilia does not resolve, suggesting an alternate biology [7-10]. In these cases, CNL is typically the only remaining differential diagnostic consideration as other myeloid neoplasms can more easily be excluded based on morphologic features and/or genetic mutations. Definitive determination of the genesis of neutrophilia (discrete neoplasm or reactive) is essential as treatments for MM and CNL are different [11,12], portending different prognoses (MM 10+ years, MM with neutrophilia poorer prognosis and CNL 2 years) [12-14].
Here we report two cases of MM with neutrophilia and a comprehensive literature review to explore the origin, course of disease, treatments and outcomes.
Methods
Following IRB approval, a retrospective evaluation of two cases of MM with neutrophilia was completed. A complete evaluation of the datapoints associated with the course of the disease to include initial presentation, diagnosis, treatments and outcomes were collected. A comprehensive literature search was completed for all MM with neutrophilia reported to exclude cases that were not available in English or those which the institution could not obtain. Information from the cases reported here and from the literature was used to create a flow chart to assist in the diagnosis of MM with neutrophilia versus concurrent MM and CNL for consideration of treatment for optimal patient care and outcomes.
Case 1
A 70-year-old male with no significant past medical history presented for further hematologic evaluation after imaging and lab work for urolithiasis showed mild splenomegaly and leukocytosis (WBC, 46.3 cells × 109 L; normal: 4.0-11.0 cells × 109 L). Follow up a month later revealed worsening leukocytosis (WBC, 56.8 cells × 109 L; absolute neutrophil count (ANC), 54.2 cells × 109 L; normal: 1.4-7.0 cells × 109 L), significant anemia (hemoglobin, 9.6 g/dL; normal: 13.5-17.5 g/dL), and mild thrombocytopenia (platelets, 120 cells × 109 L; normal: 125-390 cells × 109 L) with associated splenomegaly (spleen palpable 1-2 cm below the costal margin). The patient denied notable symptoms such as fever, chills, or weight loss, and no lymphadenopathy was detected. His creatinine was 1.2 mg/dL (normal: 0.4-1.7 mg/dL) with estimated GFR of 60 mL/min (normal: >59 mL/min) and calcium of 9.3 mg/dL (normal: 8.4-10.4 mg/dL). Dohle bodies and toxic granulation were noted on review of the smear, but there was no left shift or rouleaux (Figure 1). A myeloproliferative neoplasm was suspected. CML was excluded with negative BCR-ABL1 testing. A bone marrow biopsy demonstrated nearly 100% cellularity with prominent myeloid hyperplasia (myeloid:erythroid::12:1; normal 2-3:1), but there were no other morphologic features to suggest a myeloproliferative neoplasm and no dysplasia or increased blasts. However, a monoclonal lambda plasma cell population accounting for 20% of marrow cellularity was identified diagnostic of MM (Figures 2 and 3). Karyotype was normal, and a limited FISH panel revealed a gain of 1q, portending a poor prognosis [15,16]. Neutrophilia was speculated to be secondary to clonal plasma cell triggered production of granulocyte colony stimulating factor (G-CSF). Subsequent serum protein electrophoresis revealed a 3.6 g/dL IgA lambda monoclonal protein with serum immunoglobulin studies showing an elevated IgA level of 4068.10 mg/dL (normal; 84.50-499) but normal serum free light chain ratio of 0.80 (normal; 0.26-1.65). The patient was started on dexamethasone and bortezomib treatment with mild improvement of his leukocytosis. Lenalidomide was added with continued improvement. With less than one year of treatment, the patient had achieved complete remission of MM and resolution of the neutrophilia (IgA, 302.7 mg/dL; no detectable M spike; WBC, 8.2 cells × 109 L). Laboratory evidence of the MM fluctuated correlatively with the neutrophilia for the following 20 months (Figure 5). Additional therapies have included carfilzomib and dexamethasone. Five years after initial presentation, there is continued correlative undulation of his blood cell counts, with his most recent results showing WBC of 14.4 cells × 109 L, ANC of 12.0 cells × 109 L, and M spike of 0.22. His IgA level has stabilized to normal. His last laboratory results showed an IgA of 366 mg/dL. His kidney function and calcium levels remain normal (Cr, 1.24 mg/dL; Ca, 8.9 mg/dL). He continues on carfilzomib and dexamethasone therapy.
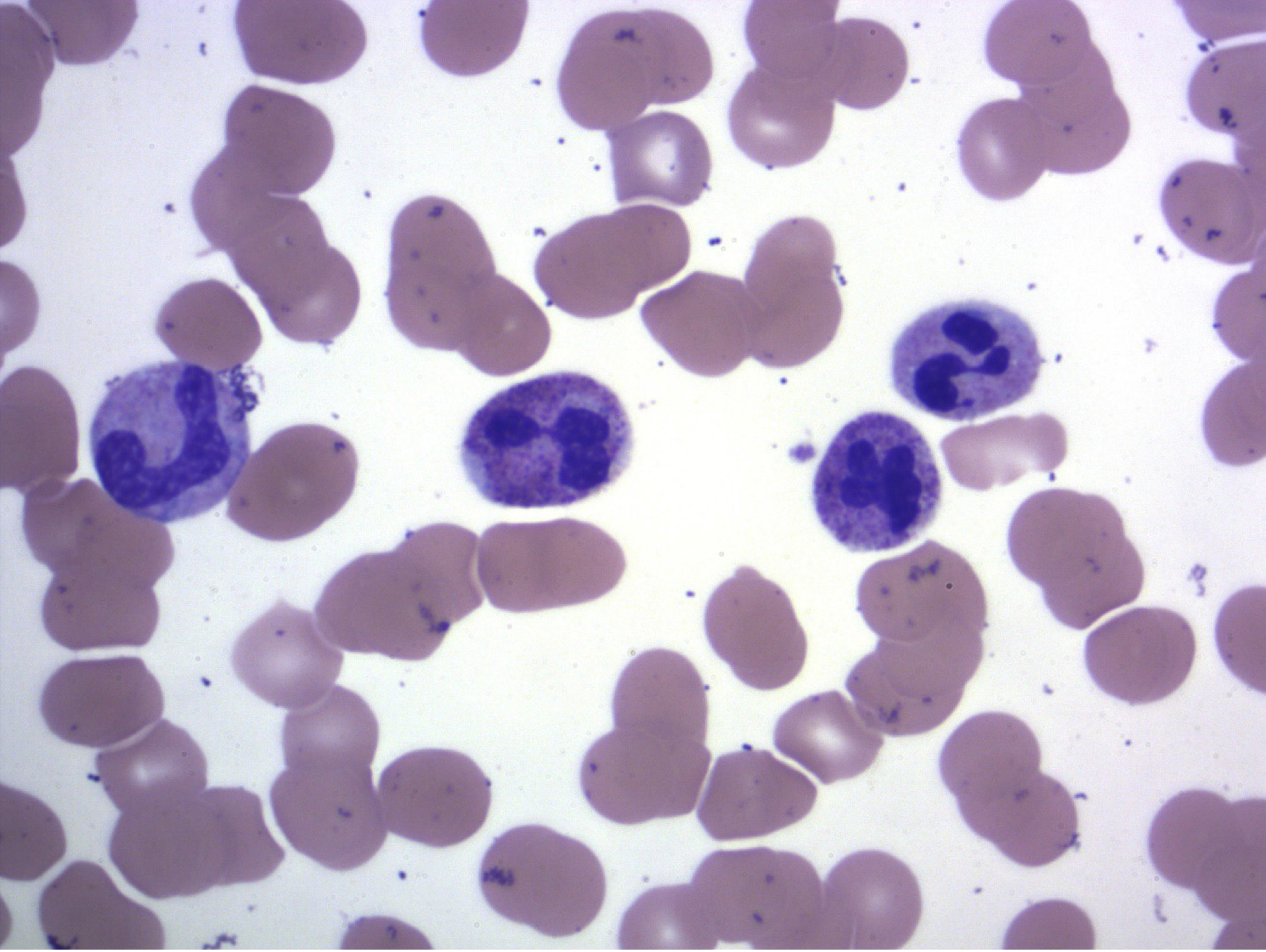
Figure 1. Peripheral blood smear. Neutrophilia with toxic granulation. No left shift beyond occasional bands (WG; 1000x).
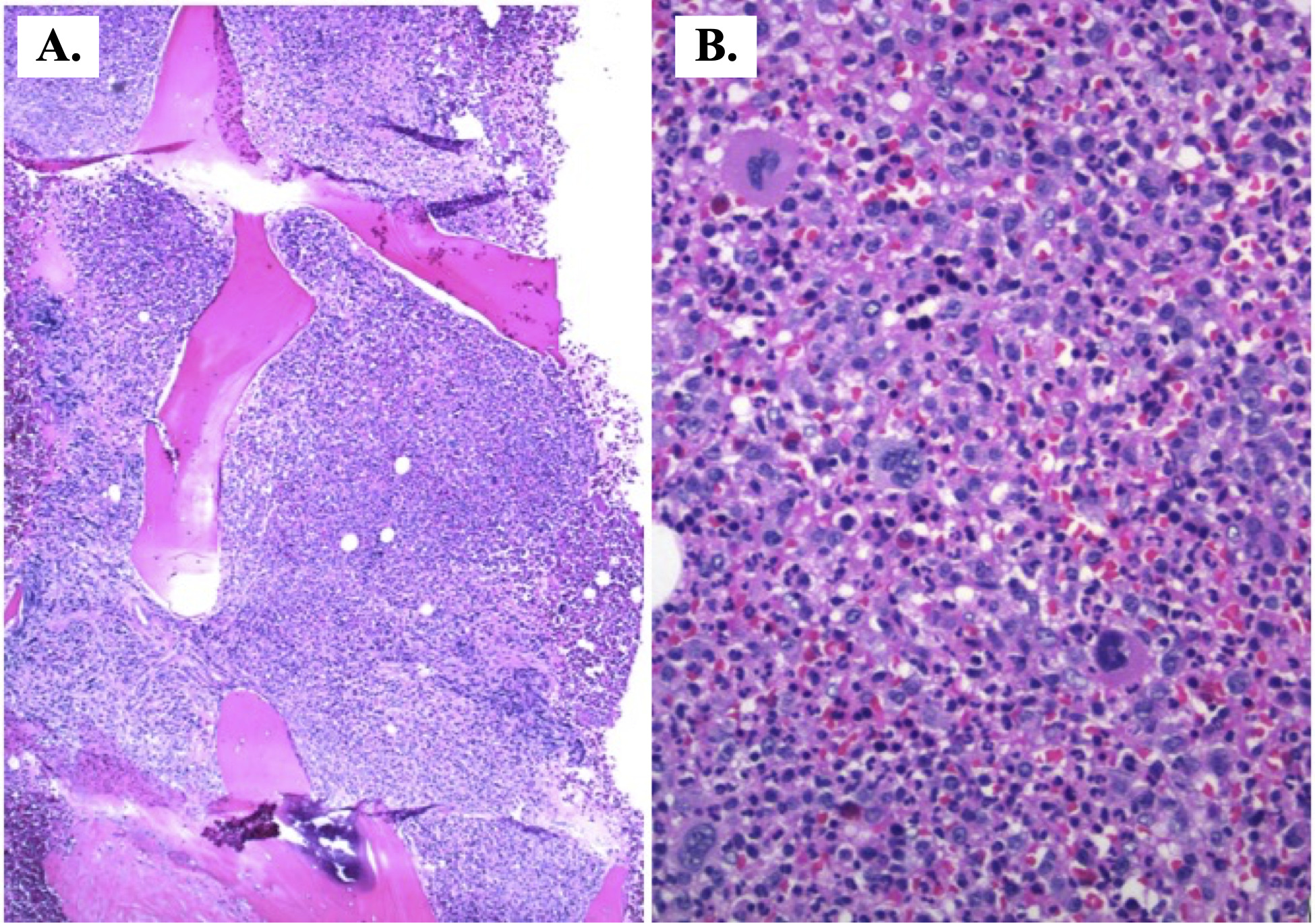
Figure 2. Hypercellular bone marrow. A. The bone marrow exhibits nearly 100% cellularity. Expected cellularity for this patient is approximately 30% (H&E; 40x). B. A predominance of myeloid maturation is visible (H&E; 200x).
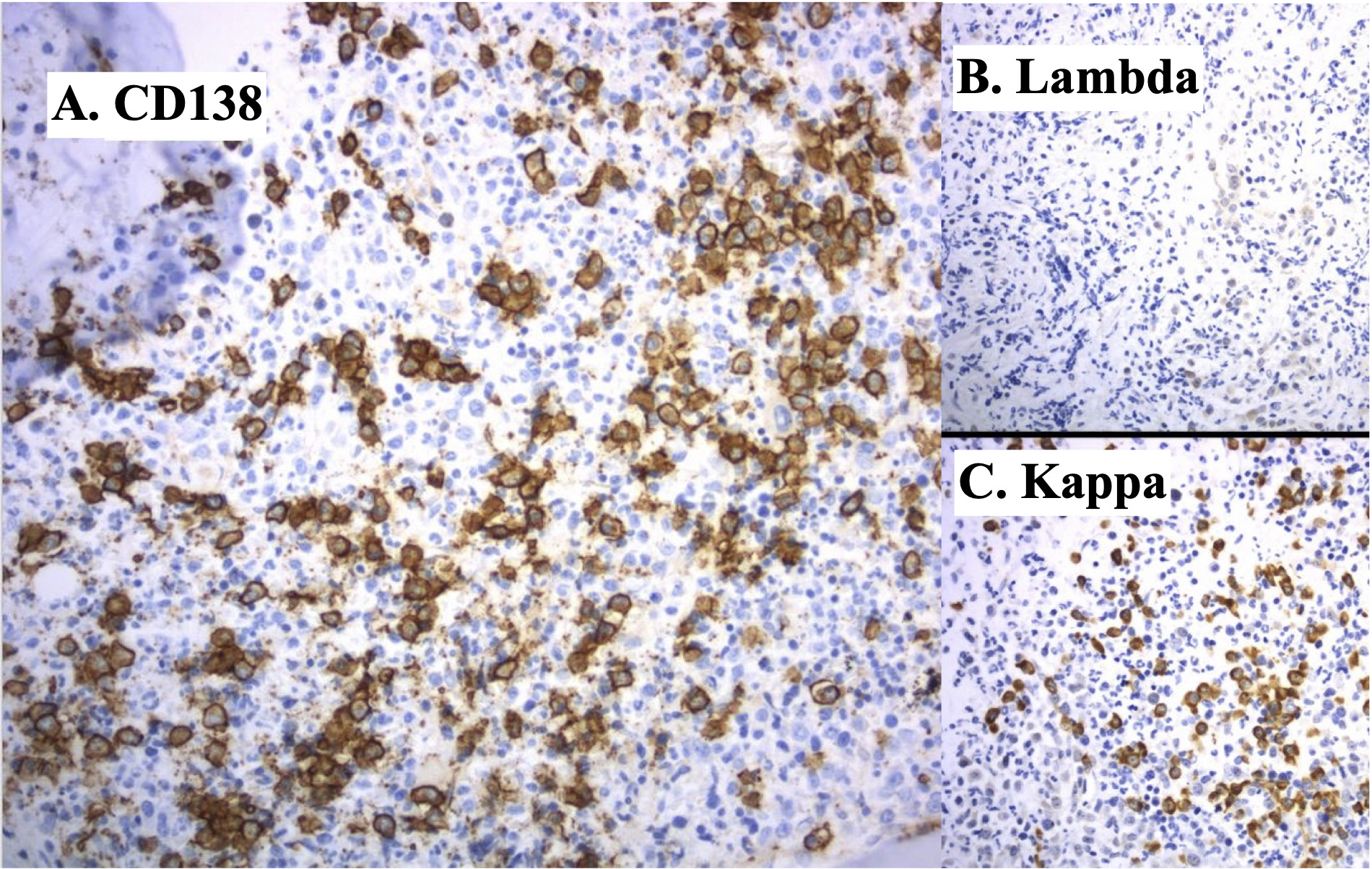
Figure 3. . Plasma cells. A. CD138 immunohistochemistry highlights approximately 20% plasma cells in the bone marrow that are difficult to see by H&E because of the myeloid hyperplasia (x200). B. Kappa and, C. Lambda light chains by in situ hybridization show the plasmsa cells are lambda light chain restricted (x200).
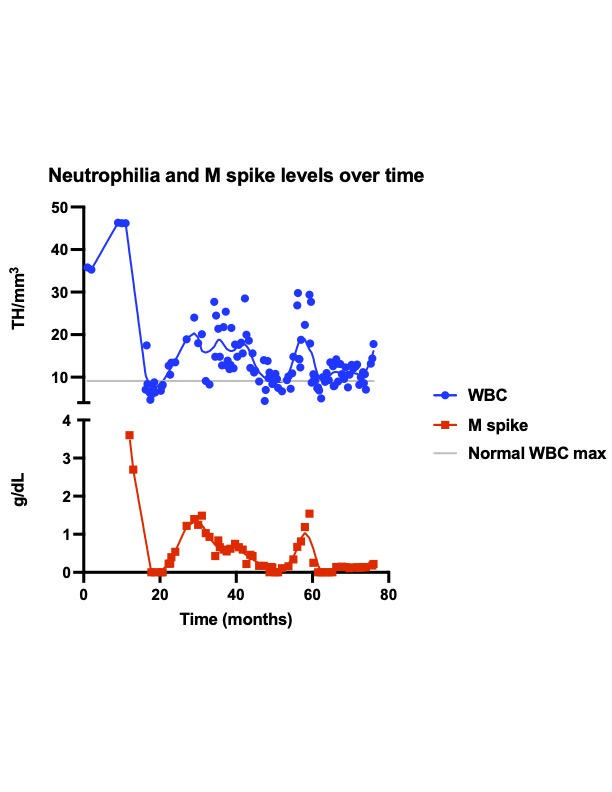
Figure 4. Neutrophilia and M spike levels over time. Note the undulating levels of neutrophilia with corresponding changes in M-spike levels throughout the course of treatment (75 weeks).
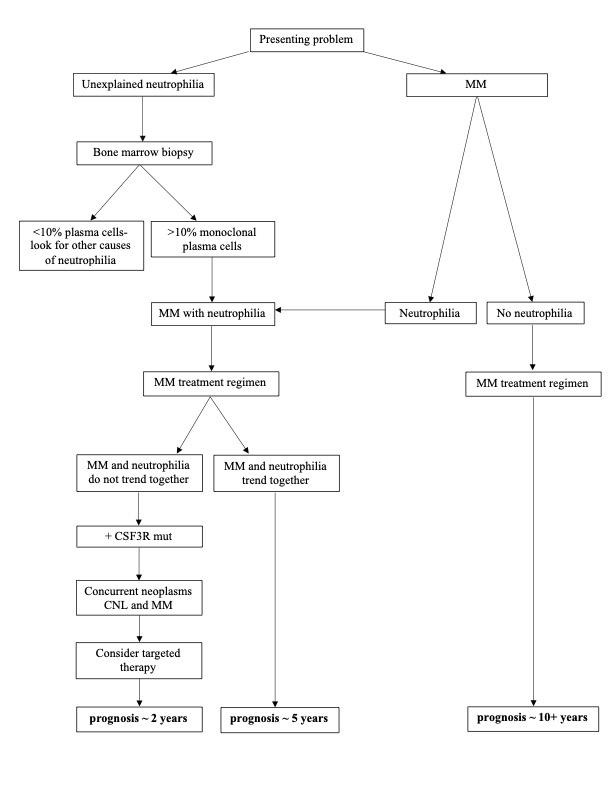
Figure 5. Diagnostic and prognostic flow chart. Algorithm for the workup of a starting diagnosis of unexplained neutrophilia or MM.
Case 2
A 67 year-old Caucasian male with a history of Factor 5 Leiden presented to his primary physician with cellulitis for which he was treated with antibiotics. During these treatments leukocytosis was noted: WBC 21 cells × 109 L and ANC 18 cells × 109 L. After successful treatment of the cellulitis, WBC decreased to 14.9 cells × 109 L. On a three-month follow-up, however, leukocytosis had worsened (WBC, 20.3 cells × 109 L) without cellulitis or swelling of the left leg and no other apparent source of infection or inflammation. The patient was referred to a hematologist oncologist for further evaluation of persistent leukocytosis.
He denied B-symptoms, and no splenomegaly was reported. A leukemoid reaction was suspected, although a myeloproliferative neoplasm remained on the differential. While further evaluation was required, no documentation was noted for two years. Eventually, a bone marrow biopsy was performed and was found to be hypercellular with a myeloid:erythroid ratio of 10:1. Additionally, a monoclonal kappa plasma cell population was identified accounting for approximately 15% of cellularity. Peripheral blood at that time showed a WBC count of 34.4 cells × 109 L with an ANC of 30.7 cells × 109 L and no left shift. Hemoglobin and platelet count were normal. Karyotype and a MM FISH panel were normal. Mutation analysis for JAK2 and BCR-ABL1 had been performed on peripheral blood and were negative. He was diagnosed with smoldering MM with the neutrophilia favored to be related to the MM. The patient elected to refrain from treatment. Nine years after presenting, he continues to decline treatment. His M spike and leukocytosis remain stable (M spike, ~1.4 g/dL; WBC, ~30 cells × 109 L; ANC, ~27 cells × 109 L). He has complaints of fatigue and night sweats but renal function, hemoglobin, and calcium levels remain normal (Cr, 1.00 mg/dL; hgb, 14.0 g/dL; calcium, 8.8 mg/dL).
Discussion
A comprehensive literature review found 35 cases of concurrent plasma cell neoplasms and neutrophilia, including the two cases listed above (Table 1). The first reported case was in 1978, with diagnoses of CNL and MM. The paper defined the CNL diagnosis using leukocytosis with predominant neutrophilia (WBC, 19 cells × 109 L; 88% segmented neutrophils and bands), splenomegaly, granulocytic hyperplasia in the bone marrow, and negative Ph chromosome [17].
| Author | Age and sex | WBC (cells*10^9L) | Segs + bandsb | Hypercell BM | Genetics | Splenomegaly | Toxic granulation | Monoclonal protein (mgd/L) | BM PC percentage | G-CSF evidence | Diagnoses | Treatment and response | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vorobiof et al. (1978) | 70 YOM | 19.0 | 88% | yes | (-) BCR-ABL | yes | - | 1400 IgA kappa | 15% | - | CNL with MM | - | 28 months; still alive |
| Watanabe et al. (1984) | 71 YOF | 86.4 | 89% | yes | (-) BCR-ABL | yes | yes | 1447 IgG lambda, 453 IgA kappa | - | G-CSF elevated in plasma | CNL with paraproteinemia | pRBC transfusion with improvement of symptoms | 38 months; death due to heart failure |
| Franchi et al. (1984) | 64 YOF | 35.0 | 80% | yes | - | yes | - | 2200 IgG lambda | 12% | - | CNL progressing to MM | busulfan, steroids with development of neutropenia | 228 months after CNL dx; 48 months after MM dx; death due to bronchopneumonia |
| Mehrotra et al. (1985) | 65 YOF | 57.0 | 88% | yes | (-) BCR-ABL | yes | yes | 2300 IgG lambda | - | - | CNL | allopurinol with worsening splenomegaly | 96 months; still alive |
| Lewis et al. (1986) | 62 YOF | 25.3 | 91% | yes | (-) BCR-ABL | yes | yes | 750 IgA lambda | 20% | - | MM | prednisolone, melphalan, cyclophosphamide without improvement of neutrophilia; added busulfan with improvement of neutrophilia | 96 months; death |
| Lewis et al. (1986) | 72 YOF | 36.3 | 91% | yes | (-) BCR-ABL | yes | - | 2100 IgA lambda | 30% | - | MM | melphalan without improvement of leukocytosis | 48 months; death due to AML |
| Zoumbos et al. (1987) | 57 YOM | 55.4 | 90% | yes | (-) BCR-ABL | yes | - | kappa light chain | 60% | - | CNL progressing to MM | allopurinol and busulfan with improvement of neutrophilia | - |
| Kubo et al. (1989) | 62 YOF | 20.1 | 82% | yes | (-) BCR-ABL | - | - | 330 IgA kappa | 5.90% | no G-CSF elevation in plasma | CNL associated with MM | - | 96 months; still alive |
| Abe et al. (1989) | 70 YOF | - | - | - | - | - | - | IgG kappa | - | - | Neutrophilia progressing to MM | - | 144 months; death due to AML |
| Standen et al. (1990) | 65 YOF | 13.7 | 86% | yes | (-) BCR-ABL | no | yes after initial txt | 500 IgG lambda | 15% | no GSF elevation in plasma | MM | tumor removal, radiotherapy with worsening leukocytosis and MM | - |
| Standen et al. (1990) | 76 YOF | 72.3 | 91% | yes | (-) BCR-ABL | yes | yes | 5600 IgG lambda | - | no GSF elevation in plasma | CNL with PCD | melphalan and prednisone with improvement of neutrophilia | - |
| Rovira et al. (1990) | 31 YOF | 23.9 | 82% | yes | (-) BCR-ABL | no | - | kappa light chain | 80% | - | CNL progressing to MM | melphalan-prednisone | 96 months; still alive |
| Dieguez et al. (1992) | - | - | - | yes | - | yes | yes | 5730 IgG lambda | - | - | CNL associated with MM | melphalan and prednisone with minimal response | - |
| Florensa et al. (1993) | 61 YOF | 66.0 | 90% | - | (-) BCR-ABL | no | yes | lambda light chain | - | - | CNL | no therapy | - |
| Cehreli et al. (1994) | 60 YOF | 62.4 | 85% | yes | (-) BCR-ABL | yes | yes | kappa light chain | - | - | CNL with MM | etoposide and hydroxyurea and doxorubicin without improvement | 4.6 months; death |
| Mori et al. (1995) | 57 YOF | 33.4 | 95% | yes | (-) BCR-ABL | yes | yes | 3628 IgA | - | - | CNL associated with MM | busulfan with minimal response | 22 months; death due to pneumonia |
| Nagai et al. (1996) | 73 YOM | 28.08 | 85% | yes | (-) BCR-ABL | - | - | 3200 IgG kappa | 7.60% | G-CSF elevated in plasma | Plasma cell dyscrasia associated with CNL | melphalan then busulfan with transient improvement followed by relapse of neutrophilia | - |
| Ito et al. (1996) | 30 YOM | 42.2 | 91% | yes | (-) BCR-ABL | yes | yes | 1269 IgG kappa | - | - | CNL with MGUS | no therapy | 144 months; still alive |
| Usada et al. (1997) | 56 YOM | 9.9 | 81% | yes | (-) BCR-ABL | yes | 1020 IgA lambda | 14% | G-CSF in BM, G-CSF elevated in plasma | MM | vincristine, doxorubicin, dexamethasone, melphalan and prednisolone and interferon with improvement of myeloma cells | 120 months; death due to respiratory failure | |
| Stevenson et al. (1998) | 60 YOF | 21.0 | 85% | yes | - | yes | - | 2100 IgA lambda | 6% | - | MM with reactive leukocytosis | cortisone injection, dexamethasone, melphalan and autologous stem cell rescue with improvement of leukocytosis | - |
| Dincol et al. (2002) | 71 YOM | 38.0 | 86% | yes | (-) BCR-ABL | yes | yes | 3993 IgG kappa | 4% | - | CNL associated with MM progressing to AML | melphalan with improvement of MM and neutrophilia | 17 months; death due to bronchopneumonia |
| Kohmura et al. (2004) | 94 YOM | 31.3 | 90.50% | yes | - | no | no | IgG | 35% | Anti-G-CSF Ab, GCSF in BM, GCSF elevated in plasma, G-CSF mRNA | MM | melphalan, prednisolone, dexamethasone with improvement of neutrophilia | - |
| Kusaba et al. (2004) | 68 YOM | 29.9 | 82% | - | (-) BCR-ABL | - | yes | lambda light chain | - | Anti-GCF Ab, G-CSF elevated in plasma | CNL associated with MM | melphalan and prednisone without response; ranimustine, vincristine, melphalan, dexamethasone with improvement of neutrophilia | - |
| Fukuno et al. (2006) | 74 YOM | 25.7 | 71% | yes | (-) BCR-ABL | yes | yes | lambda light chain | - | G-CSF in BM | CNL progressing to MM | no therapy | 24 months; death due to renal failure |
| Gnerre et al. (2007) | 40 YOF | 21.0 | yes | - | no | yes | 2128 IgM lambda | 30% | - | MM associated with neutrophilia and nephrotic syndrome | diuretics, albumin infusion, bisphosphonates, melphalan, prednisone with improvement of neutrophilia and MM | - | |
| Sebasky et al. (2008) | 56 YOM | 22.6 | - | - | (-) BCR-ABL | no | yes (nov 2007) | IgG kappa | 46% | G-CSF elevated in plasma | MM | vincristine, doxorubicin, dexamethasone with improvement of neutrophilia | 9 months; death due to MM |
| Hartley et al. (2010) | 60 YOF | 44.11 | 79% | yes | (-) BCR-ABL, (-) Jak2-V617 | no | - | 1305 IgA kappa | 7% | - | MGUS with neutrophilia | no therapy | - |
| Nedeljkovic et al. (2014) | 81 YOM | 26.8 | yes | (-) BCR-ABL, (+) Jak2-V617 | yes | no | 1600 IgG kappa | 15-20% | - | CNL w/ smoldering PC myeloma | - | - | |
| Taiwo et al. (2014) | 63 YOF | 65.59 | 91% | yes | (-) BCR-ABL | no | yes | 276.9 lambda light chain | 10% | - | smoldering MM | hydroxyurea with worsening neutrophilia; bortezomib and dexamethasone with improvement of neutrophilia | - |
| Blombery et al. (2014) | 57 YOF | 114.0 | 92% | - | (-) BCR-ABL, (-) Jak2-V617, (+) CSF3R | no | - | 600 IgG kappa | - | - | MGUS progressing to CNL | - | - |
| Shi et al. (2015) | 78 YOM | 47.5 | 86% | yes | (-) BCR-ABL | yes | - | 434 lambda light chain | 6.40% | - | CNL progressing to MM progressing to AML | hydroxyurea without improvement; cyclophosphamide, prednisone, thalidomide | 48 months; death due to AML |
| Milojkovic et al. (2015) | 52 YOM | 22.5 | 84% | - | (-) CSF3R | - | yes | 8000 IgG lambda | 10-15% | - | MM with neutrophilic leukemoid reaction | hydroxycarbamide | - |
| An et al. (2020) | 48 YOF | 57.1 | 84% | - | (-) BCR-ABL, (-) Jak2-V617, (-) CSF3R | yes | yes | 4575 kappa light chain | - | - | MM with neutrophilic leukemoid reaction | bortezomib, lenalidomide, and dexamethasone with improvement of neutrophilia | - |
| Hall et al. (2021) | 70 YOM | 46.3 | 86% | yes | (-) BCR-ABL | yes | yes | 4068 IgA | 20% | - | MM arising with leukemoid reaction | Currently, carfilzomib and dexamethasone with stable IgA and variable neutrophilia | 60 months; still alive |
| Hall et al. (2021) | 67 YOM | 20.3 | 84% | yes | (-) BCR-ABL, (-) Jak2 | no | no | 1655.8 IgA | 15% | - | Smoldering kappa MM with leukemoid reaction | Currently, refrains from treatment w/ no worsening of symptoms | 108 months; still alive |
| a) cases that were unavailable or not in English were excluded; †immunoglobulins that were not quantified are mentioned without values but the diagnosis was multiple myeloma b) Segs: Segmented neutrophils |
|||||||||||||
The definition of CNL has evolved since the first reported case. Starting in 2008, WHO criteria for CNL specifically state that a plasma cell neoplasm must be excluded, suggesting a neutrophilic reactive process may have been underlying some of the previously diagnosed cases of CNL, cases which were likely MM with neutrophilia (Table 2) [18,19]. In fact, a 2016 literature review of 150 reported cases of CNL found that only 40 cases met the current WHO criteria for the diagnosis. This was primarily because previous guidelines did not rule out underlying inflammatory processes from abnormal production of G-CSF [12]. Some have suggested the mechanism underlying this abnormal G-CSF production is through monoclonal B-cell clones (plasma cells being terminally differentiated B cells) producing cytokines that induce production of interleukins and tumor necrosis factor (TNF) that then stimulate T-cell production of G-CSF [20,21]. This theory is not novel. Ectopic production of G-CSF causing neutrophilia is seen secondary to solid organ neoplasms including lung, thyroid, gastric, gallbladder, and urinary bladder, and in other hematologic neoplasms including Hodgkin lymphoma and anaplastic large cell lymphoma [22]. Thus, high levels of G-CSF could provide evidence of a secondary response of the neutrophils in contrast to a clonal proliferation.The limitation to its use is the inavailability of either G-CSF levels or G-CSF immunostains, particularly for smaller institutions.
More recently, studies have identified a mutation in the gene encoding granulocyte colony-stimulating factor receptor (CSF3R), which regulates JAK/STAT signaling. It has been reported to be present in 80% of patients with CNL [23]. The discovery of this mutation makes diagnosis less reliant on exclusion criteria. Since its discovery, only one reported case to date has demonstrated the presence of this mutation in a patient with MM and neutrophilia to support true concurrence of CNL and MM [24]. Thus, as the likelihood of concurrent CNL and MM is exceedingly low, extended genetic evaluation for neutrophilia in the setting of newly diagnosed MM is neither economical nor timely, particularly when constraints from payers or access to molecular testing may be insurmountable hurdles. Neither of the presented cases were tested for CSF3R even though each met almost all other criteria for CNL (Table 2). While genetic testing for CSF3R may be definitive in the cause of neutrophilia if actually detected, it is not necessary to test for this specifically in cases when a bone marrow is biopsy diagnostic of MM.
| Case 1 | Case 2 | |
|---|---|---|
| -Peripheral blood WBC ≥25 x 109/L, -Segmented neutrophils plus banded neutrophils constitute ≥ 80% of the white blood cells -Neutrophils precursors (promyelocytes, myelocytes, and metamyelocytes) <10% of the white blood cells -Myeloblasts rarely observed -Monocyte count <1 x 109 L -No dysgranulopoiesis |
Yes Yes Yes Yes Noa Yes |
Yes Yes Yes Yes Noa Yes |
| -Hypercellular bone marrow -Neutrophil granulocytes increased in percentage and number -Neutrophil maturation appears normal -Myeloblasts constitute <5% of the nucleated cells |
Yes Yes Yes Yes |
Yes Yes Yes Yes |
| -Not meeting WHO criteria for BCR-ABL1 positive CML, polycythemia vera, essential thrombocythemia, primary myelofibrosis | Yes (BCR-ABL1 negative in peripheral blood) |
Yes (BCR-ABL1 and JAK2 V617Fnegative in peripheral blood |
| -No rearrangement of PDGFRA, PDGFRB, or FGFR1, and no PCM1-JAK2 fusion | Not testedb | Not testedc |
| -CSF3R T618I or another activating CSF3R mutation Or -Persistent neutrophilia ≥ 3months, splenomegaly, and no identifiable cause of reactive neutrophilia including absence of plasma cell neoplasm or, if a plasma cell neoplasm is present, demonstration of clonality of myeloid cells by cytogenetic or molecular studies. |
Not tested Mild splenomegaly but exclusionary diagnosis of MM |
Not tested Exclusionary diagnosis of MM |
| a) Relative monocyte count less than 10% b) Marrow was inaspirable, and this testing was not performed on peripheral blood. There was no eosinophilia. c) Specific FISH testing for these abnormalities was not performed. Karyotype was normal. There was no eosinophilia. |
||
In the cases where a patient presents only with unexplained neutrophilia, it is important to point out the significance of considering MM as a potential cause (Figure 4). As a bone marrow biopsy would be part of a typical workup for unexplained neutrophilia, specific evaluation for monoclonal plasma cells is an easy and efficient step for determining if neutrophilia is just the presenting symptom of a case of MM, which was true in both of our reported cases. Further, the literature shows several cases where a diagnosis of “CNL” preceded an alternate diagnosis of MM/MGUS with neutrophilia [4,5,25-28]. As cases with neutrophilia secondary to MM can show marked granulocytic hyperplasia in the marrow, which would potentially make plasma cells inconspicuous, immunohistochemical stains and/or ISH to specifically identify the plasma cells should be performed.
This overlap in presentation of CNL and MM with neutrophilia historically has led to confusion in diagnosis and can still cause misdiagnosis or delay in diagnosis of MM. Recogonition of MM with this rare presentation, however, is paramount because of differing treatments and prognosis between the two entities. Many studies have focused on differentiation between CNL and MM with neutrophilia, but few have discussed therapies for these rare disorders. Cases of CNL have the potential to be treated with JAK/STAT pathway targeted therapies [23]. Treatment for MM with neutrophilia, however, is still ill-defined. From the literature, 10 cases did not have improvement of their neutrophilia with typical treatment of their MM; however, in 13 instances, neutrophilia did improve with treatment. It is important to note that cases in this literature review span decades and treatment of MM has, of course, progressed over that time with more efficacious therapies. Thus, lack of resolution of the neutrophilia in older reported cases should not be equated to evidence of concurrent CNL. Rather, the neutrophilia could be considered another indicator of MM disease status when it is a secondary paraneoplastic-type process as was evident in case 1 (Figure 5). Consequently, it should rise and fall with myeloma laboratory results according to the response to treatment. Thus, here we suggest an alternative to extensive and expensive workup to differentiate CNL from MM with neutrophilia in instances where that testing may be unattainable. These findings, while they may not exclude CNL based on WHO criteria, suggest more justifiable evidence for a paraneoplastic process of MM. However, should the patient have declining levels of serum immunoglobulins, M spike, and serum free light chains but sustained neutrophilia with treatment, this would suggest a concurrent primary neutrophilia. In that event, CSF3R mutation analysis would be indicated.
In regards to prognosis, from the 18 reported cases that did include outcome data, MM with reactive neutrophilia averaged a five-year survival representing a better prognosis than CNL, but a worse prognosis than MM alone [12-14]. This rasies the question of whether neutrophilia in MM patients, could be considered not only as an indicator of disease, but as a prognostic factor. In our presented cases, the first patient is still living after 5 years of diagnosis and the second, 9 years after diagnosis. While treatment has not resolved neutrophilia in either case, the otherwise normal kidney function and relatively asymptomatic disease correlates with better prognosis in patients with MM with neutrophilia compared to those with CNL as seen in our literature search. However, given the time span of the reviewed cases and the overall relatively low number of cases from which to draw conclusions, this is an area that could be further evaluated in future studies.
Conclusion
In bone marrow biopsies for neutrophilia, MM needs to be considered with specific immunohistochemical stains and/or ISH for plasma cells employed as they may be inconspicuous in a background of hyperplasia. Neutrophilia in the setting of MM appears to correlate with prognosis with differing reported survival rates for MM alone, MM with secondary neutrophilia, and MM with concurrent CNL. Neutrophilia can be considered an indicator of MM disease status when it is a secondary paraneoplastic-type process, presumably secondary to elevated G-CSF. Future studies may further assess the prognostic indications of this rare presentation of MM.
Conflicts of Interest
All authors declare there are no conflicts of interest to disclose.
Funding Sources
The authors received no financial support for the research, authorship, and/or publication of this article.
References
2. Szuber N, Elliott M, Tefferi A. Chronic neutrophilic leukemia: 2020 update on diagnosis, molecular genetics, prognosis, and management. American Journal of Hematology. 2020 Feb;95(2):212-24.
3. Bain BJ, Ahmad S. Chronic neutrophilic leukaemia and plasma cell?related neutrophilic leukaemoid reactions. British Journal of Haematology. 2015 Nov;171(3):400-10.
4. Fukuno K, Tsurumi H, Kanemura N, Tanabashi S, Okamoto K, Moriwaki H. Chronic neutrophilia preceding overt aggressive light chain multiple myeloma. Leukemia & Lymphoma. 2006 Apr;47(4):762-4.
5. Rovira M, Cervantes F, Nomdedeu B, Rozman C. Chronic neutrophilic leukaemia preceeding for seven years the development of multiple myeloma. Acta Haematologica. 1990;83(2):94-5.
6. Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. In: Mayo Clinic Proceedings 2003 Jan 1;78(1):21-33.
7. Usuda H, Naito M, Ohyach K, Iizumi T. A case of multiple myeloma producing granulocyte colony?stimulating factor. Pathology International. 1997 Dec;47(12):866-9.
8. Mori H, Takahashi N, Tada J, Maeda T, Higuchi T, Shimizu T, et al. Association of chronic neutrophilic leukemia and myeloma with fibrillar inclusions in granulocytes. [Rinsho Ketsueki] The Japanese Journal of Clinical Hematology. 1995 Feb 1;36(2):121-7.
9. Diéguez JC, Amián A, Rodríguez JN, Martino ML, Cañavate M, Prados D. Chronic neutrophilic leukemia associated with myeloma. Simultaneous presentation. Sangre. 1992 Oct 1;37(5):403-6.
10. Cehreli C, Undar B, Akkoc N, Onvural B, Altungoz O. Coexistence of chronic neutrophilic leukemia with light chain myeloma. Acta Haematologica. 1994;91(1):32-4.
11. National Comprehensive Cancer Network. Multiple Myeloma (Version 5.2022). National Comprehensive Cancer Network; 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf.
12. Elliott MA, Tefferi A. Chronic neutrophilic leukemia 2016: Update on diagnosis, molecular genetics, prognosis, and management. American Journal of Hematology. 2016 Mar;91(3):341-9.
13. Landgren O, Rajkumar SV. New developments in diagnosis, prognosis, and assessment of response in multiple myeloma. Clinical Cancer Research. 2016 Nov 15;22(22):5428-33.
14. Mu S, Ai L, Fan F, Sun C, Hu Y. Prognostic role of neutrophil–lymphocyte ratio in multiple myeloma: a dose–response meta-analysis. OncoTargets and Therapy. 2018;11:499-507.
15. Walker BA, Leone PE, Chiecchio L, Dickens NJ, Jenner MW, Boyd KD, et al. A compendium of myeloma-associated chromosomal copy number abnormalities and their prognostic value. Blood, The Journal of the American Society of Hematology. 2010 Oct 14;116(15):e56-65.
16. Schmidt TM, Barwick BG, Joseph N, Heffner LT, Hofmeister CC, Bernal L, et al. Gain of Chromosome 1q is associated with early progression in multiple myeloma patients treated with lenalidomide, bortezomib, and dexamethasone. Blood Cancer Journal. 2019 Nov 25;9(12):94.
17. Vorobiof DA, Benjamin J, Kaplan H, Dvilansky A. Chronic granulocytic leukemia, neutrophilic type, with paraproteinemia (IgA type K). Acta Haematologica. 1978;60(5):316-20.
18. Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Swerdlow SH, Editor. Lyon: International agency for research on cancer; 2008 Sep 20.
19. Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood, The Journal of the American Society of Hematology. 2009 Jul 30;114(5):937-51.
20. Standen GR, Steers FJ, Jones L. Clonality of chronic neutrophilic leukaemia associated with myeloma: analysis using the X-linked probe M27 beta. Journal of Clinical Pathology. 1993 Apr 1;46(4):297-8.
21. Nagai M, Oda S, Iwamoto M, Marumoto K, Fujita M, Takahara J. Granulocyte-colony stimulating factor concentrations in a patient with plasma cell dyscrasia and clinical features of chronic neutrophilic leukaemia. Journal of Clinical Pathology. 1996 Oct 1;49(10):858-60.
22. Kohmura K, Miyakawa Y, Kameyama K, Kizaki M, Ikeda Y. Granulocyte colony stimulating factor-producing multiple myeloma associated with neutrophilia. Leukemia & Lymphoma. 2004 Jul 1;45(7):1475-9.
23. Maxson JE, Tyner JW. Genomics of chronic neutrophilic leukemia. Blood, The Journal of the American Society of Hematology. 2017 Feb 9;129(6):715-22.
24. Nedeljkovic M, He S, Szer J, Juneja S. Chronic neutrophilia associated with myeloma: is it clonal. Leuk Lymphoma. 2014 Feb 1;55(2):439-40.
25. Shi J, Ni Y, Li J, Qiu H, Miao K. Concurrent chronic neutrophilic leukemia blast crisis and multiple myeloma: A case report and literature review. Oncology Letters. 2015 May 1;9(5):2208-10.
26. Franchi F, Seminara P, Giunchi G. Chronic neutrophilic leukemia and myeloma. Report on long survival. Tumori Journal. 1984 Feb;70(1):105-7.
27. Zoumbos NC, Chrysanthopoulos C, Starakis J, Kapatais-Zoumbos K. Kappa light chain myeloma developing in a patient with chronic neutrophilic leukaemia. Br J Haematol. 1987 Apr;65(4):504-5.
28. Abe K, Imamura N, Mtasiwa DM, Inada T, Fujimura K, Kuramoto A. Multiple myeloma following chronic neutrophilia terminated with acute monocytic leukemia (AML, M 5 b). [Rinsho Ketsueki] The Japanese Journal of Clinical Hematology. 1989 Jun 1;30(6):910-4.
29. Watanabe A, Yoshida Y, Yamamoto H, Kimura S, Nagashima H, Kitajima K. A Case of Chronic Neutrophilic Leukemia with Paraproteinemia (IgG Type ? and IgA Type K). Japanese Journal of Medicine. 1984;23(1):39-44.
30. Mehrotra PK, Winfield DA, Fergusson LH. Cellular abnormalities and reduced colony-forming cells in chronic neutrophilic leukaemia. Acta Haematologica. 1985;73(1):47-50.
31. Lewis MJ, Oelbaum MH, Coleman M, Allen S. An association between chronic neutrophilic leukaemia and multiple myeloma with a study of cobalamin?binding proteins. British Journal of Haematology. 1986 May;63(1):173-80.
32. Kubo A, Kawanami M, Matsuyama E, Tamura T, Kanoh T. Chronic neutrophilic leukemia associated with monoclonal gammopathy (IgA, kappa type). [Rinsho Ketsueki] The Japanese Journal of Clinical Hematology. 1989 Jun 1;30(6):858-62.
33. Standen GR, Jasani B, Wagstaff M, Wardrop CA. Chronic neutrophilic leukemia and multiple myeloma: an association with ? light chain expression. Cancer. 1990 Jul 1;66(1):162-6.
34. Florensa L, Woessner S, Vicente P, Martí AR, Sole F, Perez A. Chronic neutrophilic leukemia associated with monoclonal gammopathy of undetermined significance. Annals of Hematology. 1993 Sep;67(3):129-31.
35. Ito T, Kojima H, Otani K, Komeno T, Mitsuhashi S, Hasegawa Y, et al. Chronic neutrophilic leukemia associated with monoclonal gammopathy of undetermined significance. Acta Haematologica. 1996;95(2):140-3.
36. Stevenson JP, Schwarting R, Schuster SJ. Analysis of clonality using X?linked polymorphisms in a patient with multiple myeloma and myelofibrosis. American Journal of Hematology. 1998 Sep;59(1):79-82.
37. Dinçol G, Nalçaci M, Doğan O, Aktan M, Küçükkaya R, Ağan M, et al. Coexistence of chronic neutrophilic leukemia with multiple myeloma. Leukemia & Lymphoma. 2002 Jan 1;43(3):649-51.
38. Kusaba N, Yoshida H, Ohkubo F, Mishima K, Shimamastu K, Okamura T, et al. Granulocyte-colony stimulating factor-producing myeloma with clinical manifestations mimicking chronic neutrophilic leukemia. [Rinsho Ketsueki] The Japanese Journal of Clinical Hematology. 2004 Mar 1;45(3):228-32.
39. Gnerre P, Ottonello L, Montecucco F, Boero M, Dallegri F. Nephrotic syndrome in a patient with IgM myeloma with associated neutrophilia. European Journal of Haematology. 2007 Jul;79(1):76-80.
40. Sebasky MM, Gupta P, Filice GA. Elevated granulocyte colony-stimulating factor, non-infectious leukocytosis and fevers in a patient with multiple myeloma. Journal of General Internal Medicine. 2008 Dec;23(12):2134-5.
41. Hartley MA, Sokol L, Caceres G, Hussein MA, List A, Pinilla-Ibarz J. Monoclonal gammopathy of undetermined significance disguised as chronic neutrophilic leukemia. Mediterranean Journal of Hematology and Infectious Diseases. 2010;2(1):e2010002.
42. Taiwo E, Wang H, Lewis R. Treatment of coexisting chronic neutrophilic leukemia and light chain multiple myeloma with hydroxyurea, bortezomib, and dexamethasone. Case Reports in Hematology. 2014 Jul 17;2014:869395.
43. Blombery P, Kothari J, Yong K, Allen C, Gale RE, Khwaja A. Plasma cell neoplasm associated chronic neutrophilic leukemia with membrane proximal and truncating CSF3R mutations. Leukemia & Lymphoma. 2014 Jul 1;55(7):1661-2.
44. Milojkovic D, Hunter A, Barton L, Cross NC, Bain BJ. Neutrophilic leukemoid reaction in multiple myeloma. American Journal of Hematology. 2015 Nov;90(11):1090.
45. An C, Zhai L, Geng H, Wang P, Zhang W. 18F-FDG PET/CT Findings in a Patient With Neutrophilic Leukemoid Reaction Associated With Multiple Myeloma. Clinical Nuclear Medicine. 2020 May 1;45(5):405-6.
