Keywords
Helicobacter pylori, Antimicrobial resistance, Treatment
Introduction
Helicobacter pylori is a gram-negative, spiral-shaped bacterium that inhabits the gastric environment of 60.3% of the global population [1]. Though most individuals infected with the bacterium remain asymptomatic, it is known that this infection plays a pivotal role in the development of diseases such as chronic gastritis, peptic ulcer, gastric cancer and gastric MALT lymphoma [2,3]. Hence, eradication of H. pylori is associated with the potential prevention of many gastric and extra gastric diseases, such as gastric cancer [4].
In the late 1990s, triple therapy with clarithromycin (500 mg), metronidazole (500 mg) or amoxicillin (1000 mg), and proton pump inhibitors (PPIs) in standard doses twice a day for 7 to 10 days became the first-line regimen for eradication of H. pylori in many countries. At the time, different clinical trials showed that this therapy achieved eradication rates of 90%, which were considered acceptable [5,6]. Nevertheless, the effectiveness of this regimen has been largely affected by clarithromycin resistance, which has reduced the eradication rate to 80% or even lower in some regions [7]. In view of this, the main guidelines for the treatment of H. pylori infection, currently recommend quadruple bismuth therapy (QBT) for first-line treatment, in preference to triple therapy, restricted to areas with low rates of resistance to clarithromycin (<15%) [8]. The QBT consists of PPI (standard dose, BID), bismuth (QID), metronidazole (400 mg, QID or 500 mg, TID-QID) and tetracycline (500 mg, QID) for 10 to 14 days. Furthermore, concomitant non-bismuth quadruple therapy with PPI (standard dose, BID), amoxicillin (1000 mg, BID), metronidazole (500 mg, BID) and clarithromycin (500 mg, BID) for 10-14 days is also recommended in regions where bismuth is not available [9-11].
Drug Resistance
In view of the pharmacological therapy currently used, it should be noted that the main medications in use may not have an effect, mainly due to drug resistance. Therefore, it is known that H. pylori is a high priority group in urgent need of new antimicrobials, considering that the efficacy of empirical treatment has decreased and that H. pylori eradication rates are directly dependent on the susceptibility of the strain to the currently used antibiotics [12,13]. Metronidazole resistance mechanism occurs due to changes in the enzymatic systems that interfere with microbial growth and development [14]. This antimicrobial demonstrates the most common antibiotic resistance in H. pylori (20-95%): 99.5% in Asia, 79.4% in America, 83.0% in Europe, and 57.0% in Oceania. Clarithromycin is the antimicrobial of first choice for H. pylori eradication and, at the moment, is of great concern due to its high resistance rates (0-50%). Its resistance mechanism occurs due to changes in genes that encode a domain of one of the subunits of the prokaryotic ribosome, in addition to other enzymes related to the process of protein synthesis [14]. Amoxicillin resistance rates are also highly variable (0-30%), mainly in regions where it can be obtained without a prescription [13]. Amoxicillin resistance is related to structural modification, such as changes in penicillin-binding proteins [14]. Levofloxacin, Rifampicin, and Furazolidone resistance in H. pylori are low and considered insignificant, but all of these antimicrobial resistance levels are increasing over time [12,13]. Thus, understanding the resistance mechanism and prevalence of the antibiotics used in H. pylori eradication is crucial to search for new drugs and improved treatment.
Novel Therapy Options
Faced with drug resistance, novel therapies can assist in the management of positive H. pylori patients. New options of PPIs as Vonoprazan (VPZ), administration of medications with different combinations and dosages, as well as other antimicrobials, probiotics and in vitro tested therapies are also able to assist in the management of positive H. pylori patients.
VPZ is a Potassium Competitive Acid Blocker that inhibits gastric acid stronger and longer since the first day of therapy [15,16]. However, it is not indicated to penicillin allergic patients, besides non-availability in some countries [17]. High Dose Double Therapy (HDDT), a combination of amoxicillin (1g tid or 750 mg qid) and PPI (standard dosage TID or QID or standard double dosage BID) for 14 days [18], showed similar effectiveness with the bismuth therapy, in addition to fewer side effects [19]. Thus, as the successful achievement of HDDT depends on an appropriate pH, double therapy with VPZ appears as a satisfactory possibility of eradicating the bacterium with few antibiotics and shorter treatment [17]. On the other hand, a new capsule containing Bismuth, Metronidazole and Tetracycline has been related to a success rate of approximately 90% in first-line and secondline treatments [20]. Furthermore, the Bismuth Quadruple Therapies (BQTs) are still effective and safe in most countries, and addition of bismuth on triple therapies improved cure rates in 30% to 40% of subpopulations with resistant strains [21]. Moreover, Rifabutin is an antimicrobial non-degradable by the gastric acid and with rare resistance to H. pylori strains that, along with PPI and amoxicillin or in BQT, achieved elimination rates of approximately 70%. Nevertheless, the high cost turns difficult the establishment of this therapy [22,23].
New pathways, such as the addition of probiotics to therapy, can inhibit colonization and adhesion of H. pylori and decrease the side effects, which improves the treatment adherence [24]. However, the use of single or multiple strains remains uncertain due to the low quality of studies currently available, which makes further research necessary [8]. Furthermore, the use of C and E vitamins can reduce oxidative stress and free radical production during the therapy, contributing to preventing tissue damage and gastric cancer development [25]. Lastly, drugs like Apigenin, Chrysin, Kaempferol and Hesperetin demonstrated in vitro antimicrobial activity to some strains resistant either by metronidazole and clarithromycin [18].
Future Perspectives
Dealing with H. pylori is an arduous task, and the main challenge against this disease is antibiotic resistance and consequent decrease of eradication rates [18,21]. The low availability of treatments capable of promoting effective bacterial eradication, especially with a single therapeutic attempt, is an issue that, along with insufficient bacterial culture with antibiotics susceptibility, makes it difficult to manage the course of the disease [26]. Of note, the World Health Organization listed, in 2017, clarithromycin-resistant H. pylori as a high-priority bacterium, which emphasizes the need to search for effective treatment regimens against this infection and for surveillance in this regard, given that high resistance rates also mean decline of possible options of treatment [14,26]. In this sense, studies assessing alternative treatments for bacterial eradication should be encouraged, in order not only to improve the effectiveness of antimicrobial drugs, but also to reduce their doses, side effects, and therapy length, enhancing the therapeutic adherence. An example is the use of VPZ combined with amoxicillin, since the association of this new acid secretion inhibitor increases the therapy success rate as well as reduces the frequency of side effects [8]. Although resistance rates remain low, the development of monitoring methods to Rifabutin effectiveness are needed in the future [27]. In addition, physicians should be instructed to choose more suitable empirical regimens for their patients, taking into consideration the local resistance profile and previous use of antimicrobial drugs by the patient. Thus, the verification of bacterial eradication or therapeutic failure should be performed in order to gather information on the effectiveness of therapeutic regimens and to characterize the local susceptibility profile [28].
Another option that has been studied is the use of antimicrobial peptides, which are compounds produced by cells as a result of innate immunity to generate protection against several pathogens. They can act on cell membranes or intracellular processes [29]. The 3 peptides with the highest anti-H. pylori activity were pexiganan, tilapia piscidin 4 and PGLa-AM1 [30]. Another approach that still lacks evidence is the use of natural compounds, such as medicinal plants, against H. pylori infection, mainly as an auxiliary agent in the treatment, but not as a monotherapy [31]. Ally to this, the use of anti-biofilm agents can also assist in the management of positive H. pylori patients. Despite being a new method, two peptides anti-biofilm (IDR-1018 e DJK-5) were identified to act against strains of the bacteria, by affecting different stages from H. pylori biofilm, which helps to circumvent drug resistance [32].
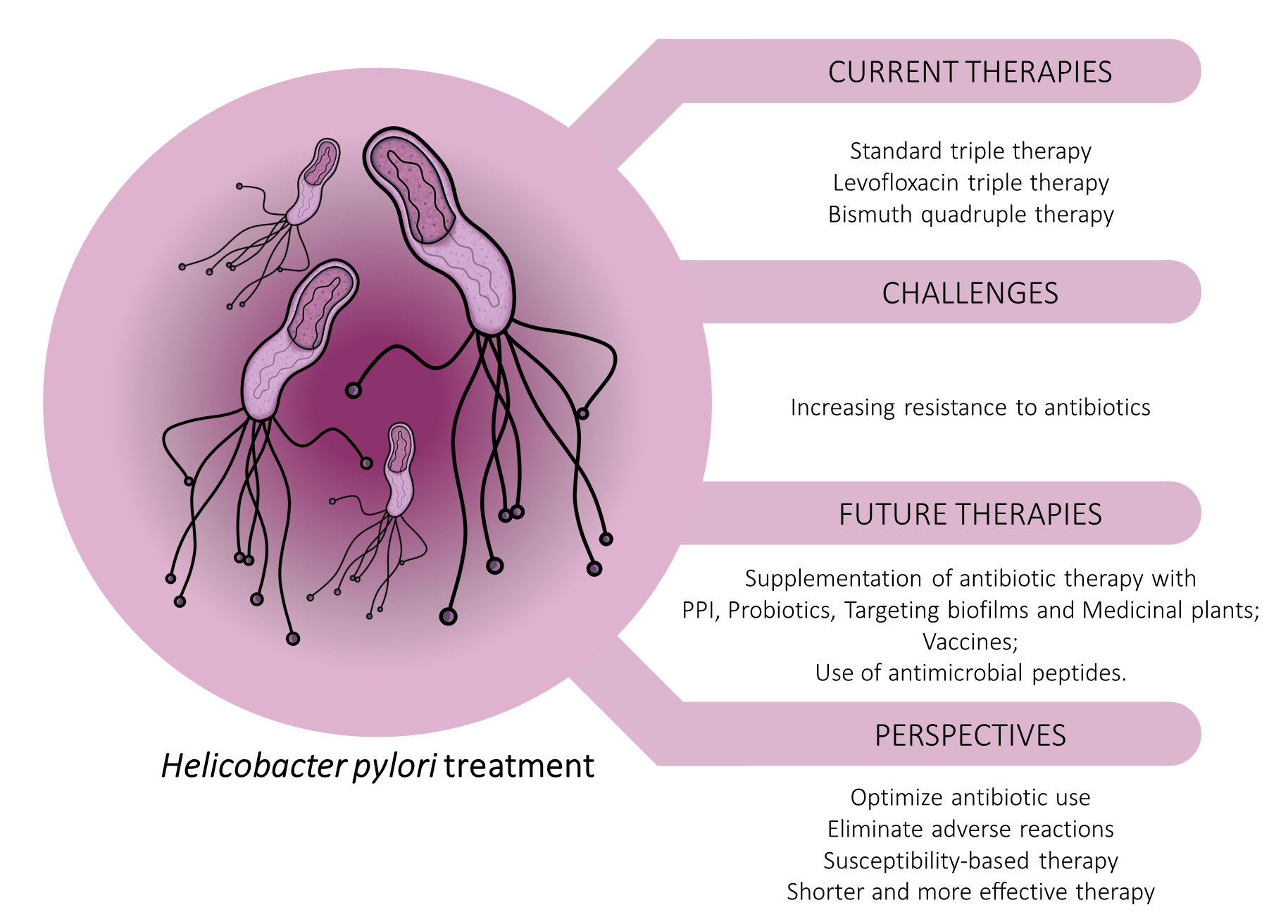
Finally, anti-H. pylori vaccines can be a potential strategy to reduce the infection prevalence and the number of unsuccessfully eradicated patients. However, the escape mechanisms of the bacteria make this process more difficult. Therefore, more studies are necessary to develop an adequate vaccine against H. pylori. [33,34]. (Figure 1) summarizes the main aspects the aspects addressed in this work related to the treatment against the H. pylori.
Figure 1. Schematic representation of currently used treatment of H. pylori infection and promising future approaches.
Conclusion
In this editorial, we outline the urgent global issue of treatment for H. pylori infection and antimicrobial resistance. It is evident that the treatment for H. pylori has become challenging, as the aforementioned standard therapies are losing effectiveness and there is an increase in antimicrobial resistance, which demands new effective eradication therapies, with medications that provide good safety profiles, minimum adverse effects and good patient compliance. The use of vonoprazan in dual therapy with amoxicillin seems to be a viable option. In addition, acid blockers, antibiotic adjuvants, anti-biofilm agents and vaccines appear to be important agents to control multidrug resistance. New controlled and randomized trials are essential to determine the future of the treatment against this bacterial infection.
References
2. Malfertheiner P, Venerito M, Schulz C. Helicobacter pylori infection: new facts in clinical management. Current Treatment Options in Gastroenterology. 2018 Dec;16(4):605-15.
3. Hu Y, Wan JH, Li XY, Zhu Y, Graham DY, Lu NH. Systematic review with meta-analysis: the global recurrence rate of Helicobacter pylori.Alimentary Pharmacology & Therapeutics. 2017 Nov;46(9):773-9.
4. Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016 May 1;150(5):1113-24.
5. Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. European Helicobacter Pylori Study Group. Gut. 1997 Jul;41(1):8-13.
6. Hunt R, Thomson AB. Canadian Helicobacter pylori consensus conference. Canadian Journal of Gastroenterology. 1998 Jan 1;12(1):31-41.
7. Zou Y, Qian X, Liu X, Song Y, Song C, Wu S, et al. The effect of antibiotic resistance on Helicobacter pylori eradication efficacy: A systematic review and meta-analysis. Helicobacter. 2020 Aug;25(4):e12714.
8. Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology. 2019 Jul 1;157(1):44-53.
9. Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016 Jul 1;151(1):51-69.
10. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Official Journal of the American College of Gastroenterology. 2017 Feb 1;112(2):212-39.
11. Malfertheiner P, Megraud F, O’morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017 Jan 1;66(1):6-30.
12. Alba C, Blanco A, Alarcón T. Antibiotic resistance in Helicobacter pylori. Current Opinion in Infectious Diseases. 2017 Oct 1;30(5):489-97.
13. Flores-Treviño S, Mendoza-Olazarán S, Bocanegra-Ibarias P, Maldonado-Garza HJ, Garza-González E. Helicobacter pylori drug resistance: therapy changes and challenges. Expert Review of Gastroenterology & Hepatology. 2018 Aug 3;12(8):819-27.
14. Gong Y, Yuan Y. Resistance mechanisms of Helicobacter pylori and its dual target precise therapy. Critical Reviews in Microbiology. 2018 May 4;44(3):371-92.
15. Sugimoto M, Yamaoka Y. Role of vonoprazan in Helicobacter pylori eradication therapy in Japan. Frontiers in Pharmacology. 2019 Jan 15;9:1560.
16. Matsumoto H, Shiotani A, Graham DY. Current and future treatment of Helicobacter pylori infections. Helicobacter Pylori in Human Diseases. 2019:211-25.
17. Suzuki S, Esaki M, Kusano C, Ikehara H, Gotoda T. Development of Helicobacter pylori treatment: How do we manage antimicrobial resistance?. World Journal of Gastroenterology. 2019 Apr 28;25(16):1907-12.
18. Hu Y, Zhu Y, Lu NH. Recent progress in Helicobacter pylori treatment. Chinese Medical Journal. 2020 Feb 5;133(3):335-43.
19. Yang X, Wang JX, Han SX, Gao CP. High dose dual therapy versus bismuth quadruple therapy for Helicobacter pylori eradication treatment: a systematic review and meta-analysis. Medicine. 2019 Feb;98(7):e14396.
20. Nyssen OP, McNicholl AG, Gisbert JP. Meta-analysis of threein- one single capsule bismuth-containing quadruple therapy for the eradication of Helicobacter pylori. Helicobacter. 2019 Apr;24(2):e12570.
21. Hu Y, Zhu Y, Lu NH. Novel and effective therapeutic regimens for Helicobacter pylori in an era of increasing antibiotic resistance. Frontiers in Cellular and Infection Microbiology. 2017 May 5;7:168.
22. Roszczenko-Jasinska P, Wojtys MI, Jagusztyn-Krynicka EK. Helicobacter pylori treatment in the post-antibiotics era—searching for new drug targets. Applied Microbiology and Biotechnology. 2020 Oct 14:9891-9905.
23. Gisbert JP. Rifabutin for the Treatment of Helicobacter pylori Infection: A Review. Pathogens. 2021 Jan;10(1):15.
24. Qureshi N, Li P, Gu Q. Probiotic therapy in Helicobacter pylori infection: a potential strategy against a serious pathogen?. Applied Microbiology and Biotechnology. 2019 Feb;103(4):1573-88.
25. González A, Salillas S, Velázquez-Campoy A, Angarica VE, Fillat MF, Sancho J, et al. Identifying potential novel drugs against Helicobacter pylori by targeting the essential response regulator HsrA. Scientific Reports. 2019 Aug 5;9(1):11294.
26. De Francesco V, Zullo A, Gatta L, Manta R, Pavoni M, Saracino IM, et al. Rescue Therapies for H. pylori Infection in Italy. Antibiotics. 2021 May;10(5):525.
27. Gong Y, Yuan Y. Resistance mechanisms of Helicobacter pylori and its dual target precise therapy. Critical Reviews in Microbiology. 2018 May 4;44(3):371-92.
28. Georgopoulos S, Papastergiou V. An update on current and advancing pharmacotherapy options for the treatment of H. pylori infection. Expert Opinion on Pharmacotherapy. 2021 Apr 13;22(6):729-41.
29. Sierra Ortigosa JM, Fusté i Domínguez E, Rabanal Anglada F, Vinuesa Aumedes T, Viñas M. An overview of antimicrobial peptides and the latest advances in their development. Expert Opinion on Biological Therapy. 2017 Apr 11;17:6:663-676.
30. Neshani A, Zare H, Akbari Eidgahi MR, Hooshyar Chichaklu A, Movaqar A, Ghazvini K. Review of antimicrobial peptides with anti- Helicobacter pylori activity. Helicobacter. 2019 Feb;24(1):e12555.
31. Lin J, Huang WW. A systematic review of treating Helicobacter pylori infection with Traditional Chinese Medicine. World Journal of Gastroenterology: WJG. 2009 Oct 7;15(37):4715-19.
32. Windham IH, Servetas SL, Whitmire JM, Pletzer D, Hancock RE, Merrell DS. Helicobacter pylori biofilm formation is differentially affected by common culture conditions, and proteins play a central role in the biofilm matrix. Applied and Environmental Microbiology. 2018 Jul 2;84(14):e00391-18.
33. Mejías-Luque R, Gerhard M. Immune evasion strategies and persistence of Helicobacter pylori. Molecular Pathogenesis and Signal Transduction by Helicobacter Pylori. 2017:53-71.
34. Dos Santos Viana I, Cordeiro Santos ML, Santos Marques H, Lima de Souza Gonçalves V, Bittencourt de Brito B, França da Silva FA, et al. Vaccine development against Helicobacter pylori: from ideal antigens to the current landscape. Expert Review of Vaccines. 2021 Aug 3:1-11.